Abstract
The aim of this study was to generate spinel-type oxides by a
continuous ultrasonic aerosol pyrolysis procedure and to evaluate the chemical
and physical properties of the product powders. The ultrasonic aerosol pyrolysis
process consists of three steps, which include “atomisation” of
an aqeous metal nitrate solution, transport to the furnace system and controlled
thermal decomposition of the precursor aerosol. The synthesis method allows
the production of pure CuMn2O4, LiMn2O4
and CuCo2O4 spinels, whereas NiMn2 − xO4
contains small quanitities of α-Mn2O3. The powders
are finely dispersed, mesoporous products exhibiting a uniform morphology.
Scanning electron microscopy reveals that the product is made up of hollow
spheres. XRD, TA and XPS results reveal that CuMn2O4
exists in a metastable state. It segregates in air already at temperatures
of 300 °C, whereas in an inert atmosphere the spinel is stable
up to 600 °C. XPS measurements indicate the presence of a redox
system in the form of 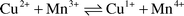 , which is one of the main features for the
high turnover rates of CO with O2 of this catalyst already at room
temperature. XPS measurements, after treatment of CuMn2O4
with CO and O2, confirm the highly reactive surface. NiMn2O4
and CuCo2O4 also show high activities in the catalytic
CO oxidation. LiMn2O4 is rather inactive. A comparison
of CuMn2O4 with LiMn2O4 points
to the crucial influence of the copper cations on the conversion rates of
the CO oxidation. In the synthesis of CuMn2O4 the introduction
of glucose into the starting metal nitrate solution revealed changed properties
of the product powders: as a consequence, the distribution of the cations
on the surface is changed and markedly smaller conversion rates for the catalytic
CO oxidation are observed.
, which is one of the main features for the
high turnover rates of CO with O2 of this catalyst already at room
temperature. XPS measurements, after treatment of CuMn2O4
with CO and O2, confirm the highly reactive surface. NiMn2O4
and CuCo2O4 also show high activities in the catalytic
CO oxidation. LiMn2O4 is rather inactive. A comparison
of CuMn2O4 with LiMn2O4 points
to the crucial influence of the copper cations on the conversion rates of
the CO oxidation. In the synthesis of CuMn2O4 the introduction
of glucose into the starting metal nitrate solution revealed changed properties
of the product powders: as a consequence, the distribution of the cations
on the surface is changed and markedly smaller conversion rates for the catalytic
CO oxidation are observed.

 Please wait while we load your content...
Please wait while we load your content...