Synthesis and reactivity of arylgold(III) complexes from aromatic hydrocarbons via C–H bond activation
Abstract
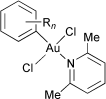
The reactions of anhydrous gold(III) chloride [AuCl3]2 with aromatic hydrocarbons (ArH) such as benzene, toluene, xylenes, mesitylene, cumene, methoxybenzene and chlorobenzene, and the following treatment with 2,6-lutidine (lut) gave stable arylgold(III) complexes [AuArCl2(lut)]. These auration reactions proceeded heterogeneously in hexane and homogeneously in diethyl ether. The 1H NMR spectra of the arylgold(III) complexes revealed that aurations towards aromatic compounds take place regiospecifically at the position with higher electron density and with less steric hindrances. The trans configuration of the arylgold(III) complexes was established by means of their far-IR spectra and confirmed for [Au(2,5-Me2C6H3)Cl2(lut)] by its single-crystal X-ray structure. The reactions of [AuArCl2(lut)] (Ar = phenyl, 2,5-xylyl) with a terminal alkyne, phenyacetylene (HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh), afforded arylated phenylacetylenes ArC
CPh), afforded arylated phenylacetylenes ArC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh.
CPh.

 Please wait while we load your content...
Please wait while we load your content...