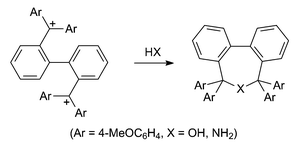
Reactions of the bis-carbenium ion 2,2′-bis[bis(p-methoxyphenyl)methyl]biphenyl ditetrafluoroborate with ammonia, n-propylamine and benzylamine have been studied with the aim of developing an acid-labile protecting group for primary amines that masks both hydrogen atoms of the NH2 group. Although the parent 5,5,7,7-tetrakis(p-methoxyphenyl)-5,7-dihydrodibenzo[c,e]azepine was isolated and fully characterised, the corresponding azepines could not be obtained in a pure state from the reactions with the primary amines. The bis-carbenium ion was prepared from the treatment of 2,2′-bis[bis(p-methoxyphenyl)hydroxymethyl]biphenyl with fluoroboric acid. Attempts to prepare the corresponding diphenyl analogue were unsuccessful using either fluoroboric acid or boron trifluoride. The only product isolated from these reactions was 5,5,7,7-tetraphenyl-5,7-dihydrodibenzo[c,e]oxepin. The oxepin and its phenyl-p-methoxyphenyl and bis(p-methoxyphenyl) analogues were efficiently obtained by dehydration of the corresponding diol, e.g. 2,2′-bis[bis(p-methoxyphenyl)hydroxymethyl]biphenyl, in the presence of 3 Å molecular sieves or Amberlite IR-200 resin. The compounds 2,2′-bis[bis(p-methoxyphenyl)hydroxymethyl]biphenyl, (R,R/S,S)-2,2′-[bis(phenyl-p-methoxyphenyl)hydroxymethyl]biphenyl, 5,5,7,7-tetraphenyl-5,7-dihydrodibenzo[c,e]oxepin, 5,5,7,7-tetrakis(p-methoxyphenyl)-5,7-dihydrodibenzo[c,e]oxepin and 5,5,7,7-tetrakis(p-methoxyphenyl)-5,7-dihydrodibenzo[c,e]azepine were characterised by crystal structure analyses. 2,2′-Bis[bis(p-methoxyphenyl)hydroxymethyl]biphenyl and 5,5,7,7-tetrakis(p-methoxyphenyl)-5,7-dihydrodibenzo[c,e]oxepin exhibited dynamic NMR properties, and free energy barriers have been determined.

 Please wait while we load your content...
Please wait while we load your content...