Determination of hydrogen concentration in ionic liquids and the effect (or lack of) on rates of hydrogenation
Abstract
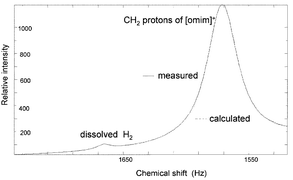
The solubility of hydrogen and the corresponding Henry coefficients for 11 ionic liquids have been determined in situ at 100 atm H2 pressure and are much lower than expected; attempts to correlate the solubility of hydrogen in the ionic liquids with the rate of reaction for the hydrogenation of benzene to cyclohexane in these solvents have been made.

 Please wait while we load your content...
Please wait while we load your content...