SelectfluorTM F-TEDA-BF4 mediated and solvent directed iodination of aryl alkyl ketones using elemental iodine†
Abstract
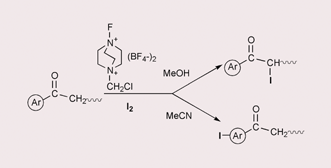
Reactions of aryl alkyl ketones with methanol solution of elemental iodine and 1-fluoro-4-chloromethyl-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (Selectfluor™ F-TEDA-BF4) result in the formation of corresponding α-iodo ketones, while switch over of the regioselectivity can be directed by using acetonitrile as the solvent and selective iodination of the aromatic site of target molecules is thus achieved.

 Please wait while we load your content...
Please wait while we load your content...