Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multifunctional lignin biocomposite for broad-spectrum water purification
Sritama
Mukherjee
 *a,
Salvatore
Lombardo
*a,
Salvatore
Lombardo
 b and
Ulrica
Edlund
b and
Ulrica
Edlund
 *a
*a
aDepartment of Fiber and Polymer Technology, School of Chemistry, Biotechnology and Health, KTH Royal Institute of Technology, Stockholm 10044, Sweden. E-mail: sritama@kth.se; edlund@kth.se
bDepartment of Materials and Environmental Chemistry, Stockholm University, Stockholm 10691, Sweden
First published on 8th December 2025
Abstract
Removal of heavy metal ions, PFAS, and synthetic dyes from anthropogenic wastewater using versatile sustainable materials is of prime importance for a clean environment and human health. This study presents a sustainably engineered lignin-based biocomposite reinforced with chitosan, designed for multifunctionality, enhanced material performance, and produced via a ‘green’ and simple one-pot synthesis in aqueous medium. The synthesized granular material, i.e. a zwitterionic lignin-chitosan composite (ZLC), was used for the removal of a wide range of chemically diverse contaminants. ZLC showed 80–90% removal of heavy metals such as Cr(VI), Cu(II), and four different cationic and anionic dyes. It was also tested against an array of five PFAS molecules, such as perfluorinated sulfonic and carboxylic acids, showing up to 86% removal for perfluorooctane sulfonic acid (PFOS). All adsorption processes followed the pseudo-second order and Langmuir models. The material also showed antifouling behavior, demonstrating robustness for real-time application. Lastly, ZLC was tested against effluent water matrices, i.e., wastewater streams from mining areas located in Sweden. The multifunctional adsorption performance of the environment-friendly material, coupled with its ease of production, cost effectiveness, and reusability, indicates that ZLC has a high potential that can garner industrial interest for simplifying multi-step filtration processes.
Environmental significanceThe release of persistent organic pollutants such as PFAS, synthetic dyes, and toxic metals into aquatic systems presents an escalating environmental concern. Addressing the dominating flow of miscellaneous pollutants into the environment requires a sustainable and multifunctional technology. We report a bio-derived adsorbent – functionalized lignin-chitosan composite (ZLC), that achieved 80–90% removal of diverse pollutants, with performance maintained through multiple regeneration cycles and antifouling properties. Leveraging the natural abundance and functionality of lignin and chitosan, ZLC represents a promising platform for scalable, recyclable, and environmentally benign water purification, contributing to the development of sustainable contaminant remediation strategies. |
1 Introduction
With the ever-growing water consumption in agriculture, industry, energy, and municipal areas, along with a limited supply of natural freshwater, it is necessary to shift towards using nontraditional water sources such as wastewater, seawater, and brackish water. Safe use of these sources requires water treatment systems that can remove nearly all dissolved constituents from contaminated water. Untreated wastewater from anthropogenic sources such as waste streams from mining areas and various industries, when released into surface water and groundwater, poses risks to drinking water safety for millions of people.1 The presence of various toxic contaminants like organic micropollutants, heavy metal ions, forever chemicals such as per- and polyfluorinated substances (PFAS), etc., even at low concentrations, can be a major contributor to water-related diseases.2 PFAS has caught attention due to its thousands of variations that are difficult to break down naturally and have widespread industrial applications, thus leading to its rampant mobilization to water, soil, and wildlife.3 Primary examples of this category include perfluorinated alkyl carboxylic and sulfonic acids, notably perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), whose production and utilization are now globally prohibited, leading to the emergence of alternative per- and polyfluoroalkyl substances with analogous characteristics.4,5 The remarkable stability of the C–F bond precludes both biological and abiotic degradation, resulting in persistent perfluorinated residues despite the transformation of certain non-fluorinated precursors, which compels the regulatory bodies to set stricter levels. These scenarios underscore the urgency for the development of ‘smart’ materials, leading to technological advancements that can facilitate access to clean water from alternative sources.6The conventional methods for water and wastewater treatment are often characterized by high levels of chemical, energy, and operational requirements, focusing on large-scale systems.7 Adsorptive materials see a wide-scale usage as they exhibit improved performance in numerous separations when compared with conventional technologies, in terms of capacity, selectivity, regenerability, and affordability.8 Polymer-derived materials, particularly biopolymers, can largely improve advanced adsorption processes from technical, socioeconomic, and environmental perspectives.
Lignin is a major structural component of lignocellulosic biomass and playing a vital role in the composition of terrestrial plant biomass.9 The lignin polymer is largely composed of three phenylpropanoid subunits: p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S), and the total carbon content accounts for ca. 60% of its structure.10 Lignin is rich in hydroxyl groups, which significantly influence its chemical and physical characteristics, including reactivity, hydrophilicity, and functionality. Chitosan is another bio-derived polymer having an amino polysaccharide structure and is well known for its versatile applicability. Both lignin and chitosan allow a wide array of chemical modifications, facilitated by their solubility in various solvents, enabling the introduction of various functional groups onto their aromatic rings and hydroxyl groups.11 Lignin and chitosan are, therefore, both from an environmental and a chemical perspective, interesting candidates for water purification materials.
Designing a multifunctional material with high adsorption capacity for a broad range of contaminants presents a significant scientific challenge due to several factors stemming from the complexity and diversity of common pollutants. Contaminants can be polar, non-polar, or amphiphilic, and they have various functional groups that interact differently with adsorbent materials. They also vary significantly in size, from small ions to large organic molecules or even nanoparticles, requiring adsorbent materials with pore sizes that can accommodate this wide range. Furthermore, achieving efficient adsorption for diverse contaminants is challenging because enhancing selectivity for one type of contaminant may reduce efficiency for others. Multifunctional materials can simplify multi-step filtration processes in water treatment industries. An ideal versatile adsorbent material must have sites that can attract and hold anionic, cationic, and neutral species effectively across a range of pH levels and offer a pore structure that can accommodate different contaminant sizes. From this perspective, zwitterionic moieties immobilized within a porous matrix stand out as an interesting approach for an adsorbent with the capacity to exert electrostatic interaction with anionic as well as cationic entities simultaneously. Zwitterionic membranes have been shown to effectively suppress bacterial and protein fouling and attract heavy metal ions.12–14 A soluble fraction of kraft lignin functionalized with zwitterionic groups showed excellent protein repellent properties.15 Few preliminary studies reported the making of chitosan-lignin composites, but with limited applicability for selective metal or dye removal.16,17 Materials offering effective broad-spectrum treatment are rare.
