Open Access Article
Open Access ArticleDriving ESG excellence in the pharmaceutical sector through an analytical approach: a roadmap for score improvement
Gursharan
Singh
 and
Ashutosh
Kumar
and
Ashutosh
Kumar
 *
*
Department of Energy & Environment, Thapar Institute of Engineering & Technology, Patiala 147004, Punjab, India. E-mail: ashu.kumar@thapar.edu
First published on 20th November 2025
Abstract
Environmental, Social, and Governance (ESG) assessment in India's active pharmaceutical manufacturing sector is gaining momentum as organizations strive to align with global sustainability benchmarks, such as EcoVadis, SEDEX, and the Dow Jones Industrial Average index. Nearly 43% of Indian pharmaceutical organizations have established dedicated ESG- or sustainability committees as of 2024. Between financial year (FY) 2021–22 and FY 2024–25, environmental factors typically received the greatest emphasis in ESG evaluations, while the social and governance dimensions were relatively underweighted, despite their critical influence on long-term corporate resilience. This imbalance highlights the need for comprehensive and integrated assessment models. The present study examines current ESG evaluation practices and identifies the crucial need for integrated frameworks, such as EcoVadis. The regulatory environment is being shaped by the rising demands of stakeholders, particularly investors, for the disclosure of this type of non-financial information, which they use as a foundation for investment decision-making. The findings of this study provide actionable insights for policymakers, rating agencies, and industry stakeholders to refine ESG reporting standards and accelerate the transition towards holistic sustainability practices across the pharmaceutical sector.
Environmental significanceIn recent years, there has been a growing interest among investors and customers in organizations that demonstrate strong adherence to Environmental, Social, and Governance (ESG) standards. This study aims to provide a comprehensive overview of non-financial disclosures within pharmaceutical organizations by analyzing strategies for enhancing ESG scores, using the EcoVadis model as a foundational framework. The ESG scores capture various dimensions of an organization's operations, including social responsibility, environmental impact, supply chain management, governance structures, internal policies, and overall business sustainability. The current study underscores the importance of integrating ESG metrics to enhance transparency and long-term value creation, while advocating the use of analytical tools to systematically monitor, report, and improve ESG performance. |
1 Introduction
In contemporary sustainability discourse, the core pillars of sustainability are defined by environmental, social, and governance (ESG) metrics, which serve as indicators of an organization's progress toward sustainable development.1 The environmental dimension assesses an organization's (small or medium, based on turnover) impact on ecological systems, including greenhouse gas (GHG) emissions, biodiversity, green belt encroachment, and pollution.2 Organizational efforts to improve ESG performance and achieve long-term sustainable growth are closely linked to ongoing environmental initiatives.3 The social pillar encompasses a company's interactions with its stakeholders, including employees, suppliers, customers, and local communities.4 The governance dimension addresses corporate governance mechanisms, such as internal control, board composition, executive compensation, audit practices, and shareholder rights.5Furthermore, with escalating legal and regulatory pressures, businesses are under growing expectations to align with evolving compliance and sustainability standards to ensure operational continuity and market relevance.6 Achieving ESG targets necessitates adherence to established sustainability benchmarks, incorporating capability building, technological advancement, and implementation of sustainable practices.7 Besides, ESG scores also reflect the degree of integration within an organization's supply chain8 and have become critical to investment decision-making by evaluating a company's environmental stewardship, social responsibility, and governance structures. These scores serve as strategic tools for investors, analysts, and stakeholders to assess non-financial risks and opportunities.9 Consequently, the standardization of ESG scoring has emerged as a critical component of contemporary market evaluation frameworks.10
Although numerous platforms provide ESG assessments, only a limited number of platforms are effectively driving improvements in an organization's ESG performance.11 ESG methodologies typically evaluate a broad array of non-financial management indicators, such as environmental impact, human rights, ethical practices, and sustainable procurement. The environmental pillar, for instance, is frequently assessed via proxies, such as environmental management systems.12 In environmentally sensitive sectors, social and governance structures, such as corporate social responsibility (CSR) initiatives, social welfare, and certifications play a pivotal role in ESG outcomes.13 Governance indicators include ownership structure, board oversight, ethical policies, and financial resilience.14 Recent studies introduced innovative approaches to ESG scoring due to its potential for enhancing brand value, attracting socially responsible investors, and mitigating regulatory risk.15 Recent studies have also identified significant gaps and challenges, emphasizing that top management commitment, stakeholder influence, and strategic alignment are critical drivers of organizational sustainability.16 Conversely, certified firms often adopt compliance-oriented pollution control measures, while uncertified firms may pursue more innovative, prevention-focused strategies.17 Empirical studies have demonstrated a strong correlation between ESG performance and financial indicators. For example, a study of 167 Indian firms between 2010 and 2020, found a positive relationship between ESG scores and return on assets, return on equity, and profitability.18 Similarly, higher ESG performance is associated with reduced downside financial risk.19 ESG incorporation and implementation of circular economy principles among leading organizations may be challenged by greenwashing practices, resulting in misleading information that can deceive the investors.20 However, ESG integration in pharmaceutical organizations faces sector-specific challenges that extend beyond general findings. Due to inherent challenges associated with handling hazardous chemicals, solvent-intensive methods, and substantial volume of effluents, the organizations are subjected to stringent regulatory regulations, making environmental performance a crucial area of attention and making it difficult to set their targets on various global platforms. Precisely, recording of energy use, water consumption, GHG emissions, and waste management practices is problematic, as these are perceived as see-through initiatives to employ sustainable manufacturing techniques and green chemicals.21 The temperatures above 200 °C are frequently required in manufacturing processes, resulting in a significant amount of fossil fuel consumption and subsequent GHG emissions. Furthermore, the higher GHG emissions lead to reporting challenges on various global platforms where GHG emission targets are disclosed.22
In the chemical industry, environmental sustainability is considered a top priority, and more systematic voluntary ESG disclosures are driven by public and governmental pressure. Listing social and health responsibilities over environmental reporting resulted in pharmaceutical organizations lagging in their ESG scores, however, their commitment to sustainability is increasing now.23 Nevertheless, significant gaps in sustainability reporting still exist in pharmaceutical organizations, especially in the underrepresentation of social and governance characteristics compared to the environmental disclosures.24 Social variables that are frequently disregarded, such as fair access, community impact, and labor standards, ultimately compromise the comprehensive understanding of sustainability performance. Additionally, the governance disclosures are inconsistent, which makes it very difficult for the stakeholders to evaluate ethical behavior and accountability.
Despite growing interest in ESG scoring, notable gaps remain in the standardization and transparency of scoring methodologies. Current models often lack consistency in data collection, validation, and weighting, resulting in significant variation in ESG ratings for comparable firms. The introduction of blockchain technology, a peer-to-peer dispersed network that enables safe and transparent value transfer, is an example of the current wave of digital growth that has shaped new avenues for enlightening ESG practices through improved sustainability reporting, accountability and traceability of organizations' disclosures.25 Furthermore, the majority of the existing literature emphasizes on theoretical frameworks with a limited practical consensus on comparative scoring mechanisms.26–29 In this context, the present study aims to provide current ESG valuation practices in Indian pharmaceutical organizations and underlines the importance of integrated frameworks like EcoVadis that holistically address all ESG dimensions and focusing specifically on non-financial disclosures, which are often underrepresented in the existing literature. This study tends to oversee the evident regulatory, operational, and disclosure complications faced by pharmaceutical organizations in India, creating a gap between ESG assessment and performance improvement. Theoretically, the limited assessment of integrated ESG frameworks in this sector limits the development of rigorous, authorized models that capture the adapted contribution of over 15 ESG categories and subcategories to the organization's overall ESG scores.
Additionally, this study provides significant empirical insights through exhaustive data analysis of ESG practices in pharmaceutical organizations, covering from FYs 2021–22 to 2024–25. By utilizing publicly accessible data from official websites and validating them with third-party assurance statements, the research ensures authenticity and transparency of the findings. Theoretically, the study expands understanding by integrating multi-dimensional ESG frameworks and scoring models based on principles adopted by leading global platforms, such as EcoVadis, SEDEX, and the Dow Jones Industrial Average index. It comprehensively analyzes important ESG categories and subcategories, determining their variable impacts on overall ESG scores. Furthermore, this work addresses sector-specific challenges related to ESG aspects, certifications, endorsements and disclosure practices, which are often underexplored in the existing literature. From a policy perspective, the study specifies practical suggestions to enhance ESG performance and disclosure quality in the pharmaceutical sector, filling a critical gap between ESG assessment and actionable corporate sustainability improvements. Finally, the study bridges the academic gaps in integrated ESG frameworks, applied gaps in sector-specific ESG data, and the practical gaps required for policy guidance tailored to industry experience.
