A new dipyridine-containing cryptand for both proton and Cu(ii) encapsulation. A solution and solid state study
Abstract
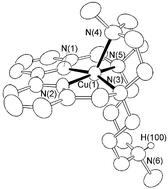
Synthesis and characterization of the new polyamine cryptand 2,5,8-triaza-5-methyl-2,8-(N-methyldipropylamino)[9]-2,2′-dipyridinophane (L) and its macrocyclic precursor 2,5,8-triaza-5-methyl[9]-2,2′-dipyridinophane (L1) are reported. Ligand L1 contains a diethylenetriamine chain linking the 6,6′ positions of a 2,2′-dipyridine moiety; in L an N-methyldipropylamine bridge links the two benzylic nitrogens of L1. Protonation and Cu(II) coordination have been studied in aqueous solution by means of potentiometric and UV-vis spectrophotometric measurements. Considering proton binding, cryptand L behaves as a proton sponge, i.e., the first protonation constant is too high to be measured in aqueous solution. Both ligands form 1 ∶ 1 Cu(II) complexes in aqueous solutions. The crystal structure of [CuL1]2+ reveals that the metal is coordinated by the five ligand donors in a strongly distorted square pyramidal geometry. Almost the same coordination environment is found in the protonated complex with L, [CuLH]3+, while the nitrogen of the N-methyldipropylamine bridge is protonated. In marked contrast, in the [CuL]2+ complex this nitrogen is involved in metal coordination. Both the solution and structural results account for the high rigidity of the macrocyclic moiety of L defined by the dipyridine unit and the diethylenetriamine chain, while coordination of the more flexible N-methyldipropylamine unit is modulated by complex protonation.

 Please wait while we load your content...
Please wait while we load your content...