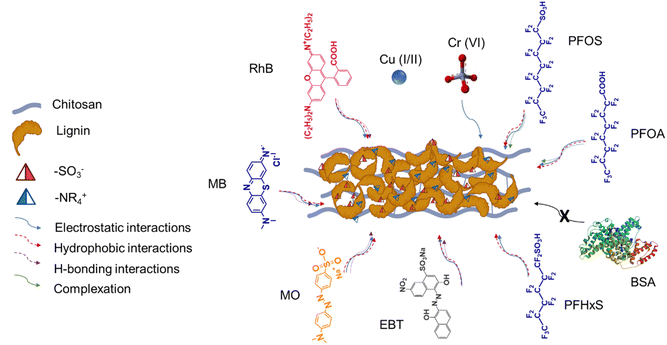
Keeping it as motivation, we present a proof-of-concept study where alkali lignin is functionalized to have zwitterionic groups and is subsequently reinforced with chitosan to produce a biocomposite using a simple and green one-pot synthetic process. While the functionalized lignin provides active sites for adsorbing and holding anionic, cationic, and neutral chemically diverse species effectively, chitosan reinforcement renders mechanical strength and stability across a range of pH levels and diverse environmental conditions, alongside avoiding biofouling synergistically, thereby making it robust for real-world applications. We developed the zwitterionic lignin biocomposite (ZLC), a versatile antifouling material for capturing a wide range of contaminants such as heavy metal ions, cationic and anionic dyes, and PFAS molecules. The processing and application of zwitterion-type lignin (ZL) and ZLC are schematically represented in Fig. 1.
2 Experimental
2.1 Materials
Kraft lignin (KL) (Sigma-Aldrich), 3-chloro-2-hydroxy-1-propansulfonic acid sodium salt hydrate (CHPSS) 95% (Sigma-Aldrich), glycidyl trimethylammonium chloride (GTMAC) ≥90% (Sigma-Aldrich), and chitosan with a medium molecular weight of 190000–310000 g mol−1 and 75–85% deacetylation (Sigma-Aldrich) were used as received. L(+)-lactic acid 95% (Acros Organics), sodium hydroxide (NaOH) (VWR chemicals), and deuterated water (D2O) (VWR chemicals) were used as received. hydrochloric acid (HCl) solution (1 M) was also used. The dialysis membrane (MWCO: 500–1000 g mol−1) was bought from Spectrum Labs. Nitrate salts of cobalt, copper, cadmium, nickel, lead, zinc, and chromium oxide were purchased from Sigma-Aldrich. Eriochrome Black T (EBT) was purchased from Acros Organics, while methyl orange (MO), methylene Blue (MB) hydrate, rhodamine B (RhB), and bovine serum albumin (BSA) were purchased from Sigma-Aldrich. Water spiked with PFAS agents perfluorobutane sulfonic acid (PFBS), perfluorohexane sulphonate (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and perfluorohexanoic acid (PFHxA) (total concentration 50 mg L−1) was procured from IVL Svenska Miljöinstitutet, Stockholm. Two samples of wastewater from a mining area in Skellefteå in Sweden were kindly provided by Boliden AB. If not otherwise stated, deionized water from Merck Millipore was used.2.2 Methods
The as-synthesized materials of ZL and ZLC were purified by dialysis and washing by centrifugation, respectively, and were kept at 60 °C overnight to dry. Large granular materials were obtained for ZLC, which were further crushed by mortar and pestle to get uniform powder material that can be used as adsorbent media.
For isotherm determination, ZL/ZLC (25 mg) was immersed in 25 mL of metal-spiked water with the concentrations 1, 5, 10, 25, 50, 75, and 100 mg L−1 at pH 5.5, and shaken (200 rpm) at room temperature for 24 h. Then, aliquots were collected from the treated water and analyzed with UV-vis/ICPOES to determine the remaining concentration of the spiked entities. The equations involved in quantifying adsorbed amounts are given in the SI.
Kinetics of BSA adsorption over ZL/ZLC was measured using Pierce™ Bradford Protein assay kit by ThermoScientific.19
Performance was quantified using ICP-OES, UV-Vis, and LC-MS/MS; characterization techniques such as SEM-EDS, mapping, XRD, FT-IR, and XPS were carried out on the materials for a qualitative study of the adsorption phenomena.
2.3 Instrumentation
3 Results and discussion
3.1 Characterization of ZL and ZLC
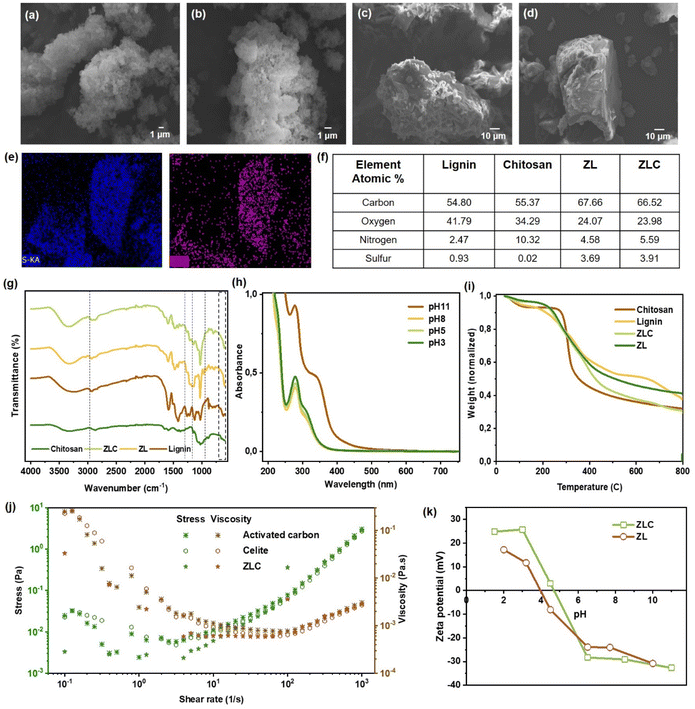
Lignin was functionalized with zwitterionic moieties and combined with chitosan in a one-pot water-based process to achieve a granular and semi-porous biocomposite. Samples were first characterized using 1H NMR spectroscopy to confirm the success of the reaction. In the set of 1H NMR spectra shown in Fig. S1 (SI), the new peak appearing at 4.43 ppm (e′) in the spectrum of ZL belongs to the protons of methine (–CH2CH(OH)–) of the attached GTMAC, while peaks at 4.25 ppm (b) and 3.66 ppm (a) correspond to methine and methylene groups of attached CHPSS, respectively. The intense signals for methyl protons from the (CH3)3N+ group observed at 3.12 ppm (g) from GTMAC overlap with those of (c) methylene protons of CHPSS in the ZL spectrum. The peaks at 3.82 ppm (d) of methylene oxirane of GTMAC, still appearing in the ZL spectrum with weaker intensity, indicate the presence of some unreacted GTMAC in the product. Overall, the appearance of the aforementioned peaks along with 4.17 ppm (a′) belonging to CHPSS methylene attached to the aromatic hydroxy group of lignin and typical GTMAC peaks at 2.96 ppm (f′) confirm the successful sulfonation and quaternization of lignin simultaneously, giving rise to the zwitterion-type functionalization. Characteristic features of chitosan at 4.8 ppm appear next to D2O in the ZLC spectrum (Fig. S2, SI), while small peaks at 3.92–3.57 ppm overlap with KL, CHPSS, and GTMAC peaks. Furthermore, a peak at 1.99 ppm from chitosan shows its tiny presence in the spectrum of the ZLC composite.ZL became a soft, fluffy material upon drying and yielded to the water pressure during batch adsorption tests. Therefore, ZL was deemed unfit for real-time water treatment applications, despite being a functional material. At the same time, ZLC was obtained as large and strong granules, which were further crushed by mortar and pestle to get a uniform powder material applicable as an adsorbent. The photographic images of ZL, ZLC granules, and powdered ZLC are provided in dried form in Fig. S3 (SI). ZLC is largely