2 Methodology and evaluation
2.1. About methodology
The behavioural patterns associated with various sustainability attributes were analysed in relation to three ESG dimensions, viz., Environmental, Social, and Governance. Drawing on the global EcoVadis assessment framework, an analytical methodology was employed to develop a structured ESG performance dashboard, aimed at enhancing the organizational ESG scores. EcoVadis utilizes a standardized rating system on a 0–100 scale, which is further categorized into five performance levels: insufficient, partial, good, advanced, and outstanding. Accordingly, the organizations were evaluated and classified based on their ESG performance (Table 1).| Parameters | Rating scale | Scoring scale |
|---|---|---|
| Environment | 0–100 | 0-Insufficient, 25-partial, 50-good, 75-advanced, 100-outstanding |
| Labour and human rights | 0–100 | 0-Insufficient, 25-partial, 50-good, 75-advanced, 100-outstanding |
| Ethics | 0–100 | 0-Insufficient, 25-partial, 50-good, 75-advanced, 100-outstanding |
| Sustainable procurement | 0–100 | 0-Insufficient, 25-partial, 50-good, 75-advanced, 100-outstanding |
2.2. Calculations and scoring
The employed methodology for ESG score calculation was consistent across all four assessment domains: environment, labour, sustainable procurement, and ethics. Each performance indicator was assessed using a defined set of seven parameters: (1) policies, (2) endorsements, (3) measures and actions, (4) certifications, audits, or labels, (5) implementation coverage, (6) reporting and key performance indicators (KPIs), and (7) 360-degree input, including disputes and awards. Furthermore, these seven parameters were consolidated into three primary themes: policies, actions, and reporting, which are illustrated in Fig. 1 with their respective weightage. These seven indicators, categorized under the three primary themes, policies, actions, and reporting, were assigned specific weights. Depending on the size and organization of the business, EcoVadis adjusts weightings to account for the various sustainability hazards and significant concerns relevant to different organizations, ultimately determining the final score for each indicator. Ecovadis assesses an organization based on evidence and measurable results reflected in documentation, which is thereafter analyzed. Moreover, 360° Watch is an AI-powered tool that scans external public sources to extract details of noncompliance or deviations, along with external audit recognition, for a transparent evaluation of the ESG score of any organization. The specific weight and calculation of the seven indicators are shown in Table 2. | ||
| Fig. 1 Three primary themes of ESG scoring, seven assessment indicators, and their weightage distribution. | ||
| Ecovadis assessment score model | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Three main indicators | S. no. | Seven parameters | Environment | Score against parameter | Score against indicator | Labour and human rights | Score against parameter | Score against indicator | Ethics | Score against parameter | Score against indicator | Sustainable procurement | Score against parameter | Score against indicator | Indicator weightage |
| Polices | 1 | Policies | 75 | 60 | 17.50 | 50 | 40 | 12.5 | 75 | 60 | 15 | 75 | 60 | 18.75 | 25% |
| 2 | Endorsement of sustainability initiatives | 50 | 10 | 50 | 10 | 0 | 0 | 75 | 15 | ||||||
| Actions | 3 | Measures and action | 100 | 65 | 36 | 75 | 48.75 | 30.75 | 75 | 48.75 | 10.50 | 75 | 48.75 | 24 | 40% |
| 4 | Certifications/audits/labels | 100 | 35 | 100 | 35 | 75 | 26.25 | 75 | 26.25 | ||||||
| 5 | Implementation coverage | 100 | 0.90 | 100 | 0.92 | 50 | 0.35 | 50 | 0.80 | ||||||
| Reporting | 6 | Reporting/KPIs | 75 | 30 | 26.25 | 75 | 30 | 26.25 | 75 | 30 | 26.25 | 100 | 40 | 29.75 | 35% |
| 7 | 360-degree input and Controversies/awards | 75 | 45 | 75 | 45 | 75 | 45 | 75 | 45 | ||||||
| Weight based on industry and size | 28% | 245.90 | 79.75 | 36% | 69.50 | 18% | 51.75 | 18% | 72.50 | ||||||
| Total score indicator | 22.33 | 25.02 | 9.31 | 13.05 | 69.71 | ||||||||||
For better understanding of scoring and calculation processes, the parameter: environment is explained here.
Example parameter: environment.
Indicators: (1) policies and (2) endorsement of sustainability initiatives.
Theme indicator: policies.
Weightage criteria: policies 80% of the total score achieved.
Endorsement of sustainability initiatives: 20% of the total score achieved.
Illustrative calculation: suppose the organization scores 75 marks for the policies indicator; the weightage is 80% of this score, yielding 60 weighted marks. Similarly, if 50 marks are scored for endorsement of sustainability initiatives, 20% of this score contributes an additional 10 weighted marks. Thus, the total weighted score under the policy theme is 70 (i.e., 60 + 10).
To derive the final theme score, the weighted score is normalized based on its contribution to the overall ESG score. If the policies theme accounts for 25% of the total ESG domain score, then the final score for this theme is calculated as: 25% of 70 = 17.5, as shown in Table 2. This approach is uniformly applied to the other three ESG domains to ensure consistency and comparability across performance indicators.
3 Results and discussion
To understand the score improvement framework better, the results of this study have been classified into three main sub-sections, namely: (a) disclosure practices and certifications, (b) ESG framework and scoring models, and (c) pharmaceutical industry-specific ESG difficulties. This framework facilitates a methodical analysis that connects international frameworks with the particulars of the pharmaceutical industry, highlighting how the certifications and changing disclosure guidelines foster transparency and accountability.3.1. Disclosure practices and certifications
Drawing on the background of existing ESG frameworks and their relevance to the pharmaceutical industry, this section analyses the scope, distribution, and functional significance of prominent ESG certifications and endorsements. Table 3 presents a comprehensive list of various ESG certifications and endorsements sourced from global platforms, sustainability reports, and recognized organizations.30,31 This table highlights the diversity of frameworks available for companies, seeking to enhance their sustainability credentials. All the key highlighted certifications and endorsements are mandatorily vital to enhance and develop a robust integrated management system to control the ESG issues. Sustainability certification audits follow a structured approach which provides a transparent picture of any organization and encourages the organization to make action plans for improving and strengthening its compliance towards ESG.32| Certifications and endorsements | Description | Environment | Social | Governance |
|---|---|---|---|---|
| ISO 9001:2015 | Quality management system | ✓ | ||
| ISO 14001:2015 | Environment management system | ✓ | ||
| ISO 45001:2018 | Occupational health and safety management system | ✓ | ||
| ISO 50001:2018 | Energy management system | ✓ | ||
| ISO 20400:2017 | Sustainable procurement standard guidelines | ✓ | ||
| ISO 27001:2022 | Information security standard | ✓ | ||
| ISO 37001:2016 | Anti-bribery management standard | ✓ | ||
| ISO 14064-1:2018 | Carbon footprint verification | ✓ | ||
| Responsible Care (RC logo) | Voluntary initiative by industries for obtaining the RC logo from Indian Chemical Council | ✓ | ✓ | ✓ |
| Aimed at improving environmental, health, safety, and security performance | ||||
| EcoVadis Rating | Global ESG performance rating platform | ✓ | ✓ | ✓ |
| Carbon Disclosure Project (CDP) | A non-profit organization for global ratings on disclosure | ✓ | ✓ | |
| It evaluates the performance of organizations on water security, climate change and forest | ||||
| United Nations Global Compact (UNGC) | Voluntary initiative to implement United Nations Sustainable Development Goals (17 SDGs) | ✓ | ✓ | ✓ |
| International Sustainability and Carbon Certification (ISCC) | Leading certification system supporting sustainable, fully traceable, deforestation-free and climate-friendly supply chains | ✓ | ✓ | ✓ |
| Science Based Targets and Initiatives (SBTi) | Monitors SBTi targets of organization in line with the Paris agreement | ✓ | ✓ | ✓ |
| Pharmaceutical Supply Chain Initiative (PSCI) | The PSCI is the leading association for pharmaceutical and healthcare companies | ✓ | ✓ | ✓ |
| SA8000:2014 | Social accountability standard for measuring social performance at workplaces | ✓ | ✓ | |
| CEO Water Mandate | Special initiative established in 2007 to advance corporate water stewardship around the world | ✓ | ✓ | |
| Supplier Ethical Data Exchange SEDEX (SMETA (Supplier Member Ethical Trade) Audit) | Assesses and monitors ethical practices within supply chains | ✓ | ✓ | ✓ |
| Dow Jones Sustainability Index (DJSI) | DJSI evaluates the sustainability performance of publicly traded companies | ✓ | ✓ | ✓ |
| BRSR and Sustainability Report | Deals with qualitative and quantitative information of an organization on environmental, social, economic and governance factors | ✓ | ✓ | ✓ |
A total of 20 certifications and endorsements were studied under the ESG fundamental framework, reflecting organizational compliance and their commitment towards ESG.32 Among these certifications, governance exhibited the highest coverage with 16 certifications and endorsements, followed by 14 focused on the environment and 11 addressed the social dimension. It is to be noted that these certifications and endorsements included standards from the International Organization for Standardization (ISO), the United Nations Global Compact (UNGC), the Dow Jones Sustainability Index (DJSI), and EcoVadis among others. Each certification served a unique purpose in guiding the organizations toward improved sustainability practices, emphasizing the importance of standardized benchmarks in fostering corporate responsibility.33
In the environmental pillar, standards such as ISO 14001 (Environmental Management System), ISO 50001 (Energy Management System), and ISO 14064-1 (Greenhouse Gas Emissions Accounting) serve as the core compliance tools, guiding the organizations toward environmental excellence. These frameworks efficiently guide the organizations in operationalizing environmental sustainability through systematic resource management, emission reduction, and climate risk mitigation. ESG protocols have become indispensable for organizing business strategies with global sustainability objectives, especially in sectors like energy that are sensitive to environmental issues.34 Furthermore, the initiatives, such as Carbon Disclosure Project (CDP) and CEO Water Mandate introduced rigorous disclosure-based performance measures, focusing on corporate transparency in climate and water governance. Besides, International Sustainability and Carbon Certification (ISCC Plus) plays a complementary role by emphasizing material traceability, sustainable procurement, and circular resource utilization.
