Bridging mode flexibility of 1,3-dithiacyclohexane in silver(i) co-ordination polymers†
Abstract
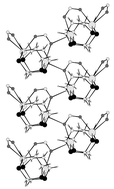
Co-ordination polymers with Ag∶1,3-dithiane molar ratios of 1∶1, {[Ag2(1,3-dithiane)2][X][Y]}∞ (X = Y = NO3, 1; X = Y = PF6, 2; X = Y = BF4, 3; X = BF4, Y = Cl, 4; X = Y = NO2, 5, and 2∶1, {[Ag2(1,3-dithiane)(SO4)(H2O)2]·H2O}∞6, the extended structures of which are 1-D chains and 2-D sheets, respectively, have been synthesised and structurally characterised. The recurrent structural motif is the [Ag2(μ-1,3-dithiane)2]2+ cationic dimer in which two chair-conformation 1,3-dithiane molecules act as two-connecting units to bridge two Ag(I) centres. In 1, 2, and 6, the dimers are linked into near-linear chains by pairs of bridging anions via formation of an Ag2X2 rhomboid dimer. In 3, instead of forming the Ag2X2 (X = F) rhomboid dimer, two [BF4−] anions form μ2-F,F′-bridges which support weak Ag–S interactions between cations to give a saw-tooth chain structure. This extra Ag–S contact makes the 1,3-dithiane a three-connecting unit. In 4 and 5, the Ag(I) centres of the cationic dimer are bridged not only by two 1,3-dithiane molecules but also by a Cl− or NO2− anion, respectively, to give [Ag2(μ-1,3-dithiane)2(μ2-Cl)]+ (4) and [Ag2(μ-1,3-dithiane)2(μ2-O,O′-NO2)]+ (5). In 4, the Cl− anion acts as a μ4-bridge to link three [Ag2(μ-1,3-dithiane)2]2+ moieties and generate a zig-zag chain. In 5, one oxygen of the bridging NO2− anions acts as a μ2-bridge to link two [Ag2(μ-1,3-dithiane)2]2+ dimers and generate a saw-tooth chain. Although the chains in 1, 2, 3 and 5 are uncharged, that in 4 is cationic and that in 6 is anionic. Charge balance in 4 is maintained by a non-co-ordinated [BF4−] anion; that in 5 is maintained by ‘Ag(H2O)2+’, the co-ordination polyhedron of which is completed by sulfur donors from separate 1,3-dithiane molecules, which thus act as four-connecting units, and by two oxygens of separate SO42− anions to give a two-dimensional sheet structure.

 Please wait while we load your content...
Please wait while we load your content...