amorphous, as shown by p-XRD data. Fig. S4 (SI) shows XRD pattern (I) having two diffraction peaks at about 2θ = 10.7° and 20.6° that are attributed to the hydrated crystal structure and amorphous region of chitosan. II shows a typical broad peak of lignin between 2θ = 20–23°, indicating its amorphous nature, which is wider in case of ZL (III), after functionalization of lignin. Pattern IV of the ZLC composite show a weaker and wider peak around 2θ = 28°, which shows that the chitosan has changed into an amorphous state after being combined with lignin. ZL and ZLC were imaged with SEM, as shown in Fig. 2a and c. It was observed that ZLC, being a composite with chitosan, showed higher aggregation and formed almost 10 times larger particles, while ZL was finer. While the parent lignin powder showed porous near-spherical structures with visible cracks and openings (Fig. 1), the composite showed entirely different morphology of irregular-shaped granular structures, resulting in particle sizes in the range of 20–100 µm for ZLC and 5–20 µm for ZL. Surface morphology of ZLC cross-section at higher magnification is shown by SEM images in Fig. S5 (SI). Specific surface area (SSA) and pore volume were determined for the lignin precursor and ZLC using BET N2 adsorption. SSA and pore volume of lignin were 0.60 m2 g−1 and 3.4 × 10−3 cm3 g−1, respectively, while that of ZLC was 1.86 m2 g−1 and 7.8 × 10−3 cm3 g−1, respectively. Although composite formation increased the SSA and porosity by 2–3 times compared to the precursor, the values are still lower than those of nanomaterial-based adsorbents like MOFs, metal oxides, or carbon-based adsorbents like biochar, activated carbon etc.; indicating the zwitterionic functionalization of ZLC playing the major role in defining the uptake capacity of ZLC. No change in particle morphology was observed upon exposure to various pollutant concentrated solutions, which implies that adsorption is the mode of interaction (Fig. 2b and d). The absence of a drastic change in particle size indicates that ZLC does not disintegrate upon agitation and that the composite remains stable in water. EDS analysis (Fig. 2f) indicates that both the precursor and resulting materials are primarily composed of carbon, followed by oxygen. Notably, the nitrogen and sulfur content increased after zwitterionic modification of lignin with NH4+ and SO3− functional groups in ZL and ZLC, as further illustrated in the elemental mapping (Fig. 2e).
The FT-IR spectra of the precursor and the product materials are shown in Fig. 2g. Sharpening of O–H peaks in the 3500–3000 cm−1 region of ZLC and ZL compared to that of lignin and chitosan imply reduction in H-bonding due to bonding/substitution with other functional groups. Aside from the characteristic peaks contributed by lignin and chitosan, ZL and ZLC spectra show extra bands at 1145 and 1345 cm−1 corresponding to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching coming from R–SO3− addition by CHPSS, shown by dotted lines. The C–N stretch of the quaternary ammonium found at around 1475 cm−1, C–N(CH3)3+ symmetric stretch bands between 950–800 cm−1, and 3040 cm−1 for C–H stretch of +NCH3,20 were all coming from the quaternary ammonium functional group incorporated from GTMAC. The chemical stability of ZLC was assessed in the pH range of 3 to 11 (Fig. 2h), and no disintegration over the acidic to the basic range was detected; however, in the extreme alkaline region (pH ≥ 11), lignin may start isolating itself from the composite. The thermal decomposition behaviour is not significantly affected by the chemical modification. Chitosan undergoes major degradation around 270 °C, which is associated with the depolymerization of the polysaccharide backbone, dehydration of saccharide rings, and decomposition of acetylated units. Lignin, on the other hand, shows significant weight loss at approximately 190 °C, due to degradation of its polyphenolic backbone and cleavage of weak ether and ester linkages. In ZLC, the observed Tmax of 230 °C indicates a shift in the thermal stability compared to the individual components, suggesting interactions between chitosan and lignin during thermal decomposition. Furthermore, differences in the char residue were evident. Lignin typically leaves a higher char yield due to its aromatic structure, which favors carbonaceous residue formation. Chitosan, in contrast, produces less char because of its relatively linear polysaccharide backbone and lower aromatic content. ZLC displayed an intermediate char residue, implying partial stabilization of chitosan by the aromatic framework in lignin (Fig. 2i). High mechanical strength and robustness is crucial for an adsorbent to be used in a filter module and able to withstand the water flux and for avoiding pressure drop in the column filter by clogging. Hence, ZLC was analyzed for rheological properties and compared to commercial benchmark materials such as activated carbon and Celite (Fig. 2j). Both the shear stress–shear rate curve and the viscosity–shear rate curve of ZLC follow closely the behavior and values of commercial activated carbon and celite (kieselguhr) with a distinct non-Newtonian behavior. In the process of adsorption, the overall surface charge of the material plays a significant role in influencing electrostatic interactions with various charged contaminants in water. Hence, pH-dependent zeta potential was recorded for ZL and ZLC dispersed in water (0.05 wt%) in the pH range of 1–11, shown in Fig. 2k. Isoelectric points of ZL and ZLC appeared at pH 4 and 4.7, respectively. ZLC showed higher dispersion stability than ZL at low and high pH regions.
