Asymmetric synthesis of β-pyridyl-β-amino acid derivatives
Abstract
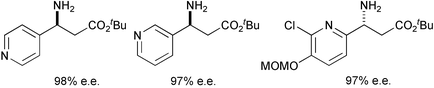
The conjugate additions of homochiral lithium (R)-N-benzyl-N-α-methyl-4-methoxybenzylamide to tert-butyl 3-(3-pyridyl)- and tert-butyl 3-(4-pyridyl)-prop-2-enoates proceed in 84% de, and after subsequent recrystallisation and oxidative N-deprotection furnish the (S)-3-(3-pyridyl)- and (S)-3-(4-pyridyl)-β-amino acid derivatives in 97% ee and 98% ee respectively. Conjugate additions of lithium N-benzyl-N-α-methyl-4-methoxybenzylamide to tert-butyl 3-(2-pyridyl)prop-2-enoates proceed with low levels of diastereoselectivity unless the 3-(2-pyridyl) ring is substituted. Application of this methodology allows the asymmetric synthesis of (R)-tert-butyl 3-(2-chloro-3-methoxymethoxy-6-pyridyl)-3-aminopropanoate, the protected β-amino ester component of kedarcidin, in 97% ee.

 Please wait while we load your content...
Please wait while we load your content...