Intramolecular Diels–Alder reaction of cyclopenta-1,3-diene derivatives generated in situ from 4-(pent-4-enyl)cyclopent-2-enone ethylene ketals
Abstract
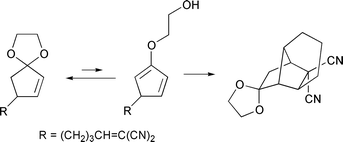
Intramolecular Diels–Alder reaction of cyclopenta-1,3-diene derivatives 16 generated in situ from the corresponding 4-(pent-4-enyl)cyclopent-2-enone ethylene ketals 15 is investigated. 4-[(E)-5-(Ethoxycarbonyl)pent-4-enyl]cyclopent-2-enone ethylene ketal 15a and 4-(5,5-dicyanopent-4-enyl)cyclopent-2-enone ethylene ketal 15b were prepared in 5 steps from cyclopent-2-enone ethylene ketal 5. Upon heating at 120 °C, compound 15a undergoes intramolecular Diels–Alder reaction via the 4-substituted cyclopenta-1,3-dien-2-yl enol ether 17a to produce tricyclo[5.2.1.0]decan-8-one derivative 19a as the sole product. On the other hand, 15b undergoes intramolecular Diels–Alder reaction at the same temperature via the 5-substituted cyclopenta-1,3-dien-2-yl enol ether 16b to give tricyclo[4.4.0.0]decan-10-one derivative 18b as the major product. The molecular structures of these cyclization products are unequivocally elucidated by X-ray crystallographic analyses. The latter reaction represents, to our knowledge, the first direct trapping of 5-substituted cyclopenta-1,3-diene derivative having a simple three-carbon tether by Diels–Alder cyclization prior to isomerization by 1,5-hydrogen migration.

 Please wait while we load your content...
Please wait while we load your content...