O stretching coming from R–SO3− addition by CHPSS, shown by dotted lines. The C–N stretch of the quaternary ammonium found at around 1475 cm−1, C–N(CH3)3+ symmetric stretch bands between 950–800 cm−1, and 3040 cm−1 for C–H stretch of +NCH3,20 were all coming from the quaternary ammonium functional group incorporated from GTMAC. The chemical stability of ZLC was assessed in the pH range of 3 to 11 (Fig. 2h), and no disintegration over the acidic to the basic range was detected; however, in the extreme alkaline region (pH ≥ 11), lignin may start isolating itself from the composite. The thermal decomposition behaviour is not significantly affected by the chemical modification. Chitosan undergoes major degradation around 270 °C, which is associated with the depolymerization of the polysaccharide backbone, dehydration of saccharide rings, and decomposition of acetylated units. Lignin, on the other hand, shows significant weight loss at approximately 190 °C, due to degradation of its polyphenolic backbone and cleavage of weak ether and ester linkages. In ZLC, the observed Tmax of 230 °C indicates a shift in the thermal stability compared to the individual components, suggesting interactions between chitosan and lignin during thermal decomposition. Furthermore, differences in the char residue were evident. Lignin typically leaves a higher char yield due to its aromatic structure, which favors carbonaceous residue formation. Chitosan, in contrast, produces less char because of its relatively linear polysaccharide backbone and lower aromatic content. ZLC displayed an intermediate char residue, implying partial stabilization of chitosan by the aromatic framework in lignin (Fig. 2i). High mechanical strength and robustness is crucial for an adsorbent to be used in a filter module and able to withstand the water flux and for avoiding pressure drop in the column filter by clogging. Hence, ZLC was analyzed for rheological properties and compared to commercial benchmark materials such as activated carbon and Celite (Fig. 2j). Both the shear stress–shear rate curve and the viscosity–shear rate curve of ZLC follow closely the behavior and values of commercial activated carbon and celite (kieselguhr) with a distinct non-Newtonian behavior. In the process of adsorption, the overall surface charge of the material plays a significant role in influencing electrostatic interactions with various charged contaminants in water. Hence, pH-dependent zeta potential was recorded for ZL and ZLC dispersed in water (0.05 wt%) in the pH range of 1–11, shown in Fig. 2k. Isoelectric points of ZL and ZLC appeared at pH 4 and 4.7, respectively. ZLC showed higher dispersion stability than ZL at low and high pH regions.
3.2 Adsorption of heavy metals
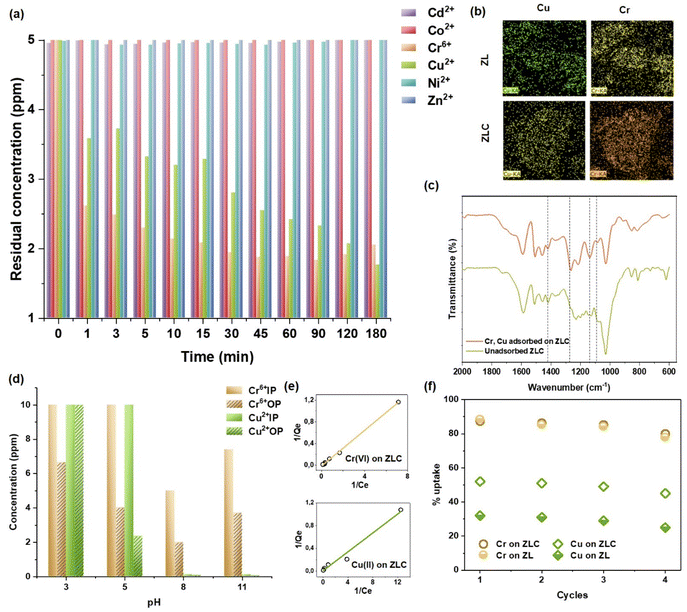
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, symmetric and asymmetric stretch of S–O groups alongside a band at 1420 cm−1 due to –OH bending, which either diminishes or vanishes in the metal-adsorbed ZLC. Also, weak bands at 918 and 968 cm−1 stemming from +N(Me)3 of ZLC cannot be seen in the metal-loaded ZLC, which suggest that these are the N- and S- active sites participating in metal adsorption, whereas new peaks at 908 and 1138 cm−1 can be attributed to Cr and Cu ion adsorption.22,23 To improve the adsorption efficiency, it is interesting to explore the pH range where ZLC performs best. pH-dependent adsorption experiments showed that maximum Cr(VI) and Cu(II) adsorption occurred at pH 5 (Fig. 3d). Alkaline pH promotes precipitation of the dissolved metal salts in the input water samples, thus making it an unsuitable condition to work in. Fig. 3e represents the Langmuir isotherm plots for the adsorption of heavy metals and dyes on ZLC to observe its maximum uptake capacity for each contaminant. The isotherm data were also modeled for the Freundlich isotherm, and both models were applied to ZL as well to compare the uptake capacities (Table 1). Langmuir isotherm showed higher R2 values in every case; linear fits are shown in Fig. S8 (SI).
O stretching, symmetric and asymmetric stretch of S–O groups alongside a band at 1420 cm−1 due to –OH bending, which either diminishes or vanishes in the metal-adsorbed ZLC. Also, weak bands at 918 and 968 cm−1 stemming from +N(Me)3 of ZLC cannot be seen in the metal-loaded ZLC, which suggest that these are the N- and S- active sites participating in metal adsorption, whereas new peaks at 908 and 1138 cm−1 can be attributed to Cr and Cu ion adsorption.22,23 To improve the adsorption efficiency, it is interesting to explore the pH range where ZLC performs best. pH-dependent adsorption experiments showed that maximum Cr(VI) and Cu(II) adsorption occurred at pH 5 (Fig. 3d). Alkaline pH promotes precipitation of the dissolved metal salts in the input water samples, thus making it an unsuitable condition to work in. Fig. 3e represents the Langmuir isotherm plots for the adsorption of heavy metals and dyes on ZLC to observe its maximum uptake capacity for each contaminant. The isotherm data were also modeled for the Freundlich isotherm, and both models were applied to ZL as well to compare the uptake capacities (Table 1). Langmuir isotherm showed higher R2 values in every case; linear fits are shown in Fig. S8 (SI).
| Sample no. | Effluent | Material | Isotherm | |||
|---|---|---|---|---|---|---|
| Langmuir | Freundlich | |||||
| Uptake (mg g−1) | R 2 value | Uptake (mg g−1) | R 2 value | |||
| 1 | Cr(VI) | ZL | 71.4 | 0.99 | 7.4 | 0.97 |
| 2 | Cr(VI) | ZLC | 108.6 | 0.99 | 6.8 | 0.99 |
| 3 | Cu(II) | ZL | 156.2 | 0.97 | 7.7 | 0.98 |
| 4 | Cu(II) | ZLC | 196.0 | 0.98 | 14.6 | 0.98 |
| 5 | MB | ZLC | 192.3 | 0.94 | 18.2 | 0.69 |
| 6 | MO | ZLC | 4166.6 | 0.90 | 40.7 | 0.77 |
| 7 | RhB | ZLC | 71.9 | 0.93 | 6.7 | 0.93 |
| 8 | EBT | ZLC | 1030.9 | 0.88 | 13.8 | 0.81 |
Although ZL was tested for heavy metals in initial experiments to check its functionality, it was not further used for organic dyes and PFAS removal due to its soft and weak structure, that made it difficult to handle.
