Bond energy M–C/H–C correlations: dual theoretical and experimental approach to the sensitivity of M–C bond strength to substituents†
Abstract
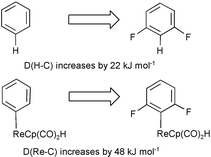
DFT methods are used to quantify the relationship between M–C and H–C bond energies; for MLn = Re(η5-C5H5)(CO)2H and fluorinated aryl

 Please wait while we load your content...
Please wait while we load your content...