The SA8000 standard, categorized under the social pillar, emerges as one of the few certification systems with structured audits and performance-based metrics, enabling the monitoring of labour conditions, child labour prohibition, and equitable remuneration.35 ESG parameters operationalize and measure the socially responsible activities under corporate social responsibility (CSR). A company's commitment to society is reflected through CSR; however ESG converts this commitment into measurable outcomes for investment decisions and helps in enhancing the ESG score.36 Diversity in boardrooms, organizations, and open discovery of ESG activities enhance investor trust and market efficiency, while presenting fresh viewpoints on how corporate governance and sustainability strategies interact.37 The UNGC and Supplier Ethical Data Exchange (SEDEX/SMETA) focused on the broader social sustainability, aligned with UN Sustainable Development Goals (SDGs) and transparent evaluation of ethical labour practices across global supply chains.
In the governance pillar, most certifications emphasize mostly on internal accountability, anti-corruption, and data security. ISO 27001 establishes systematic control for information security, particularly critical for pharmaceutical firms, handling intellectual property and patient-sensitive data.38 ISO 37001 offers a proactive anti-bribery framework for enhancing the compliance and ethical transparency. ESG assessment platforms, such as the Dow Jones Sustainability Index (DJSI) and EcoVadis were capable of performance benchmarking and rating, respectively across sectors by integrating ESG parameters into a multi-stakeholder evaluation system. Notably, Responsible Care (RC) represents a sectoral governance commitment, particularly within the chemical and pharmaceutical industries, reinforcing operational integrity and ethical stewardship.
The ISO 27001 information security standard focuses on an organization's action towards data security of employees and its stakeholders. More precisely, ISO 27001 focuses on how organizations are managing their database on digital platforms as well as in hard copies.39 The ISO 37001 anti-bribery standard focused on strengthening governance policies and compliance to restrict bribery at site, which can indirectly impact the brand image of the organization.40 ISO 37001 audit and certification were found to be supporting the organization in building faith and self-assurance among stakeholders and strengthening the legal compliance of the site.41 It also focused on due diligence of corporate partners and reducing the hazard of third-party bribery. The CDP focused on evaluating performance, particularly towards water security, climate action and forest (if applicable to any specific organization). Effective water and wastewater measurement can be meticulously assessed through a multiple range of enquiries, covering water monitoring, recycling, reuse, data recording, and reduction initiatives under water security. The initiatives toward GHG emission reduction, emission discoveries under scope 1, 2, and 3 and its methodologies used for calculation, targets considered for emission reduction, energy efficiency and use, and SBTi targets were scrutinized through the climate change questionnaire.42 In addition, organizations adopt the Responsible Care (RC) logo that shows their commitment towards seven codes which eventually improves environment, health, safety and sustainability standards with responsible business conduct. In this regard, the RC auditors from different regions conduct assessments to ensure a neutral audit of an organization.43 The RC logo is sanctioned by the International Council of Chemical Associations which emphasizes seven codes, such as process safety, pollution prevention, emergency response, product stewardship, distribution, security, environment, and health and safety for checking the critical logical control.44
The supplier ethical data exchange audit and portal mainly focuses on the 4-pillar methodology for assessing the ethical and responsible occupational practices across value supply chains. It covers the control and evaluation of labour practices at the organization level where buyers can easily access any organizational transparency data towards EHS and labour practices through the online SEDEX platform. This audit is also accepted by all global platforms, as it gives a clear depiction of an organization with a SEDEX score after the evaluation process.45 European customers usually demand ISCC Plus certification from pharmaceutical organizations to promote bio-circular materials used for manufacturing in pharmaceutical and speciality chemical products with a focus on material traceability and chain of custody from the point of origin till distribution in the market.46 Besides, the certification supports organizations to boost their product sustainability for long term nourishment in the market using bio-circular materials.47,48 The closeness of ESG certifications and endorsements (Table 3) underscores the variety of available certifications and their strategic relevance to pharmaceutical organizations, aiming to enhance their ESG performance. Overall, Table 3 emphasizes that ESG compliance is not a singular effort but rather a composite strategy requiring simultaneous engagement with multiple certification bodies and thematic domains. By providing a structured overview of available certifications and endorsements, the table serves as a strategic guide for ESG integration, particularly in highly regulated and publicly scrutinized industrial sectors, such as the pharmaceutical sector. Furthermore, it highlights that the comprehensive ESG performance can only be realized through a multi-certification strategy that addresses environment, labour, and governance dimensions in a holistic and verifiable manner.
Table 4 provides a comparative insight into the landscape of ESG certifications and endorsements, specifically tailored for prominent pharmaceutical industries. The comparative analysis shows how various certifications align with industry specific challenges and regulatory requirements. By examining the criteria and benefits associated with each certification, Table 4 revealed that some certifications are entirely an organization's internal domain specific. For instance, SA8000 emphasizes on social accountability practices, particularly in industries where significant labour practices are followed.35 SA8000 certification relied on strengthening the labour compliances, focusing on an organization's action towards child labour, forced labour, discrimination, good health and wellbeing, equal remuneration, etc., trailed by six monthly surprise audits, which will ultimately help the organizational assessment on neutral grounds.
| Organization | ESG certifications and endorsements | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISO 9000 | ISO 14000 | ISO 45000 | ISO 50000 | ISO 20400 | ISO 27001 | ISO 37001 | ISO 14064 | RC | EcoVadis | CDP | UNGC | ISCC | SBTi | PSCI | SA 8000 | CEO WM | SR | SEDEX SMETA | DJSI | Total score | |
| Company A (A) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | 13/20 |
| Luna Chemicals Industries Pvt. Ltd (B) | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 1/20 |
| SMS Pharmaceuticals Ltd (C) | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | 7/20 |
| Solara Active Pharma Sciences Ltd (D) | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | 5/20 |
| BASF (E) | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | 15/20 |
| Hubei Granules Biocause Company Ltd (F) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 3/20 |
| Shandong Xinhua Pharmaceutical Co Ltd (G) | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 3/20 |
| Wanbury limited (H) | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 5/20 |
| Aarti drugs limited (I) | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 4/20 |
| Abhilash Chemicals and Pharmaceuticals private Ltd (J) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | 9/20 |
| Harman Finochem Ltd (K) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 7/20 | |
| Auro Lab. Ltd (L) | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 2/20 |
| USV Private Limited (M) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 4/20 |
| Sohan Healthcare Pvt Ltd (N) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | 6/20 |
| Granules India Limited (O) | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | 12/20 | |
| Exemed Pharmaceuticals (P) | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | 7/20 |
| Farmson Pharmaceuticals Gujarat Private Limited (Q) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 4/20 |
| Sri Krishna Pharmaceuticals Limited (R) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 6/20 | |
| Para Products Pvt Ltd (S) | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | 2/20 |
| Meghmani organics (T) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | 4/20 |
| Atabay Kimya Sanayi ve Ticaret Anonim Sirketi (U) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | 12/20 |
| Aurobindo Pharma Ltd (V) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | 9/20 |
| Cadila Healthcare Ltd (W) | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 1/20 |
| Dr. Reddy's Lab. Limited (X) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | 19/20 |
| Ind swift Lab. Limited (Y) | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | 10/20 |
| Metrochem API Private Ltd (Z) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 8/20 | |
| MSN lab. Pvt. Ltd (A1) | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | 13/20 | |
| Praveen Lab. Pvt. Ltd (B1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | 5/20 |
| Saurav Chemicals Ltd (C1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 4/20 |
| Smilax Lab. Limited (D1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 3/20 |
| Hereto Drugs Limited (E1) | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | 16/20 | |
| Nosch Labs Private Limited (F1) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 4/20 |
| Rakshit Pharmaceuticals Ltd (G1) | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 1/20 |
| Vasudha Pharma Chem Ltd (H1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | 10/20 |
| Zydus Takeda Healthcare Pvt. Ltd (I1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | 8/20 |
| Divis Laboratories Ltd (J1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | 10/20 |
| Hetero Labs Limited (K1) | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | 15/20 |
| Lupin Limited (L1) | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | 12/20 |
| Neuland Laboratories Ltd (M1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | 9/20 |
| Alembic Pharmaceuticals Ltd (N1) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | 16/20 |
| Aarti Industries (O1) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | 17/20 | |
| UPL (P1) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 16/20 |
| Tagoor Lab. Pvt. Ltd (Q1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 6/20 | |
| Laxmi Organics (R1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | 6/20 |
| Jubilant Ingrevia (S1) | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | 12/20 |
| SRF (T1) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | 12/20 |
| Jubilant Pharmova (U1) | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | 11/20 |
Table 4 also presents a consolidated ESG scoring which is structured to reflect adherence to ESG criteria that are increasingly significant to stakeholders and investors.49 Organizations with 16 or more certifications indicated advanced ESG maturity. For example, Dr Reddy's Laboratories, Aarti Industries, Alembic Pharmaceuticals, and Hetero Labs consistently adopted a wide range of certifications which span across all three ESG pillars. Their certification portfolio includes critical governance instruments, such as ISO 37001 and CDP in addition to environmental standards and social accountability frameworks like SA8000, stemming from a high level institutional commitment, proactive stakeholder engagement, and alignment with global investor expectations. Organizations at an intermediate position, ranging between 8 and 12 certifications, were either undergoing ESG integration or selectively compliant with high priority standards. These organizations, viz., Solara Active Pharma Sciences, Jubilant Pharmova, Metrochem API, and Vasudha Pharma typically exhibited adherence to baseline environmental certifications, such as ISO 9001 and ISO 14001, however, may not be aligned with more rigorous social or governance indicators. The position of associated organizations suggested a transition phase, where firms were found to be expanding their ESG capabilities either in response to the regulatory mandates, investor pressure, or global supply chain requirements. Furthermore, organizations with five or fewer certifications exhibited relatively low ESG adoption, viz., Luna Chemicals, Auro Lab, and Farmson Pharmaceuticals which may possess only foundational certifications (e.g., ISO 9001 for quality management) and lacked formal alignment with climate action frameworks, labour standards, or ethical governance indicators. The absence of multi-dimensional certification suggested limited ESG engagement, which may result from constrained institutional capacity, low stakeholder scrutiny, or absence of regulatory compulsion, particularly in domestic or non-exporting market segments.
