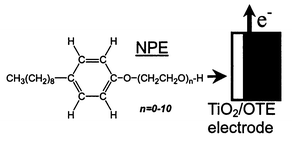
Photocurrent is generated during the photooxidation of nonylphenol polyethoxylate surfactants (NPE-n; n = 1, 5, 7, 9 and 10), precursors of 4-nonylphenol (NP), on illuminated TiO2/OTE electrodes on which TiO2 semiconductor particles were immobilized by a pasting (PA), a sol–gel (SG), or both techniques (HY). The photooxidative process of NP and NPEs was examined by absorption spectroscopic methods monitoring the cleavage of the benzene ring moiety, and by the evolution of CO2 gas. The kinetics of photooxidation and the magnitude of the generated photocurrent scaled with the length of the ethoxyl side-chain for NPEs where n = 0, 1, 5, 7 and 10. The relative order of adsorption of NPEs on the positive TiO2 surface was predicted from calculated partial negative point charges of all the C and O atoms in the NPEs using molecular orbital methods. Both photodegradation and photocurrent generation were enhanced for the NPE-9 substrate using the HY TiO2/OTE electrode compared to the PA and SG electrodes. Although, biodegradation of NPEs yields NP in rivers, no formation of NP was observed during the photooxidation of NPE-9 because of predominant opening of the benzene ring. Photooxidation of NPEs is facilitated under an applied bias of +0.3 V and scales with the number of ethoxylate groups.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...