Conjugate additions of organocuprates to a 3-methylene-6-isopropyldiketopiperazine acceptor for the asymmetric synthesis of homochiral α-amino acids
Abstract
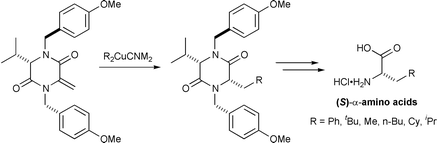
Addition of a range of organocuprates to (S)-N,N′-bis(p-methoxybenzyl)-3-methylene-6-isopropylpiperazine-2,5-dione 8 affords cis-3-isopropyl-6-alkyldiketopiperazines in excellent yield and >95% de. Subsequent deprotection and

 Please wait while we load your content...
Please wait while we load your content...