Across all adoption tiers, governance certifications and endorsements remain dominant within pharmaceutical organizations due to the sector's rigorous regulatory environment and the critical nature of its products. These organizations are subject to rigorous oversight by regulatory agencies which enforce strict quality, safety, and compliance standards through certifications like ISO 9001 (quality management), ISO 37001 (anti-bribery) and other ISO standards as discussed in this study. However, there is a lack of social certifications because factors, such as reasonable access, working conditions, and public impact, are tougher to measure and steadily certify and are less regulated. Therefore, social standards continue to be more subjective, despite governance contexts having explicit requirements and homogenous audits, which leads to a slower implementation rate and fewer uniform certifications throughout the sector. The pattern of certification adoption across the organizations, also provides strategic insight into ESG benchmarking and supply chain resilience. Firms with extensive certification portfolios are better positioned to meet the expectations of international clients, institutional investors, and procurement frameworks. From a public policy perspective, these findings point to the need for enabling mechanisms, such as subsidies, fiscal incentives, or mandatory reporting requirements to encourage broader ESG certification uptake, particularly among small- and mid-sized organizations. Table 5 serves as a critical tool for assessing the sustainability performance of organizations within the pharmaceutical industry. Each organization is assigned with a score that categorizes them into different levels of achievement, such as bronze, silver, or gold, based on their performance metrics.50Table 5 highlights the disparities in ESG scores among organizations, indicating varying levels of commitment to sustainable practices. Additionally, it provides insights into how these scores correlate with specific certifications and endorsements, allowing for a subtle understanding of what drives higher ESG ratings. By analyzing this information, researchers can identify trends and benchmarks within the industry, facilitating a deeper discussion on best practices and areas of further improvement.51–53
| Organization | Assessment year | |||
|---|---|---|---|---|
| FY 2021–22 | FY 2022–23 | FY 2023–24 | FY 2024–25 | |
| Medal obtained bronze (50–58), silver (59–69), gold (70–77) | Medal obtained bronze (50–58), silver (59–69), gold (70–77) | Medal obtained bronze (56–63), silver (64–71), gold (72–79) | Medal obtained bronze (58–65), silver (66–72), gold (73–80) | |
| a X organizations with information not publicly disclosed; # organizations with silver medal or above. | ||||
| Company A# | Bronze (53) | Bronze (57) | Bronze (61) | Silver medal (71) |
| Luna Chemicals Industries Pvt. Ltd | X | X | X | Gold medal (76) |
| SMS Pharmaceuticals Ltd | Bronze medal | X | X | X |
| Solara Active Pharma Sciences Ltd | No medal | Bronze medal | Bronze medal | X |
| BASF | X | X | X | X |
| Hubei Granules Biocause Company Ltd | X | X | X | X |
| Shandong Xinhua Pharmaceutical Co Ltd | X | X | X | X |
| Wanbury limited | X | X | X | X |
| Aarti drugs limited | X | X | X | X |
| Abhilash Chemicals and Pharmaceuticals Private Ltd | X | X | X | X |
| Harman Finochem Ltd | Silver medal | X | X | X |
| Auro Lab. Ltd | X | X | X | X |
| USV Private Limited | X | X | X | X |
| Sohan Healthcare Pvt Ltd | X | X | X | X |
| Granules India Limited | X | X | X | Gold |
| Exemed Pharmaceuticals | Bronze | X | X | X |
| Farmson Pharmaceuticals Gujarat Private Limited | X | X | X | X |
| Sri Krishna Pharmaceuticals Limited | X | X | X | X |
| Para Products Pvt Ltd | X | X | X | X |
| Meghmani organics | X | X | X | X |
| Atabay Kimya Sanayi ve Ticaret Anonim Sirketi | X | X | X | X |
| Aurobindo Pharma Ltd | X | X | X | X |
| Cadila Healthcare Ltd | X | X | X | X |
| Dr. Reddy's. Lab. Limited# | 47 | Gold medal | Gold medal | Gold medal |
| Ind swift Lab. Limited. (Synthimed Labs) | X | Bronze medal | Bronze medal | Silver medal |
| Metrochem API Private Ltd | Bronze medal | X | X | X |
| MSN lab. Pvt. Ltd | Bronze medal | X | X | X |
| Praveen Lab. Pvt. Ltd | X | X | X | X |
| Saurav Chemicals Ltd | X | X | X | X |
| Smilax Lab. Limited | X | X | X | X |
| Hereto Drugs Limited# | Silver medal | Gold medal | Silver medal | X |
| Nosch Labs Private Limited | X | X | X | X |
| Rakshit Pharmaceuticals Ltd | X | X | X | X |
| Vasudha Pharma Chem Ltd | Bronze medal | Silver medal | Silver medal | Gold |
| Zydus Takeda Healthcare Pvt. Ltd | No medal | X | X | X |
| Divis Laboratories Ltd | Silver medal | Bronze medal | Bronze medal | X |
| Hetero Labs Limited | Silver | Silver | Gold medal | X |
| Lupin Limited# | X | X | Silver (51) | Silver (69) |
| Neuland Laboratories Ltd | Silver medal | Silver medal | Silver medal | Silver medal (72) |
| Alembic Pharmaceuticals Ltd | No medal (31 percentile) | X | 44 | X |
| Aarti Industries# | Silver (56) | Gold (68) | Gold (68) | Gold (78) |
| UPL | X | X | X | X |
| Tagoor Lab. Pvt. Ltd | X | X | X | X |
| Laxmi Organics | X | X | X | X |
| Jubilant Ingrevia# | Bronze (52) | Bronze (55) | Gold | Gold |
| SRF# | Bronze (52) | Silver (62) | Gold | X |
| Jubilant Pharmova | Silver medal | Gold medal | Silver medal | Silver medal |
Table 5 also depicts the details of ESG scores in a particular FY on the global platform EcoVadis,54 highlighting the trends and shifts in corporate sustainability practices over the specified financial years. The data indicate a general upward trajectory in ESG scores from FY 2021–22 to FY 2024–25, reflecting an increased commitment among organizations to enhance their ESG practices.55,56 In FY 2021–22, the scoring range categorized the organizations into bronze (50–58), silver (59–69), and gold (70–77) medals.57 However, in FY 2024–25, the thresholds of these categories were adjusted slightly with bronze scores now ranging from 56–63, silver from 64–71, and gold from 72–79.58 The adjustment was due to the fact that EcoVadis had raised its benchmarks for assessing ESG performance, compelling the organizations to adopt more rigorous sustainability measures to achieve higher ratings.59 The improvement in scores also implies that companies are increasingly recognizing the importance of ESG factors in their operational strategies, potentially driven by stakeholder pressures and regulatory requirements.60 Overall, the evolving landscape of corporate sustainability and the critical role of platforms like EcoVadis are identified as major factors for promoting responsible business practices.61
Company A, an organization demonstrating consistent improvement (moving from bronze to silver) during FY 2021–22 and FY 2024–25, reflects successful implementation of ESG strategies and possibly investments in certifications, supplier audits, reporting mechanisms, and stakeholder engagement. Granules India, showed a jump from a score of 47 (no medal) in FY 2021–22 to achieving gold in FY 2024–25, indicating a significant transformation. These upward trajectories signal that ESG performance improvements are possible even for firms starting from a relatively weak baseline, provided there is institutional commitment.
Conversely, some organizations remain under assessment or unranked for consecutive FYs, reflecting challenges in meeting the baseline reporting requirements, delays in certification or audit cycles, or lack of verifiable data for third party evaluation. Additionally, organizations, such as Divis Laboratories fluctuated between silver and bronze medals, illustrating the sensitivity of EcoVadis scoring to minor changes in documentation, implementation depth, or coverage scope. These cases underscore the critical role of consistency and documentation in effective ESG management.
