Transition-metal imido-boroxide complexes: a structural and spectroscopic investigation of the influence of boron
Abstract
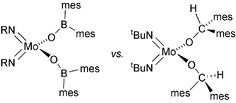
The four coordinate compounds Mo(NR)2[OB(mes)2]2 (1, R = tBu; 2, R = Ar = 2,6-iPr2C6H3) have been obtained from the reaction of [(mes)2BOLi(Et2O)n]x with the corresponding Mo(NR)2Cl2(dme) starting material; for structural comparison, Mo(NtBu)2[OCH(mes)2]2 (3) was also synthesised. The related five-coordinate complexes Ti(NtBu)[OB(mes)2]2(py)2 (4) and Ti(NtBu)[OCH(mes)2]2(py)2 (5) were made using analogous procedures. X-Ray structural investigations of 1–5 were performed to assess the effect that the boron atom has on the metal–oxygen and metal–nitrogen(imido) bond lengths and angles. On average, both classes of compound displayed longer metal–oxygen bonds and shorter metal–nitrogen(imido) bonds for the boroxide derivatives. Spectroscopic investigation of the Δδ values for the tert-butylimido derivatives revealed that complexes incorporating the [OB(mes)2]− anion exhibit a reduction in the electron density at the imido

 Please wait while we load your content...
Please wait while we load your content...