Electrochemical treatment of distillery effluent using catalytic anodes
Abstract
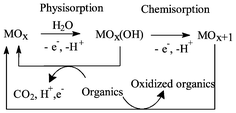
Electrochemical treatment processes can provide valuable contributions to the protection of the environment through the minimization of waste and toxic materials in effluents. Complete decolourisation of the distillery effluent is a difficult task. The industry treated effluent still has a brown colour. This paper reports complete colour removal of industry treated distillery effluent through an electrochemical technique. Electrolysis of distillery effluent was carried out in a static electrochemical reactor. Two different types of anodes, planar graphite (Gr) and titanium substrate insoluble anodes (TSIA) were chosen for the treatment of distillery effluent. Lead dioxide coated on titanium (PbO2–Ti) and ruthenium oxide coated on titanium (RuO2–Ti) electrodes were used as TSIA. Current density (C.D.) was varied from 1.5 to 5.5 A dm−2. Complete decolourisation was obtained in both cases. A maximum of 92% of chemical oxygen demand (COD) reduction, 98.1% of biological oxygen demand (BOD) reduction and 99.5% of absorbance reduction were obtained in the set up in which RuO2–Ti as anode and stainless steel as cathode were used. A probable mechanism has been proposed for the oxidation of organics present in the effluent.

 Please wait while we load your content...
Please wait while we load your content...