Science based targets initiative (SBTi) is also a key driving factor for reducing greenhouse gas emissions and subsequently contributing to carbon neutrality aligned with the Paris agreement.62 SBTi, a non-profit organization which follows a structured process to evaluate organization goals to reduce greenhouse gas carbon emissions.63 SBTi follows a detailed five stage process starting with taking commitment from the organization in stage-1, developing carbon reduction goals in stage-2, submitting targets for evaluation and communicating the approved targets to all stakeholders in stage-3 and stage-4, and disclosing yearly progress in stage-5 for emission reduction trends against the baseline.
The organizations highlighted in Table 5 reflect efforts made by organizations towards ESG, which are visible through their annual sustainability reports published on their organizational website. Sustainability reports should be aligned with key minimum global standard requirements, such as BRSR (Business Responsibility and Sustainability Report), GRI (Global Reporting Initiative), UNGC (United Nations Global Compact), PSCI (Pharmaceutical Supply Chain Initiative) with third-party verified independent assurance statement against all indicators, which will help the organizations to disclose their details transparently and accurately on various platforms, showing credibility of the organization towards ESG.64 A report published in line with the GRI helps organizations boost business risk management and supports the UN SDGs.65 Disclosing the initiatives towards achieving 17 SDGs on the UNGC portal will help to better affiliate organizational targets linked with specific goals and align the sustainability roadmap to achieve long term business goals.66
With the help of SDGs, organizations are getting clear goals, such as introduction of a zero liquid discharge facility under clean water and sanitation (SDG 6), and waste to wealth initiatives under responsible consumption and production (SDG 12).66 In the evolving pharmaceutical market, organizations are following the PSCI maturity model. Its principles provide an edge to strengthen the non-financial disclosures in a strong demanding market which also attracts customers that require durable ESG compliance.67 The EcoVadis evaluation process examines an organization on the basis of a variety of criteria with an emphasis on openness and responsibility. The organizations looking for improvement in their standing, should consider incorporating sustainable practices into their fundamental strategies along with integration of ESG certifications in a defined process.68 EcoVadis uses a seven parameter assessment approach for assessing every angle of the organization to strengthen environment, social, labour and human rights, and sustainable procurement pillars.
3.2. ESG framework and scoring models
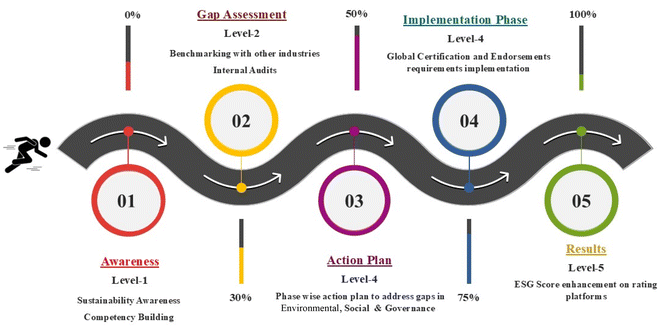
An organization’s environmental, social and governance performance can be effectively estimated and compared across various industries using standardized techniques, offered by ESG frameworks and scoring models. Various scoring models are being employed by organizations for better evaluation of the organizational ESG score. Fig. 2 illustrates the progressive roadmap for increasing the ESG score by following a 5-level process. It begins with awareness (level 1) wherein the sustainability concepts are introduced. Thereafter, during gap assessment (level 2), internal audits are conducted for evaluating the current position of the organization, followed by action plan (level 3) wherein a robust action plan is executed for eliminating the gaps and implementing controls (level 4) required for fortifying the ESG compliances. Finally, the organization will be represented on the global ESG platform for ratings, reflecting measurable improvements during the results stage (level 5).This five-level action plan not only offers a systematic roadmap for ESG improvement but also permits reasonable benchmarking against top international platforms like EcoVadis, SEDEX and the DJSI. With a durable scoring system, EcoVadis emphasizes environmental, labour, human rights, ethics, and sustainable procurement management. SEDEX underlines responsible sourcing and ethical trade practices, and the DJSI provides detailed ESG evaluations for more general corporate sustainability leadership. Eventually, all these platforms promote ESG enrichment and boost adherence and strengthen the overall compliance and transparency. EcoVadis differentiates itself by providing organizations with an inclusive view of their sustainability position and supporting them in accurately aligning their ESG principles with international standards and legal obligations. Many pharmaceutical organizations utilize EcoVadis due to its comprehensive evaluation system and global reputation, as highlighted in the studies above.
Nurturing awareness across its four main themes is the top priority of EcoVadis, which emphasizes the importance of understanding chemical safety, material safety and datasheet (MSDS) management, environmental challenges, GHG emissions, information security, risk assessment, ethics and sustainable sourcing. This foundational familiarity confirms that organizations are aware of the scope of their ESG accountabilities. Level 2 is also connected with platform requirements as it focuses on comprehensive internal or external audits to identify compliance gaps, which is a necessary step for accurate reporting of current ESG performance covering all four indicators of the EcoVadis platform, followed by a detailed action plan to mitigate the risk related to environment health safety along with product, personal information security, and bribery involved in transactions and business dealings.
The combination of a five-level action plan with these global rating platforms enables pharmaceutical organizations to systematically progress, address areas for improvement, and gain global recognition for their sustainability credentials. Fig. 3 illustrates a radar plot of 47 organizations for ESG certifications and endorsements in which organizations are denoted as A-Z and A1-U1 (denoted in Table 4), showing various acquired certifications which contribute to their increasing revenues. Non-financial disclosures demonstrate strong relation with the financial performance of an organization when it has a high ESG score. As shown in Fig. 4 and Table 6, in addition to demonstrating improved financial performance, a few Indian chemical and pharmaceutical organizations have progressed significantly in their EcoVadis sustainability ratings. They have jumped from bronze and silver to gold medal recently. For example, Dr Reddy's Laboratories established stable revenue growth from $2417.84m in FY 21–22 to $3671.29m in FY 24–25, reflecting its improved ESG integration and sustainable profitable expansion. Despite a short-term decline in FY 23–24, Aarti Industries recovered with substantial revenue increases, demonstrating resilience and the ability to direct expansion concerning sustainable practices.
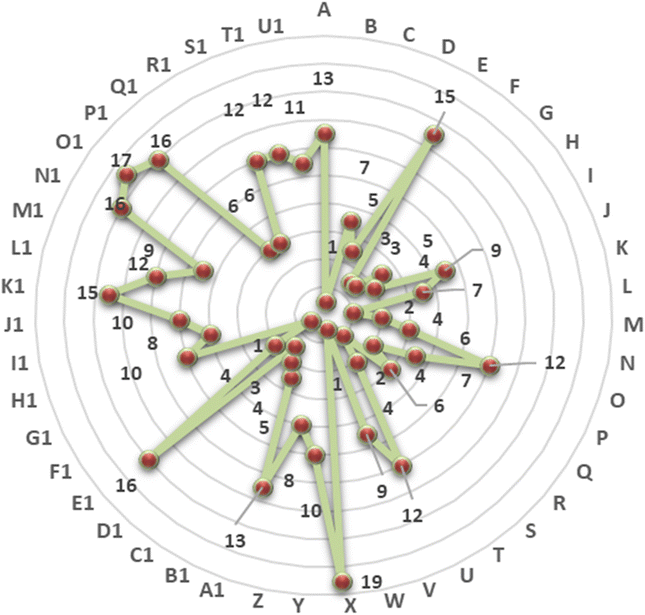 | ||
| Fig. 3 Radar plot of pharmaceutical organizations for ESG certifications and endorsements (organizations' name as denoted in Table 4). | ||
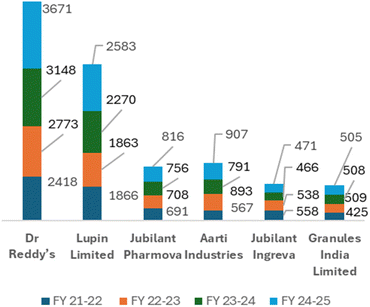 | ||
| Fig. 4 Growth in organization's revenue (million USD) between FY 2021–2022 and 2024–2025.95,116,121–126 | ||
| Name of organizations | Revenue (million USD) | |||
|---|---|---|---|---|
| Industries | FY 21–22 | FY 22–23 | FY 23–24 | FY 24–25 |
| Dr Reddy's | 2418 | 2773 | 3148 | 3671 |
| Lupin Limited | 1866 | 1863 | 2270 | 2583 |
| Jubilant Pharmova | 691 | 708 | 756 | 816 |
| Aarti Industries | 567 | 893 | 791 | 907 |
| Jubilant Ingreva | 558 | 538 | 466 | 471 |
| Granules India Limited | 425 | 509 | 508 | 505 |
3.3. Pharmaceutical industry-specific ESG difficulties
The various challenges faced by pharmaceutical industries on environment, social, and governance fronts are discussed in this sub-section. The pharmaceutical organizations need to hold themselves to higher industry standards that set the standard for ESG excellence in addition to comparing themselves to direct industry participants to overcome these obstacles. This benchmarking enables them to identify routine gaps, learn from leaders in other organizations and adapt best practices that bring them in line with their unique operational and ethical responsibilities. A strong forward looking ESG policy which covers labor practices, governance frameworks, environmental commitments must be developed to implement an integrated strategy. Regular due diligence confirms that these policies stay relevant, robust and approachable to emerging risks, while periodic third-party independent evaluations and certifications can provide credibility and external authentication.Additionally, ongoing assessment on international platforms, such EcoVadis ratings or comprehensive sustainability directories, enables pharmaceutical organizations to present themselves openly to investors, regulators, and stakeholders. By integrating organizational goals with more general social and environmental objectives, such proactive engagement promotes trust, strengthens competitive advantage, and enhances long-term sustainability. Ultimately, this dynamic cycle of benchmarking, development of policies, careful monitoring, and open review helps the pharmaceutical industry to reduce its ESG-specific problems, while promoting innovation and responsibility in every aspect of the pharmaceutical sector. Organizations can improve stakeholder trust, manage regulatory expectations, and promote long-term value creation beyond compliance by integrating (a) disclosure practices and certifications, (b) ESG framework and scoring models, and (c) pharmaceutical industry-specific ESG difficulties.
