Aroylthioureas: new organic ionophores for heavy-metal ion selective electrodes†
Abstract
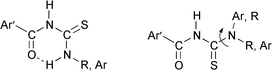
Thiourea derivatives (46 aroylthioureas) having different substituents close to the sulfur atom were synthesized and their ionophore potential in ion selective electrodes (ISEs) was examined. Structural considerations were taken into account based on the corresponding heavy-metal ISE parameters. As ionophores, some 1-furoyl-3-substituted thioureas (series 2) gave the best results in Pb(II), Hg(II) and Cd(II) ISEs. The strong intramolecular hydrogen bond in series 2 allows ligand interaction only through the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group. Substituents on the furan and phenyl rings give rise to low solubility in the membrane plasticizer. 3-Alkyl substituted furoylthioureas improve solubility but enhance oxidative processes with chain length. New X-ray diffraction (XRD) structures and theoretical DFT calculations were considered in the analysis of the substituent influence on the selectivity of ISEs. These new ionophores have advantages because of their stability, simple synthesis and easy modification of the sulfur binding ability resulting from substitution.
S group. Substituents on the furan and phenyl rings give rise to low solubility in the membrane plasticizer. 3-Alkyl substituted furoylthioureas improve solubility but enhance oxidative processes with chain length. New X-ray diffraction (XRD) structures and theoretical DFT calculations were considered in the analysis of the substituent influence on the selectivity of ISEs. These new ionophores have advantages because of their stability, simple synthesis and easy modification of the sulfur binding ability resulting from substitution.

 Please wait while we load your content...
Please wait while we load your content...