Möbius aromaticity in bipyramidal rhodium-centered bismuth clusters
Abstract
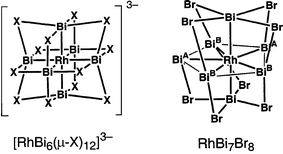
The chemical bonding topologies in the mixed rhodium–bismuth clusters [RhBi6(µ-X)12]3− (X = Br, I) and RhBi7Br8 are compared. The octahedral cluster [RhBi6(µ-X)12]3− can be considered as a d6 Rh(III) complex containing six equivalent BiX2− ligands with interligand halogen bridging but no Bi–Bi bonding. However, the pentagonal bipyramidal cluster RhBi7Br8 has only two similar BiBr2− ligands in the axial positions. The five equatorial bismuth atoms in RhBi7Br8 form a bonding pentagon, which is an example of a five-atom Möbius aromatic system with a five-center four-electron bond. This unusual bond involves σ-type overlap between p orbitals in the plane of the pentagon with the single phase change required for Möbius aromaticity.

 Please wait while we load your content...
Please wait while we load your content...