4 Conclusions and future prospects
The findings of this study revealed that environmental factors have primarily shaped ESG initiatives in India's pharmaceutical manufacturing sector. In contrast, social and governance factors have lagged, despite their critical role in achieving comprehensive sustainability. The study assesses the ESG performance of pharmaceutical organizations in India by aligning and integrating assessment methods with global platforms. Between FYs 2021–22 and 2024–25, environmental factors received the most significant emphasis in ESG evaluations, while social and governance dimensions received relatively lower importance, despite their substantial impact on overall scores. The incremental advancement in EcoVadis scores primarily reflected organizations' responses to elevated global benchmarks, indicating a pattern of adaptive fulfillment rather than intense transformational change. This underscores the imperative need for a more reasonable and integrated ESG approach that emphasizes governance integrity, social responsibility, and environmental stewardship. Considering the proactive implementation of specific environmental, social, and governance measures in response to changing stakeholder expectations and standards, Company A's EcoVadis score increased significantly from 53 at the start of the period to 71 by FY 2024–2025. This continuous growth demonstrates the tangible benefits of a comprehensive ESG commitment and underscores that consistent progress in all ESG areas can lead to quantifiable improvements in external assessments.Moreover, a strong and comprehensive roadmap for pharmaceutical sectors to adopt ESG certifications and improve their ESG scores through a structured, five-level process is provided through this study, which also makes a more practical contribution by including ESG enhancement within compliance and competence-building agendas. Future research and industrial policies must focus on mainstreaming these instruments to support broader sectoral and city level transitions using AI-driven analytics toward sustainable development that could enhance clarity and comparability in non-financial disclosures. Decision-makers may also evaluate BRSR-linked disclosures and real-time frameworks to enhance corporate organizations’ trustworthiness. Non-financial disclosures have a direct and substantial impact on financial results, as demonstrated by the fact that organizations with good ESG execution or commitment have seen better sales growth. Six prominent pharmaceutical organizations in India, for example, have demonstrated notable revenue growth in tandem with their strong ESG promises and increased ESG ratings as discussed above in the study. ESG initiatives improve sustainability and boost financial success, as this correlation demonstrates.
To achieve fair ESG weighting, regulators must mandate disclosure requirements and third-party audits. To endorse openness and demonstrate a sincere corporate commitment, the Securities and Exchange Board of India (SEBI) requires that the top 1000 listed organizations in India obtain a reasonable level of assurance statement from independent third-party before disclosing ESG information. Moreover, to satisfy changing stakeholder expectations and regulatory requirements, organizations must prioritize environmental certifications as well as develop their social and governance spheres. Diverse certification sets are reliable pointers of ESG maturity that investors, mainly in emerging countries, should consider when making investment decisions. Altogether, these actions strengthen stakeholder trust, encourage accountability, and raise the general legitimacy of ESG disclosures. Finally, the study can help in strengthening India's position as a reasonable pharmaceutical focal point, which is capable of paired profitability with purpose.
Author contributions
GS: writing – original draft, data curation, methodology. AK: writing – review & editing, visualization, supervision, resources, methodology, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
All data used in this study are publicly available and can be accessed through the references cited in this manuscript.Acknowledgements
The authors sincerely thank Thapar Institute of Engineering & Technology for providing necessary infrastructural support and research facilities for this study. The continued encouragement and resources were instrumental in carrying out this research.References
- S. Drempetic, C. Klein and B. Zwergel, J. Bus. Ethics, 2020, 167, 333–360, DOI:10.1007/s10551-019-04164-1.
- M. Baldini, L. D. Maso, G. Liberatore, F. Mazzi and S. Terzani, J. Bus. Ethics, 2018, 150, 79–98, DOI:10.1007/s10551-016-3139-1.
- Q. B. Baloch, S. Maher, N. Iqbal, S. N. Shah, M. Sheeraz, F. Raheem and K. I. Khan, Bus. Process Manag. J., 2022, 28, 131–149, DOI:10.1108/BPMJ-02-2021-0084.
- I. Jin, J. Sustainable Finance Invest., 2022, 12, 1125–1145, DOI:10.1080/20430795.2020.1837501.
- A. Fatemi, M. Glaum and S. Kaiser, Glob. Finance J., 2018, 38, 45–64, DOI:10.1016/j.gfj.2017.03.001.
- A. Alshehhi, H. Nobanee and N. Khare, Sustainability, 2018, 10, 494, DOI:10.3390/su10020494.
- M. Geissdoerfer, D. Vladimirova and S. Evans, J. Clean. Prod., 2018, 198, 401–416, DOI:10.1016/j.jclepro.2018.06.240.
- R. Rajesh, J. Clean. Prod., 2020, 247, 119600, DOI:10.1016/j.jclepro.2019.119600.
- G. Friede, T. Busch and A. Bassen, J. Sustainable Finance Invest., 2015, 5, 210–233, DOI:10.1080/20430795.2015.1118917.
- J. P. Hawley and A. T. Williams, The Rise of Fiduciary Capitalism: How Institutional Investors Can Make Corporate America More Democratic, University of Pennsylvania Press, Philadelphia, 1st edn, 2000 Search PubMed.
- A. Behl, P. R. Kumari, H. Makhija and D. Sharma, Ann. Oper. Res., 2022, 313, 231–256, DOI:10.1007/s10479-021-04189-8.
- L. H. Pedersen, S. Fitzgibbons and L. Pomorski, J. Financ. Econ., 2021, 142, 572–597, DOI:10.1016/j.jfineco.2020.11.001.
- N. Attig, S. El Ghoul, O. Guedhami and J. Suh, J. Bus. Ethics, 2013, 117, 679–694, DOI:10.1007/s10551-013-1714-2.
- D. Cumming, M. Meoli, A. Rossi and S. Vismara, J. Bus. Ventur., 2024, 39, 106362, DOI:10.1016/j.jbusvent.2023.106362.
- C. Flammer, Manag. Sci., 2015, 61, 2549–2568, DOI:10.1287/mnsc.2014.2038.
- C. Wijethilake and T. Lama, Bus. Strategy Environ., 2019, 28, 143–154, DOI:10.1002/bse.2245.
- D. Simpson and R. Sroufe, Int. J. Oper. Prod. Manag., 2014, 34, 830–852, DOI:10.1108/IJOPM-02-2012-0063.
- V. Veeravel, V. P. Murugesan and V. Narayanamurthy, Q. Rev. Econ. Finance, 2024, 95, 193–202, DOI:10.1016/j.qref.2024.03.008.
- A. G. Hoepner, I. Oikonomou, Z. Sautner, L. T. Starks and X. Y. Zhou, Rev. Finance, 2024, 28, 483–510, DOI:10.1093/rof/rfad034.
- D. L. Todaro and R. Torelli, Corp. Soc. Responsib. Environ. Manag., 2024, 31, 4034–4046, DOI:10.1002/csr.2786.
- S. Rawal, Nirma Univ. J. Pharm. Sci., 2022, 9, 1–16 Search PubMed.
- X. Ma, Q. He, L. Zhang, W. Wu and W. Li, Energy, 2025, 326, 135981, DOI:10.1016/j.energy.2025.135981.
- S. Calciolari, M. Cesarini and M. Ruberti, J. Clean. Prod., 2024, 447, 141511, DOI:10.1016/j.jclepro.2024.141511.
- S. Sundarasen, R. Kumar, K. Tanaraj, A. A. Alsmady and U. Rajagopalan, Res. Glob., 9, 100259, DOI:10.1016/j.resglo.2024.100259.
- A. Hassanein, K. B. Benameur, M. M. Mostafa, W. A. Shattarat and N. H. Magar, Cogent Bus. Manag., 12, 2513638, DOI:10.1080/23311975.2025.2513638.
- M. Statman and D. Glushkov, Financ. Anal. J., 2009, 65, 33–46, DOI:10.2469/faj.v65.n4.5.
- A. de Souza Barbosa, M. C. B. C. da Silva, L. B. da Silva, S. N. Morioka and V. F. de Souza, Humanit. Soc. Sci. Commun., 2023, 10, 1–18, DOI:10.1057/s41599-023-01919-0.