| Real water samples | pH | Cr(VI) removal | Cu(II) removal |
|---|---|---|---|
| Sample 1 | 8.1 | 62% | 97% |
| Sample 2 | 8.3 | 63% | 89% |
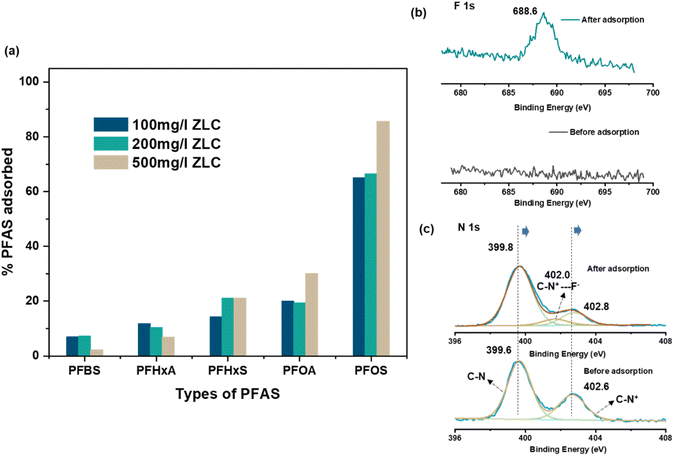
3.3 Adsorption of organic contaminants: PFAS
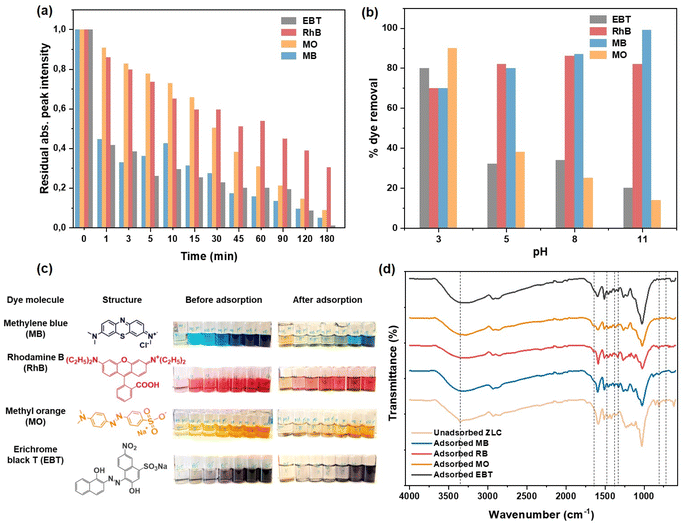
3.4 Adsorption of organic contaminants: cationic and anionic dyes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations in heterocyclic groups of MB), 1383 and 1328 cm−1 (due to C–N, C
N vibrations in heterocyclic groups of MB), 1383 and 1328 cm−1 (due to C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S+ stretch of heterocycles), 1268 cm−1 for C–H bending, 884 cm−1 for dimers or H-aggregates, while 646 cm−1 for γ and δ (C–S–C) groups confirm the presence of adsorbed MB on the surface of ZLC.27 In case of RhB adsorbed on ZLC, a peak at 1648 cm−1 for stretching vibrations of C
S+ stretch of heterocycles), 1268 cm−1 for C–H bending, 884 cm−1 for dimers or H-aggregates, while 646 cm−1 for γ and δ (C–S–C) groups confirm the presence of adsorbed MB on the surface of ZLC.27 In case of RhB adsorbed on ZLC, a peak at 1648 cm−1 for stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups of aromatic rings, 1340 and 1244 cm−1 for C–C and C–O–C stretching, and 683 cm−1 for aromatic C–H wagging vibrations confirm the presence of adsorbed RhB on the ZLC surface.28,29 In the MO-adsorbed ZLC spectrum, weak bands appeared at 1649 and 1600 cm−1 attributed to N–H bending/C
N groups of aromatic rings, 1340 and 1244 cm−1 for C–C and C–O–C stretching, and 683 cm−1 for aromatic C–H wagging vibrations confirm the presence of adsorbed RhB on the ZLC surface.28,29 In the MO-adsorbed ZLC spectrum, weak bands appeared at 1649 and 1600 cm−1 attributed to N–H bending/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch and N
C stretch and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations, respectively. Peaks at 1264 and 1215 cm−1 stemmed from C–N stretching of aliphatic amines, and a weak band at 814 cm−1 due to the 1,4-disubstituted (para)benzene ring confirmed the presence of the azo dye on ZLC surface.30,31 Apart from the azo-related peaks at 1654 and 1597 cm−1, peaks appearing at 1334 and 1215 cm−1 due to –NO2 and –SO3 groups, respectively, confirmed the EBT adsorption on the ZLC surface.32
N stretching vibrations, respectively. Peaks at 1264 and 1215 cm−1 stemmed from C–N stretching of aliphatic amines, and a weak band at 814 cm−1 due to the 1,4-disubstituted (para)benzene ring confirmed the presence of the azo dye on ZLC surface.30,31 Apart from the azo-related peaks at 1654 and 1597 cm−1, peaks appearing at 1334 and 1215 cm−1 due to –NO2 and –SO3 groups, respectively, confirmed the EBT adsorption on the ZLC surface.32
A schematic representation summarizing various possible molecular interactions between ZLC and different contaminants for adsorption is provided as Fig. 7.
