1,2-Migration in β-(acyloxy)ethyl radicals revisited—concerted or stepwise?†
Abstract
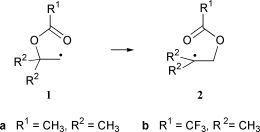
The acyloxy rearrangement in 2-(acetyloxy)-2-methyl-1-propyl radical (1a) and in 2-(trifluoroacetyloxy)-2-methyl-1-propyl radical (1b) has been investigated with a number of theoretical methods. In both systems the most favorable reaction pathway for 1,2-acyloxy rearrangement leads through a five-membered ring transition state in a concerted fashion. A second pathway through a three-membered ring transition state is only slightly less favorable, while the addition–elimination process through a cyclic 1,3-dioxolan-2-yl radical intermediate has significantly higher barriers. Stationary points corresponding to a contact ion pair could not be found. With respect to the most favorable reaction pathway, the barrier difference between substrates 1a and 1b amounts to 2.5 ± 0.1 kcal mol−1
at a variety of theoretical levels.

 Please wait while we load your content...
Please wait while we load your content...