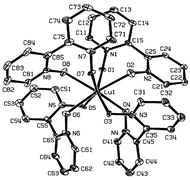
Single crystals of [Ln(2,2′-bipyridine-1,1′-dioxide)4](ClO4)3
(Ln = Nd, Lu) have been obtained and the crystal structures determined. By comparison with previous structural determinations, it is demonstrated that steric crowding associated with the decreasing lanthanide ions radius causes changes of the angles between the rings of the ligand. This leads to a deformation of the coordination polyhedron from an almost ideal cube (La, Ce) to a dodecahedron distorted towards a square antiprism (for Nd and heavier lanthanides), and slightly distorted square antiprism for Lu. Electronic absorption spectra of Nd(bpyO2)43+ and Eu(bpyO2)43+
(bpyO2 = 2,2′-bipyridine-1,1′-dioxide) at room temperature and 4 K were measured in CH3CN and CH3NO2 and in the solid state. Emission and excitation spectra, and lifetime measurements of Tb(bpyO2)43+ at 293 and 77 K are presented and used to characterize the excited state photophysics of this species. Comparisons are made to previous results, and generalizations concerning molecular and electronic structure for the series of complexes are presented.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...