3.5 Protein fouling
Protein adsorption is a ubiquitous fouling process that can have undesirable consequences for any adsorbent operating in the field or with industrial effluents. Thus, ZL and ZLC were tested for their protein resistance by exposing them to a model protein (bovine serum albumin, BSA) at room temperature, and the adsorption process was analyzed in a time-dependent manner using the Pierce™ Bradford Protein assay. The percentage of BSA absorbed over time was calculated from the absorbance data (Fig. 8). It was observed that the BSA molecules initially interacted slightly with the ZLC surface, but no adsorption was observed beyond 60 min. This can be due to the positive and negative functional groups on ZL and ZLC surfaces, making the material very hydrophilic, and the electrostatic interactions among the groups can result in their reorientation towards the interface of the aqueous medium, giving rise to a hydration layer that can effectively promote protein resistance.33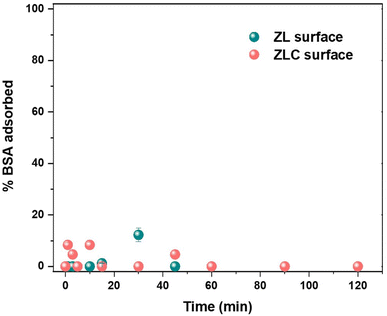 | ||
| Fig. 8 Time-dependent performance of ZL and ZLC tested against adsorption of BSA using Pierce™ Bradford Protein assay method. | ||
3.6 Comparison with the literature
Several conventional and novel sorbents have been used for the removal of various contaminant species from aqueous solution for broad-spectrum water purification. While, most of them show sensitivity towards one or two types of contaminant species, multifunctionality of ZLC can be clearly observed when compared with other adsorbents. These findings are summarized together with data from this work (Table 3).| Adsorbent | Removal capacity (mg g−1 or %) | Antifouling properties | Reference | ||
|---|---|---|---|---|---|
| Heavy metals | PFAS | Organics | |||
| ZLC | 71.4 Cr(VI), 156.2 Cu(II) | PFOS 86%, PFOA 30% | MB 192.3, MO 4166.6, RhB 71.9, EBT 1030.9 | Yes | This work |
| Walnut shell activated carbon | 51.3 Cr(VI), 204.1 Cu(II) | — | — | — | 34 |
| Sorghum bicolor | 20.7 Cr(VI), 24.8 Cu(II) | — | — | — | 35 |
| Biosilica/Silk fibroin/Polyurethane | 201.56 Cr(VI), 31.69 Cu(II) | — | — | — | 36 |
| ZIF-67/BC/CH aerogel | 152.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr(VI), 200.6 Cu(II) Cr(VI), 200.6 Cu(II) |
— | Active red X–3B ∼100% | — | 37 |
| γFe2O3 NP@cellulose/Activated carbon | — | — | MB 1.40(4.39 × 10−3 mmol g−1), MO 2.12 (6.48 × 10−3 mmol g−1) | — | 38 |
| Chitosan grafted-benzaldehyde/montmorillonite/algae | — | — | BG 899.5, RB19 213.6 | — | 39 |
| MgO–SiO2/lignin at 313 K | 35.30 Cu(II), 69.42 Cd(II) | — | — | — | 40 |
| Carboxymethylated lignin/Fe3O4 | 189.85 Pb(II), 124.43 Cu(II), 106.97 Ni(II) | — | — | — | 41 |
| Amino-functionalized lignin microspheres | 74.84 Cd(II), 54.20 Cr(VI), 53.12 As(V), 49.42 Ni(II) | — | — | — | 42 |
| Quaternized lignin | — | — | RB 41.85, MB 49.47, AB-92 110.53, CR 134.61 | Biocompatible | 43 |
| Fe3O4/C-ACLS carbon coated magnetic ferric oxide@calcium lignosulfonate (ACLS) | — | — | CR 198.8, TY 192.3, EBB-R 198.5 | — | 44 |
| Lignin-based polyurethane foam | 98.2 Cu(II) | — | MB 67.1, RhB 96.1 | — | 45 |
| Iron anchored graphene (G-fe) nanosheets from dried seaweed (ulva fasciata) | 645 Pb(II) | — | CR 970, CV 909, MO 664, MB 402 | — | 46 |
| Cationic quaternized nanocellulose | — | PFOS 559, PFOA 405, PFBS 319, PFBA 121 | — | — | 47 |
| Chitosan/F–COF | — | PFOS 8307.1, PFOA 6177.1, GenX 4603.3 | — | — | 48 |
| Rice straw-derived biochar-ALG composite beads | — | PFOS ∼99%, PFBS 40% | — | — | 49 |
| Reduced graphene oxide modified zinc ferrite immobilized chitosan beads (rGO-ZF@CB) | — | PFOA 16.07, PFOS 21.64 | — | — | 50 |
| Silane-modified zirconium-based coagulant DZHC | 94% Cu(II), 100% Pb(II), 99% Cr(VI), 32% Zn(II) | PFOS 100%, PFOA 99%, PFHxS 99%, PFHxA 96%, PFBS 98% and PFBA 85% | — | — | 51 |
| Modified coal fly ash (CFA) | — | PFDoDA 3.5,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFBA 1.8, PFHxA 1.8, PFOA 2.3, PFDA 3.0 PFBA 1.8, PFHxA 1.8, PFOA 2.3, PFDA 3.0 |
— | — | 52 |
| Hardwood-derived biochar | Cd(II) | PFOS 16.1, PFOA 7.7, GenX 4.8 | — | — | 53 |
| GAC | 92% Cd(II), 81% Cu(II), 84% Pb(II), of 72% Ni(II) and 52% Zn(II) | PFOS/PFOA 4–5 mg mg−1 | Pharma molecules | TSS dependent | 54 |
| Zr-based porphyrinic MOF (PCN-224) | — | PFOS 963, PFHxS 517, PFBS 395 | — | — | 55 |
Conventional materials such as activated carbon (GAC and PAC) dominate commercially due to their efficiency and cost-effectiveness, while ion exchange resins are expensive but known for their selective removal capacity. These materials come with high energy demands, frequent replacement needs, and CO2 emissions. ZLC offers significant advantages over these alternatives as it captures a wide range of contaminants, including PFAS, heavy metals, and dyes, across a broad pH range while resisting fouling. It is produced via a simple, green, water-based one-pot process at 70–80 °C, unlike activated carbon, which requires high-temperature activation (700–800 °C), and is often not regenerated due to low cost, causing untreated spent materials to end up in landfills. ZLC is mainly made from lignin and chitosan – easily available and inexpensive raw materials, making it cost-effective and it can be regenerated multiple times. It is thermally stable and chemically robust, with high mechanical stability (comparable to activated carbon and Celite shown in Fig. 2), thus making ZLC comparable to commercial filter materials. With further enhancement of its uptake capacity, its multifunctionality, ease of production, and reusability make it a scalable, sustainable alternative for industrial applications.
