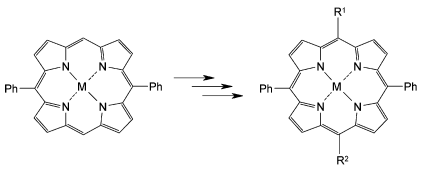
Functionalized tri- (A2B-type) and tetra- (A2BC-type) meso-substituted asymmetric porphyrins bearing highly reactive centers like -NH2, -OH, -C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, -CHO in substituents at the meso positions were synthesized in good yields via the reaction of 5,15-diphenylporphyrin with corresponding functionalized organolithium reagents. Through further transformation other functional groups like carboxylate, iodophenyl, thiocarboxylate, tertiary amines and quaternary ammonium salts are easily available. Such porphyrins serve as precursors for highly complex tetrapyrrolic systems. As examples, several novel porphyrins with potentially useful chemical and physical properties such as amphiphilicity, water solubility, and electrochemical redox activity were synthesized. In contrast to existing methods such compounds are now accessible regioselectively via one-step or two-step reactions in high yields. In addition, protocols were developed to prepare porphyrins with meso-aryl substituents bearing functional groups at the para, meta, or ortho position. Thus, starting materials for various specifically superstructured tetrapyrroles are available via rational syntheses.
CH, -CHO in substituents at the meso positions were synthesized in good yields via the reaction of 5,15-diphenylporphyrin with corresponding functionalized organolithium reagents. Through further transformation other functional groups like carboxylate, iodophenyl, thiocarboxylate, tertiary amines and quaternary ammonium salts are easily available. Such porphyrins serve as precursors for highly complex tetrapyrrolic systems. As examples, several novel porphyrins with potentially useful chemical and physical properties such as amphiphilicity, water solubility, and electrochemical redox activity were synthesized. In contrast to existing methods such compounds are now accessible regioselectively via one-step or two-step reactions in high yields. In addition, protocols were developed to prepare porphyrins with meso-aryl substituents bearing functional groups at the para, meta, or ortho position. Thus, starting materials for various specifically superstructured tetrapyrroles are available via rational syntheses.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, -CHO in substituents at the meso positions were synthesized in good yields via the reaction of
CH, -CHO in substituents at the meso positions were synthesized in good yields via the reaction of 

 Please wait while we load your content...
Please wait while we load your content...