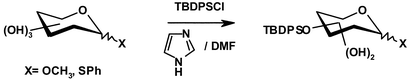
The regioselective protection of secondary hydroxy groups of gluco-, galacto-, manno-, rhamno- and fucopyranosides using TBDPSCl with imidazole in DMF has been studied. It was found that the relative spatial arrangement of the OH groups modulates the silylation selectivity which arises from the combination of kinetic factors and the intramolecular migrations of the secondary TBDPS groups. The rearrangement of the TBDPS groups has a much larger effect on the α-D-manno- and α-L-rhamnopyranosides, allowing the protection of the OH groups at positions 2, 3 or 2 and 4, in synthetically useful yields, by changing the reaction conditions. The relative reactivity of the secondary OH groups seems particularly likely to be governed by steric factors. This trend provides a valuable approach to the synthesis of 3-O-tert-butyldiphenylsilyl-1-thio-β-L-fucopyranoside.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...