- Y. Lian, Y. Li and H. Cao, Front. Environ. Sci., 2023, 11, 1170582, DOI:10.3389/fenvs.2023.1170582.
- A. Clément, É. Robinot and L. Trespeuch, J. Enterp. Communities People Places Glob. Econ., 2025, 19, 92–110, DOI:10.1108/JEC-10-2022-0147.
- W. Ali, J. G. Frynas and Z. Mahmood, Corp. Soc. Responsib. Environ. Manag., 2017, 24, 273–294, DOI:10.1002/csr.1410.
- A. F. Cicchiello, F. Marrazza and S. Perdichizzi, Corp. Soc. Responsib. Environ. Manag., 2023, 30, 1121–1128, DOI:10.1002/csr.2408.
- S. Mittelbach-Hörmanseder, K. Hummel and M. Rammerstorfer, Eur. Account. Rev., 2021, 30, 309–348, DOI:10.1080/09638180.2020.1763818.
- Y. C. Chen, M. Hung and Y. Wang, J. Account. Econ., 2018, 65, 169–190, DOI:10.1016/j.jacceco.2017.11.009.
- G. Makridou, M. Doumpos and C. Lemonakis, Int. J. Energy Sect. Manag., 2024, 18, 873–895, DOI:10.1108/IJESM-01-2023-0012.
- H. B. Christensen, L. Hail and C. Leuz, Rev. Account. Stud., 2021, 26, 1176–1248, DOI:10.1007/s11142-021-09609-5.
- F. García, J. González-Bueno, F. Guijarro and J. Oliver, Sustainability, 2020, 12, 3324, DOI:10.3390/su12083324.
- A. Hassanein and N. Elsayed, Int. J. Finance Econ., 2025 DOI:10.1002/ijfe.70009.
- E. Perrault Crawford and C. Clark Williams, Corp. Gov. Int. J. Bus. Soc., 2010, 10, 512–526, DOI:10.1108/14720701011069722.
- ISO 27001:2022, Information Security, Cybersecurity and Privacy Protection—Information Security Management Systems—Requirements, International Organization for Standardization, 3rd edn, 2022, https://www.iso.org/standard/27001 Search PubMed.
- ISO 37001:2025, Anti-bribery Management Systems—Requirements with Guidance for Use, International Organization for Standardization, 2nd edn, 2025, https://www.iso.org/standard/37001 Search PubMed.
- N. Jagodzińska, Zeszyty Nauk, Organ. Zarz. Politechnika Śląska, 2021 Search PubMed.
- D. C. Matisoff, D. S. Noonan and J. J. O'Brien, Bus. Strategy Environ., 2013, 22, 285–305, DOI:10.1002/bse.1741.
- Responsible Care, Responsible Care, International Council of Chemical Associations, 2025, https://icca-chem.org/focus/responsible-care/.
- E. A. Holtmaat, C. Adolph and A. Prakash, World Dev., 2020, 127, 104735, DOI:10.1016/j.worlddev.2019.104735.
- J. C. Marques, Regul. Gov., 2019, 13, 157–176, DOI:10.1111/rego.12252.
- K. Mononen, Master's thesis, Lut University, 2023, https://urn.fi/URN:NBN:fi-fe20230929137783.
- ISCC, ISCC PLUS, International Sustainability and Carbon Certification, 2010, https://www.iscc-system.org/certification/iscc-certification-schemes/iscc-plus/.
- A. L. Friedman and S. Miles, J. Manag. Stud., 2002, 39, 1–21, DOI:10.1111/1467-6486.00280.
- K. Hussainey and A. Salama, J. Appl. Account. Res., 2010, 11, 229–241, DOI:10.1108/09675421011088152.
- I. C. Lourenço, J. L. Callen, M. C. Branco and J. D. Curto, J. Bus. Ethics, 2014, 119, 17–28, DOI:10.1007/s10551-012-1617-7.
- A. Pérez, Corp. Commun. Int. J., 2015, 20, 11–29, DOI:10.1108/CCIJ-01-2014-0003.
- G. Michelson, N. Wailes, S. Van Der Laan and G. Frost, J. Bus. Ethics, 2004, 52, 1–10, DOI:10.1023/B:BUSI.0000033103.12560.be.
- M. Nekhili, A. Boukadhaba, H. Nagati and T. Chtioui, Int. J. Hum. Resour. Manag., 2021, 32, 3061–3087, DOI:10.1080/09585192.2019.1629989.
- M. Cappucci, J. Appl. Corp. Finance, 2018, 30, 22–28, DOI:10.1111/jacf.12296.
- A. Buallay, Manag. Environ. Qual. Int. J., 2019, 30, 98–115, DOI:10.1108/MEQ-12-2017-0149.
- J. E. Humphrey, D. D. Lee and Y. Shen, Aust. J. Manag., 2012, 37, 135–151, DOI:10.1177/0312896211410081.
- E. Avetisyan and K. Hockerts, Bus. Strategy Environ., 2017, 26, 316–330, DOI:10.1002/bse.1919.
- J. Entine, Organ. Environ., 2003, 16, 352–368, DOI:10.1177/1086026603256283.
- G. Friede, T. Busch and A. Bassen, J. Sustainable Finance Invest., 2015, 5, 210–233, DOI:10.1080/20430795.2015.1118917.
- Y. Eliwa, A. Aboud and A. Saleh, Crit. Perspect. Account., 2021, 79, 102097, DOI:10.1016/j.cpa.2019.102097.
- J. Carrillo-Hermosilla and T. Konnola, J. Ind. Ecol., 2010, 14, 541–557, DOI:10.1111/j.1530-9290.2010.00259.
- SBTi, Ambitious corporate climate action – Science Based Targets Initiative, Science Based Targets Initiative, 2015, https://sciencebasedtargets.org/ Search PubMed.
- J. Giesekam, J. Norman, A. Garvey and S. Betts-Davies, Sustainability, 2021, 13, 1657, DOI:10.3390/su13041657.
- A. Fonseca, M. L. McAllister and P. Fitzpatrick, J. Clean. Prod., 2014, 84, 70–83, DOI:10.1016/j.jclepro.2012.11.050.
- J. D. Sachs, Lancet, 2012, 379, 2206–2211, DOI:10.1016/S0140-6736(12)60685-0.
- UNGC, About the UN Global Compact, United Nations Global Compact, 2006, https://unglobalcompact.org/about/foundation Search PubMed.
- PSCI, Pharmaceutical Supply Chain Initiative, Pharmaceutical Supply Chain Initiative, 2015, https://pscinitiative.org/.
- Ecovadis, Understanding EcoVadis Surveys, Ecovadis, 2025, https://idp.ecovadissurvey.com/Account/Login?ReturnUrl=%2Fconnect%2Fauthorize%2Fcallback%3Fclient_id%3Dbellucci%26redirect_uri%3Dhttps%253A%252F%252Fwww.ecovadis-survey.com%252Fapp%252F%2523%252Fsso-callback%253F%26response_type%3Did_token%2520token%26scope%3D Search PubMed.
- ISO 9001:2015, Quality Management Systems—Requirements, International Organization for Standardization, 5th edn, 2015, https://www.iso.org/standard/62085.html Search PubMed.
- ISO 14001:2015, Environmental Management Systems—Requirements with Guidance for Use, International Organization for Standardization, 3rd edn, 2015, https://www.iso.org/standard/60857.html Search PubMed.
- ISO 45001:2018, Occupational health and safety management systems—Requirements with guidance for use, International Organization for Standardization, 1st edn, 2018, https://www.iso.org/standard/63787.html Search PubMed.
- ISO 50001:2018, Energy Management Systems—Requirements with Guidance for Use, International Organization for Standardization, 2nd edn, 2018, https://www.iso.org/standard/69426.html Search PubMed.
- ISO 20400:2017, Sustainable Procurement—Guidance, International Organization for Standardization, 1st edn, 2017, https://www.iso.org/standard/63026.html Search PubMed.
- ISO 14064-1:2018, Greenhouse Gases—Part 1: Specification with Guidance at the Organization Level for Quantification and Reporting of Greenhouse Gas Emissions and Removals, International Organization for Standardization, 2nd edn, 2018, https://www.iso.org/standard/66453.html Search PubMed.
- CDP, CDP – Disclosure, Insight, Action, CDP, 2025, https://www.cdp.net/en Search PubMed.
- SA8000:2014, SA8000® Standard - SAI, Social Accountability International, 2025, https://sa-intl.org/programmes/sa8000/.
- CEO Water Mandate, CEO Water Mandate | Sign the Commitment to Water Stewardship, CEO Water Mandate, 2025, https://ceowatermandate.org/.
- SEDEX, Sedex: Sustainable Business and Supply Chain Solutions, SEDEX, 2025, https://www.sedex.com/ Search PubMed.
- DJSI, DJSI Index Family | S&P Global, S&P Global, 2025, https://www.spglobal.com/esg/djsi Search PubMed.
- BRSR, SEBI | BRSR Core - Framework for assurance and ESG disclosures for value chain, Securities and Exchange Board of India, 2023, https://www.sebi.gov.in/legal/circulars/jul-2023/framework-for-assurance-and-esg-disclosures-for-value-chain_73629.html.