4 Conclusions
This study demonstrates the preparation and performance of a unique multifunctional composition that is capable of adsorbing anionic, cationic, and neutral chemically diverse contaminants effectively across a range of pH levels, while avoiding biofouling, making it a robust choice for real-world applications. Functionalized lignin with a structural reinforcement added by chitosan (ZLC) were prepared using a green, simple, and water-based one-pot synthetic procedure. Morphological characterization of the materials conveyed that they are micron-sized granulated, amorphous, mechanically and thermally stable, while chemically they are stable in a wide pH range. Lignin modified with sulphonyl and quaternary ammonium groups gives the composite a zwitterion-type functionalization that acts as active sites for adsorption. ZLC were tested as adsorbents and demonstrated excellent performance for an array of water contaminants such as Cr and Cu ions, PFAS molecules such as PFOS, and the synthetic dyes MB, MO, RhB, and EBT. The materials also performed well for real water samples from mining areas and are reusable for multiple cycles. The adsorption process was in each case studied using XPS and IR spectroscopy. XPS data confirmed the interaction of contaminants with the N and S active sites of the materials. Further, the adsorption process followed pseudo-second-order kinetics and Langmuir isotherm, which showed very high uptake capacities for the large number of contaminants. This sustainable, low-cost material presents a viable solution for improving broad-spectrum water purification systems worldwide, addressing the constraints of conventional treatment approaches.Author contributions
S. M., conceptualization, methodology, data collection, investigation, writing-original draft, and editing; S. L., investigation, editing, and calculations; U. E., conceptualization, supervision, writing-review, editing, and acquired funding. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Data availability
Data generated or analysed during this study are included in this published article and its supplementary information (SI) . Supplementary information: includes figures and tables that provides formulae and equations; 1H NMR and pXRD data for lignin, chitosan, ZL and ZLC; Langmuir and Freundlich isotherm models for adsorption; FT-IR spectra of all dyes; parameters of adsorption kinetics, and physicochemical parameters of real water samples. See DOI: https://doi.org/10.1039/d5va00282f.Acknowledgements
The authors thank the Swedish Foundation for Strategic Environmental Research (Mistra) for their financial support (project name Mistra TerraClean, project number 2015/31). Fei Ye and Joydeep Dutta, Functional materials, Applied Physics, KTH, are thanked for providing access to and assistance with Inductively coupled plasma optical emission spectrometry. Tove Mallin, RISE, Sweden, and Linda Önnby, IVL, Sweden, are thanked for providing PFAS-spiked water samples and for the analysis of PFAS concentration. Aji Mathew and Varvara Apostolopoulou are thanked for the instrumentation used at Stockholm University. Matthew Fielden, KTH is thanked for assistance with XPS. Master's students Helina Tafesse Belachew and Kaveri Mani are thanked for their assistance in the experiments and sample analysis. Boliden is thanked for providing mining wastewater samples.References
- N. A. A. Qasem, R. H. Mohammed and D. U. Lawal, npj Clean Water, 2021, 4, 1–15 CrossRef.
- G. Sigmund, M. Venier, M. Ågerstrand, I. T. Cousins, J. DeWitt, M. L. Diamond, J. Field, A. T. Ford, S. Joudan, S. van Leeuwen, R. Lohmann, C. Ng, M. Scheringer, A. Soehl, N. Suzuki, X. Trier, S. Valsecchi, P. Vlahos, C. J. Young and Z. Wang, Environ. Sci. Technol. Lett., 2025, 12(9), 1104–1106, DOI:10.1021/ACS.ESTLETT.5C00478.
- S. Mantripragada, S. O. Obare and L. Zhang, Acc. Chem. Res., 2023, 56, 1271–1278 CrossRef CAS PubMed.
- N. K. Khalid, A. Aker, S. St´, S. Lair, S. ´ Ebastien Sauvé and S. Sauvé, Environ. Sci. Adv., 2025, 4, 1599–1611, 10.1039/D5VA00061K.
- C. Ching, Z. W. Lin, W. R. Dichtel and D. E. Helbling, ACS ES&T Eng., 2023, 3, 661–670 Search PubMed.
- S. Mukherjee, J. Shantha Kumar, A. Nagar and T. Pradeep, ACS Symp. Ser., 2022, 1412, 625–657 CrossRef CAS.
- M. Ahmed, M. O. Mavukkandy, A. Giwa, M. Elektorowicz, E. Katsou, O. Khelifi, V. Naddeo and S. W. Hasan, npj Clean Water, 2022, 5, 1–25 CrossRef.
- E. Barry, R. Burns, W. Chen, G. X. De Hoe, J. M. M. De Oca, J. J. De Pablo, J. Dombrowski, J. W. Elam, A. M. Felts, G. Galli, J. Hack, Q. He, X. He, E. Hoenig, A. Iscen, B. Kash, H. H. Kung, N. H. C. Lewis, C. Liu, X. Ma, A. Mane, A. B. F. Martinson, K. L. Mulfort, J. Murphy, K. Mølhave, P. Nealey, Y. Qiao, V. Rozyyev, G. C. Schatz, S. J. Sibener, D. Talapin, D. M. Tiede, M. V. Tirrell, A. Tokmakoff, G. A. Voth, Z. Wang, Z. Ye, M. Yesibolati, N. J. Zaluzec and S. B. Darling, Chem. Rev., 2021, 121, 9450–9501 CrossRef CAS PubMed.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344(6185), 1246843, DOI:10.1126/SCIENCE.1246843.
- X. Liu, X. Duan, W. Wei, S. Wang and B. J. Ni, Green Chem., 2019, 21, 4266–4289 RSC.
- M. Fazeli, S. Mukherjee, H. Baniasadi, R. Abidnejad, M. Mujtaba, J. Lipponen, J. Seppälä, O. J. Rojas and O. J. Rojas, Green Chem., 2024, 26, 593–630 RSC.
- S. K. Lau and W. F. Yong, ACS Appl. Polym. Mater., 2021, 3, 4390–4412 CrossRef CAS.
- G. Liu, C. Matindi, M. Hu, X. Li, X. Ma and J. Li, 60 Years Loeb-Sourirajan Membr. Princ. New Mater. Model. Charact. Appl., 2022, 349–389 Search PubMed.
- D. Georgouvelas, H. N. Abdelhamid, J. Li, U. Edlund and A. P. Mathew, Carbohydr. Polym., 2021, 264, 118044 CrossRef CAS.
- L. An, Y. H. Yu, J. Chen, J. H. Bae, D. H. Youn, H. M. Jeong and Y. S. Kim, Ind. Crops Prod., 2021, 167, 113514 CrossRef CAS.
- V. Nair, A. Panigrahy and R. Vinu, Chem.–Eng. J., 2014, 254, 491–502 CrossRef CAS.
- S. Sohni, R. Hashim, H. Nidaullah, J. Lamaming and O. Sulaiman, Int. J. Biol. Macromol., 2019, 132, 1304–1317 CrossRef CAS PubMed.
- Y. Guo, W. Gao, F. Kong and P. Fatehi, Int. J. Biol. Macromol., 2019, 140, 429–440 CrossRef CAS.
- Pierce™ Bradford Protein Assay Kit, https://www.thermofisher.com/order/catalog/product/23200, accessed 14 July 2024.
- N. B. Colthup, L. H. Daly and S. E. Wiberley, Introd. to Infrared Raman Spectrosc., 1975, 341, 321–334 Search PubMed.
