A kinetic study of the reaction of NO2 with HI over the temperature range 278 to 333 K
Abstract
The rate constant, k, for the reaction, NO2
+ HI → products, has been measured directly for the first time. The rate was found to be − d[NO2]/dt
=
k[NO2][HI] with k
= (6.9 ± 1.1) × 10−19 cm3 molecule−1 s−1 at 303 K. The measurements were made under pseudo-first-order conditions with HI in excess over NO2. NO2 loss rate was monitored by 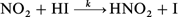 as the initiation step based on the kinetic and product studies, followed by a heterogeneous reaction between HNO2 and HI. A comparison of the reactions between NO2 with HX is made. This reaction is unlikely to play a major role in the removal of HI and as a source for HNO2 in the atmosphere because of its slow rate.
as the initiation step based on the kinetic and product studies, followed by a heterogeneous reaction between HNO2 and HI. A comparison of the reactions between NO2 with HX is made. This reaction is unlikely to play a major role in the removal of HI and as a source for HNO2 in the atmosphere because of its slow rate.

 Please wait while we load your content...
Please wait while we load your content...