- Ecovadis, Understanding EcoVadis Medals and Badges, Ecovadis, 2025, https://support.ecovadis.com/hc/en-us/articles/210460227-Understanding-EcoVadis-Medals-and-Badges Search PubMed.
- Luna Chemicals Industries Pvt. Limited, RLG GROUP | Factory-Luna Chemical, Luna Chemicals Industries Pvt. Limited, 2025, http://www.lunachemical.com/.
- SMS Pharmaceuticals Limited, SMS Pharmaceuticals Ltd – an Integrated Pharma Company with World Class Bulk Drugs, SMS Pharmaceuticals Limited, 2025, https://www.smspharma.com/ Search PubMed.
- Solara Active Pharma Sciences Ltd, Solara Annual Report 2023–24, Solara Active Pharma Sciences Ltd, 2024, https://solara.co.in/uploads/2024/11/Solara-AR-23-24.pdf Search PubMed.
- BASF, Downloads – BASF Report 2024, BASF, 2024, https://www.basf.com/global/en/investors/reporting-and-downloads.html Search PubMed.
- Hubei Granules Biocause Company Limited, About Us, Hubei Granules Biocause Company Limited, 2025, http://en.biocause.com/new/english/index.php/index-show-tid-2.html.
- Shandong Xinhua Pharmaceutical Co Limited, About Us - Shandong Xinhua Pharmaceutical I&E Co., Ltd, Shandong Xinhua Pharmaceutical Co Limited, 2025, http://www.xhpharma.com/.
- Wanbury Limited, Wanbury, Wanbury Limited, 2025, http://www.wanbury.com/ Search PubMed.
- Aarti Drugs Limited, Sustainability Report – Aarti Drugs, Aarti Drugs Limited, 2025, http://www.aartidrugs.com/sustainability-report Search PubMed.
- Abhilash Chemicals and Pharmaceuticals Private Limited, Home - Abhilash Chemicals and Pharmaceuticals/Abhilash Life Sciences, Abhilash Chemicals and Pharmaceuticals Private Limited, 2025, http://www.abhilashchemicals.com/.
- Harman Finochem Limited, Harman Finochem - Leading manufacturer and exporter of metformin & allopurinol, Harman Finochem Limited, 2025, https://www.harmanfinochem.com/ Search PubMed.
- Auro Lab. Limited, Manufacturer of Pharma Bulk Drugs, Active Pharma Ingredients, Bulk Active Ingredients, Auro Lab. Limited, 2025, https://www.aurolab.com/ Search PubMed.
- USV Private Limited, Healthcare, Pharma, Pharmaceutical Companies in India, Mumbai, USV Private Limited, 2025, https://www.usvindia.com/ Search PubMed.
- Sohan Healthcare Pvt Limited, Sohan Healthcare, Sohan Healthcare Pvt Limited, 2025, http://www.sohanhealthcare.com/ Search PubMed.
- Granules India Limited, Granules Integrated Report 2024-2025, Granules India Limited, 2024, Granules_Integrated-Report-2024-25.pdf Search PubMed.
- Exemed Pharmaceuticals, Home - Exemed Pharma, Exemed Pharmaceuticals, 2025, https://www.exemedpharma.com/.
- Farmson Pharmaceuticals Gujarat Private Limited, Farmson Basic Drugs Private Limited, Farmson Pharmaceuticals Gujarat Private Limited, 2025, http://www.farmson.com/.
- Sri Krishna Pharmaceuticals Limited, SKPL Sustainability Report, Sri Krishna Pharmaceuticals Limited, 2023, http://www.srikrishnapharma.com/sustainability-report Search PubMed.
- Meghmani Organics, Sustainability – Meghmani Organics Ltd, Chemistry of Success at Work, Meghmani Organics, 2025, https://www.meghmani.com/sustainability/.
- Atabay, Atabay – Geçmişten Geleceğe Sağlık, Atabay, 2025, https://www.atabay.com/.
- Aurobindo Pharma Limited, Aurobindo Pharma Limited – Annual Report 2023-24, Aurobindo Pharma Limited, 2025, https://www.aurobindo.com/investors/annual-reports Search PubMed.
- Cadila Healthcare Limited, Indian Multinational Pharmaceuticals Company | Cadila Pharmaceuticals, Cadila Healthcare Limited, 2025, https://www.cadilapharma.com/ Search PubMed.
- Metrochem API Private Limited, Metrochem, Metrochem API Private Limited, 2025, https://www.metrochemapi.com/.
- MSN Laboratories Pvt. Limited, MSN Laboratories|Leading Pharmaceutical Company, MSN Laboratories Pvt. Limited, 2025, https://www.msnlabs.com/.
- Praveen Laboratories Pvt. Limited, Praveen Labs, Praveen Laboratories Pvt. Limited, 2025, http://www.praveenlabs.com/.
- Saurav Chemicals Limited, Pharma APIs and Intermediates Manufacturers - SCL Lifesciences, Saurav Chemicals Limited, 2025, https://www.scllifesciences.com/.
- Smilax Laboratories Limited, Home - Smilax Labs, Smilax Laboratories Limited, 2025, https://www.smilaxlabs.com/.
- Hetero, Hetero Sustainability Report (2022-23), Hetero, 2023, https://www.hetero.com/sustainability-report-2022-23 Search PubMed.
- Nosch Labs Private Limited, Nosch Labs, Nosch Labs Private Limited, 2025, https://www.noschlabs.com/ Search PubMed.
- Rakshit Pharmaceuticals Limited, Rakshit Drugs - Pharmaceutical Company in Hyderabad, Rakshit Pharmaceuticals Limited, 2025, https://www.rakshitdrugspvtltd.com/ Search PubMed.
- Vasudha, Vasudha Sustainability Report 2024, Vasudha, 2024, https://www.vasudhapharma.com/sustainability-report-2024.pdf Search PubMed.
- Zydus Takeda Healthcare Pvt. Limited, Company Information | Zydus Takeda Healthcare Private Limited, Zydus Takeda Healthcare Pvt. Limited, 2025, https://www.zydustakeda.com/company-information Search PubMed.
- Divis Laboratories, Sustainability Report 2022-23 - Divis Laboratories World's Largest API Manufacturing Facility, Divis Laboratories, 2023, https://www.divislabs.com/sustainability-report-2022-23 Search PubMed.
- Neuland Laboratories, Integrated Annual Report 2023–24, Neuland Laboratories, 2023, https://www.neulandlabs.com/investor-relations/annual-reports Search PubMed.
- Alembic Pharmaceuticals Ltd, ESG Ratings, Alembic Pharmaceuticals Ltd, 2023, https://www.alembicpharmaceuticals.com/esg-ratings Search PubMed.
- Aarti Industries, Integrated Annual Report 2024-2025, Aarti Industries, 2024, Integrated-Annual-Report-2024-25.pdf Search PubMed.
- UPL, Sustainability Reports 2023–24, UPL, 2023, https://www.upl-ltd.com/downloads/Sustainability-reports-2023-24.pdf Search PubMed.
- Tagoor Laboratories Pvt. Limited, TAGOOR LABORATORIES Pvt. Ltd, Tagoor Laboratories Pvt. Limited, 2025, https://www.tagoorlabs.com/.
- Laxmi Organics, Acetyl Intermediates | Essentials Business | Speciality Business | Laxmi Organic Industries Ltd, Laxmi Organics, 2025, https://www.laxmi.com/.
- SRF, Celebrating Excellence: Awards and Accolades, SRF, 2023, https://annualreport.srf.com/celebrating-excellence-awards-and-accolades.php Search PubMed.
- Jubilant Ingrevia, Annual Report 2024-25, Jubilant Ingrevia, 2024, annual-report-2024-25.pdf Search PubMed.
- Jubilant Pharmova, Jubilant Pharmova Sustainability Report 2023-24, Jubilant Pharmova, 2023, https://www.jubilantpharmova.com/sustainability-report-2023-24.pdf Search PubMed.
- Lupin Limited, Ten Years Financial Summary 2024-25, Lupin Limited, 2024, https://www.lupin.com/wp-content/uploads/2025/07/ten-years-financial-summary-ir-2024-2025.pdf Search PubMed.
- Dr Reddy's Laboratories Limited, Integrated Annual Report 2024-25, Dr Reddy's Laboratories Limited, 2024, Integrated Annual Report 2024-25.pdf Search PubMed.
- Jubilant Pharmova, Jubilant Pharmova Annual Report 2024-25, Jubilant Pharmova, 2024, JPM_AnnualReport_2024-25.pdf Search PubMed.
- Jubilant Pharmova, Jubilant Pharmova Annual Report 2022-23, Jubilant Pharmova, 2023, JPM_AR-2022-23.pdf Search PubMed.
- Synthimed Labs, Synthimed Final Report 2023-24, Synthimed Labs, 2024, Synthimed-Final-Report.pdf Search PubMed.
- Para Products Private Limited, Para Products Private Limited, 2024, https://www.zaubacorp.com/PARA-PRODUCTS-PRIVATE-LIMITED-U74900DL1998PTC096414.
| This journal is © The Royal Society of Chemistry 2026 |