- T. A. Saleh, Interface Sci. Technol., 2022, 34, 65–97 Search PubMed.
- J. Wang, L. Zhao, W. Duan, L. Han and Y. Chen, Ind. Eng. Chem. Res., 2012, 51, 13655–13662 CrossRef CAS.
- L. Ramrakhiani, S. Ghosh, S. Sarkar and S. Majumdar, Mater. Today Proc., 2016, 3, 3538–3552 CrossRef.
- J. W. Heo, L. An, M. S. Kim, D. H. Youn and Y. S. Kim, Int. J. Biol. Macromol., 2023, 253, 127421 CrossRef CAS.
- M. Szabó, J. Kalmár, T. Ditrói, G. Bellér, G. Lente, N. Simic and I. Fábián, Inorganica Chim. Acta, 2018, 472, 295–301 CrossRef.
- O. Tkachenko, D. Diment, D. Rigo, M. Stro̷mme and T. M. Budnyak, Biomacromolecules, 2024, 25, 4292–4304 CrossRef CAS PubMed.
- O. V. Ovchinnikov, A. V. Evtukhova, T. S. Kondratenko, M. S. Smirnov, V. Y. Khokhlov and O. V. Erina, Vib. Spectrosc., 2016, 86, 181–189 CrossRef CAS.
- A. A. M. Farag and I. S. Yahia, Opt. Commun., 2010, 283, 4310–4317 CrossRef CAS.
- W. Xiao, Z. N. Garba, S. Sun, I. Lawan, L. Wang, M. Lin and Z. Yuan, J. Clean. Prod., 2020, 253, 119989 CrossRef CAS.
- L. Wu, X. Liu, G. Lv, R. Zhu, L. Tian, M. Liu, Y. Li, W. Rao, T. Liu and L. Liao, Sci. Reports, 2021, 11, 1–11 Search PubMed.
- K. Akansha, D. Chakraborty and S. G. Sachan, Biocatal. Agric. Biotechnol., 2019, 18, 101044 CrossRef.
- P. Veerakumar, T. Jeyapragasam, S. Surabhi, K. Salamalai, T. Maiyalagan and K. C. Lin, J. Chem. Eng. Data, 2019, 64, 1305–1321 CrossRef CAS.
- C. M. Xing, F. N. Meng, M. Quan, K. Ding, Y. Dang and Y. K. Gong, Acta Biomater., 2017, 59, 129–138 CrossRef CAS PubMed.
- R. Xie, H. Wang, Y. Chen and W. Jiang, Environ. Prog. Sustain. Energy, 2013, 32, 688–696 CrossRef CAS.
- S. Choudhary, V. Goyal and S. Singh, Clean Technol. Environ. Policy, 2015, 17, 1039–1051 CrossRef CAS.
- P. S. Prasad, T. Gomathi, P. N. Sudha, M. Deepa, K. Rambabu and F. Banat, Environ. Technol. Innov., 2022, 28, 102741 CrossRef CAS.
- D. Li, X. Tian, Z. Wang, Z. Guan, X. Li, H. Qiao, H. Ke, L. Luo and Q. Wei, Chem.–Eng. J., 2020, 383, 123127 CrossRef CAS.
- X. Luo and L. Zhang, J. Hazard. Mater., 2009, 171, 340–347 CrossRef CAS PubMed.
- A. H. Jawad, A. S. Abdulhameed, S. N. Surip and Z. A. Alothman, J. Clean. Prod., 2023, 393, 136334 CrossRef CAS.
- Ł. Klapiszewski, K. Siwińska-Stefańska and D. Kołodyńska, Chem.–Eng. J., 2017, 330, 518–530 CrossRef.
- B. Du, L. Chai, Q. Zheng, Y. Liu, X. Wang, X. Chen, S. Zhai, J. Zhou and R. C. Sun, Int. J. Biol. Macromol., 2023, 234, 123668 CrossRef CAS PubMed.
- A. L. Popovic, J. D. Rusmirovic, Z. Velickovic, Z. Radovanovic, M. Ristic, V. P. Pavlovic and A. D. Marinkovic, Int. J. Biol. Macromol., 2020, 156, 1160–1173 CrossRef CAS PubMed.
- B. Du, W. Li, L. Chai, W. Li, X. Wang, X. Chen, J. Zhou and R. C. Sun, J. Mol. Liq., 2023, 377, 121566 CrossRef CAS.
- C. Jiang, X. Wang, D. Qin, W. Da, B. Hou, C. Hao and J. Wu, J. Hazard. Mater., 2019, 369, 50–61 CrossRef CAS PubMed.
- J. Chen, J. Wu, Y. Zhong, X. Ma, W. Lv, H. Zhao, J. Zhu and N. Yan, Sep. Purif. Technol., 2023, 311, 123284 CrossRef CAS.
- A. Mahto, A. Kumar, J. P. Chaudhary, M. Bhatt, A. K. Sharma, P. Paul, S. K. Nataraj and R. Meena, J. Hazard. Mater., 2018, 353, 190–203 CrossRef CAS.
- D. Li, C. S. Lee, Y. Zhang, R. Das, F. Akter, A. K. Venkatesan and B. S. Hsiao, J. Mater. Chem. A, 2023, 11, 9868–9883 RSC.
- C. He, Y. Yang, Y. J. Hou, T. Luan and J. Deng, Sep. Purif. Technol., 2022, 294, 121195 CrossRef CAS.
- I. M. Militao, F. Roddick, L. Fan, L. C. Zepeda, R. Parthasarathy and R. Bergamasco, J. Water Process Eng., 2023, 53, 103616 CrossRef.
- S. S. Elanchezhiyan, J. Preethi, K. Rathinam, L. K. Njaramba and C. M. Park, Carbohydr. Polym., 2021, 267, 118165 CrossRef CAS PubMed.
- D. Xiao, W. Dong, H. Wang, J. Cao, J. Liu, Y. Wang, Y. Wu, F. Ma and H. Yi, Chem.–Eng. J., 2025, 519, 164887 CrossRef CAS.
- H. V. Patel, M. Greer, B. Brazil, W. Yu, S. Hamoush, L. Zhang and R. Zhao, J. Hazard. Mater., 2025, 484, 136763 CrossRef CAS PubMed.
- A. Chaudhary, M. Usman, O. Mašek, S. Haderlein and K. Hanna, J. Water Process Eng., 2025, 74, 107875 CrossRef.
- R. Mailler, O. Danel, M. Esperanza, S. Courtois and A. Gonzalez Ospina, Sci. Total Environ., 2024, 952, 175918 CrossRef CAS PubMed.
- Y. Zhang, K. Kong, Q. Wu, T. Ma, J. Liang and R. Wang, ChemSusChem, 2024, 17, e202400069 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |