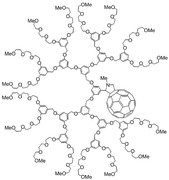
Poly(aryl ether) dendritic branches terminated with peripheral triethyleneglycol chains have been attached to C60. The resulting fullerodendrimers have been characterised by electrospray mass spectrometry (ESMS), which appears to be a particularly interesting analytical tool for the unambiguous structural assignment of such high molecular weight fullerene derivatives. Their photophysical properties have been systematically investigated in three solvents, namely toluene, dichloromethane, and acetonitrile. The changes observed in the photophysical properties along the series suggest an increasing interaction between the poly(aryl ether) dendritic wedges and the fullerene core, which brings about an increasing isolation of the central chromophore from the exterior. Finally, thanks to their high solubility, fullerodendrimers 1–4 have been easily incorporated in mesoporous silica glasses and preliminary measurements
on the resulting doped samples have revealed efficient optical limiting properties.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...