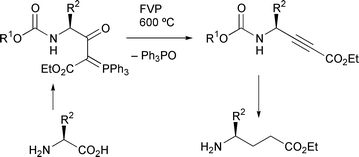
Flash vacuum pyrolysis of stabilised phosphorus ylides. Part 17.1 Preparation of aliphatic amino acid derived γ-alkoxycarbonylamino-β-oxo ylides and pyrolysis to give α,β-acetylenic γ-amino acid and GABA analogues
Abstract
A series of eleven α-aminoacyl stabilised ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Et using a
CHCO2Et using a

 Please wait while we load your content...
Please wait while we load your content...