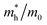Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solution-based synthesis of nanocrystalline KBiS2 films at low temperatures and study of photoinduced charge generation
Marco Sigl a,
Melissa Egger
a,
Melissa Egger a,
Daniel Knez
a,
Daniel Knez b,
Harald Fitzekc,
Dmytro Neshchadin
b,
Harald Fitzekc,
Dmytro Neshchadin d,
Ison Haue,
Thomas Webbe,
Fernando Warchomicka
d,
Ison Haue,
Thomas Webbe,
Fernando Warchomicka f,
Jiawen Han
f,
Jiawen Han e,
Ruiqi Wu
e,
Ruiqi Wu e,
Alex M. Ganose
e,
Alex M. Ganose e,
Georg Gescheidt
e,
Georg Gescheidt d,
Gerald Kothleitnerb,
Gregor Trimmel
d,
Gerald Kothleitnerb,
Gregor Trimmel a,
Saif A. Haque
a,
Saif A. Haque *e and
Thomas Rath
*e and
Thomas Rath *a
*a
aInstitute for Chemistry and Technology of Materials, NAWI Graz, Graz University of Technology, Stremayrgasse 9, 8010 Graz, Austria. E-mail: thomas.rath@tugraz.at
bInstitute of Electron Microscopy and Nanoanalysis, Graz University of Technology, Steyrergasse 17, 8010 Graz, Austria
cGraz Centre for Electron Microscopy, Steyrergasse 17, 8010 Graz, Austria
dInstitute of Physical and Theoretical Chemistry, Graz University of Technology, Stremayrgasse 9, 8010 Graz, Austria
eDepartment of Chemistry, Molecular Sciences Research Hub, Imperial College London, White City Campus, 82 Wood Lane, W12 0BZ London, UK. E-mail: s.a.haque@imperial.ac.uk
fInstitute of Materials Science, Joining and Forming, Graz University of Technology, Kopernikusgasse 24, 8010 Graz, Austria
First published on 19th December 2025
Abstract
Ternary metal sulfides have been widely investigated in recent years as solar absorber materials in photocatalysis and photovoltaics, where they have the potential to replace expensive or harmful materials like noble metals or lead-based compounds. Potassium bismuth sulfide has two polymorphs showing promise for solar applications. However, the preparation of KBiS2 is hardly investigated beyond the originally proposed highly energy demanding solid state or salt melt syntheses. In order to facilitate the applicability of KBiS2 films, we investigated the formation of cubic and rhombohedral KBiS2 from metal xanthate precursors. The use of xanthates offers the advantages of low conversion temperatures and good solubility of the precursors in non-halogenated and non-aromatic solvents. We successfully prepared KBiS2 films with rhombohedral and cubic crystal structures, which we could confirm with XRD and HR-STEM experiments, and found that the cubic phase forms at a relatively low temperature of 200 °C. Our findings provide a facile low temperature method to prepare KBiS2 thin films and demonstrate well-suited optoelectronic properties of this material. In particular, the long charge recombination lifetime of 41.1 microseconds in TiO2/KBiS2/spiro-OMeTAD films highlights the suitability of KBiS2 for potential applications in solar energy conversion devices.
Introduction
With rising energy demands and the need to replace fossil fuels, solar energy is gaining increasing attention as a sustainable alternative. Materials with strong light absorption, suitable bandgaps, and low cost are essential for future applications in photovoltaics1–4 and photocatalysis,5–8 including solar fuel production and wastewater treatment. Recently, increasing interest has fallen onto metal chalcogenides, as they commonly show very suitable optical properties for solar energy conversion. A large group of metal chalcogenides are ternary metal sulfides, which combine two different metal cations and a sulfur anion to yield a plethora of structures. Ternary metal sulfides, such as CuInS2, Cu3BiS3, AgBiS2, ZnIn2S4 and CuSbS2, typically show a bandgap in the visible range, making them promising candidates for utilization of sunlight.4,9–13Bismuth sulfide itself is known to have high absorption coefficients and a low bandgap around 1.3 eV in the visible range.14 Furthermore, introducing an additional metal ion to create ternary bismuth sulfides can lead to an improved charge carrier concentration.15 Aluminum- or nickel-doped bismuth sulfides and ternary phases such as CuBiS2, Cu3BiS3 and AgBiS2 have been reported in photovoltaic or photocatalytic applications.16–22
An emerging and versatile ternary bismuth-based sulfide is potassium bismuth sulfide. Depending on the ratio of potassium to bismuth, several crystal structures, like orthorhombic,23 rhombohedral24 and cubic24,25 crystal phases, are reported. The cubic KBiS2 crystallizes in a simple rock salt structure (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and is reported with an optical bandgap of 1.61 eV.4,26 While the properties of this material are very interesting, its synthesis typically involves high temperature processes. KBiS2 is usually synthesized via solid state or salt melt syntheses using powdered Bi, K2CO3 and elemental sulfur at high temperatures of around 800 °C for several hours.24,27 The K2S5 salt flux based synthesis of rhombohedral KBiS2 reported by Qu et al. takes place at 600 °C for a prolonged time.28 Even the K3BiS3 intermediate phases reported by McClain et al. still require 450 °C for 48 h.24
m) and is reported with an optical bandgap of 1.61 eV.4,26 While the properties of this material are very interesting, its synthesis typically involves high temperature processes. KBiS2 is usually synthesized via solid state or salt melt syntheses using powdered Bi, K2CO3 and elemental sulfur at high temperatures of around 800 °C for several hours.24,27 The K2S5 salt flux based synthesis of rhombohedral KBiS2 reported by Qu et al. takes place at 600 °C for a prolonged time.28 Even the K3BiS3 intermediate phases reported by McClain et al. still require 450 °C for 48 h.24
Recently, Yang et al. reported a successful hot-injection synthesis, using the long chained oleylamine and oleic acid. With this approach, they obtained nanocrystalline KBiS2 at 250 °C. However, the obtained crystallites are very small (7.4 nm) and the remaining ligands at the surface can interfere with the optoelectronic properties. Nonetheless, the photodetectors manufactured with these nanoparticles proved to show an excellent photoresponse.26 However, in order to utilize KBiS2 as a broadly applicable semiconductor for solar energy conversion, a synthetic method yielding crystalline thin films at low temperature and short reaction times would be highly beneficial.
A promising approach to prepare KBiS2 with lower energy demand is via the use of metal alkyl xanthates. Metal xanthates (also known as metal dithiocarbonates) are the metal salts of xanthogenic acid. They can act as single source precursors for the corresponding metal sulfides, meaning they are both metal and sulfur sources.13,29–33 Metal xanthates can be easily prepared for most metals either using commercially available potassium xanthates as precursors or by preparing them directly from metal hydroxides or alkoxides. Additionally, metal alkyl xanthates are soluble in various solvents, depending on the used alkyl moiety. This enables a versatile method to prepare defined metal sulfide thin films from these precursors.10,34–40
In this study, we present a method to prepare rhombohedral and cubic potassium bismuth sulfide (KBiS2) thin films from potassium and bismuth xanthates at significantly lower temperatures compared to the common solid-state approach (see Fig. S1). Using the ethyl xanthates of these metals offers good availability, as potassium ethyl xanthate is inexpensive and commercially available. The bismuth analogue can be synthesized via simple ligand exchange from potassium xanthate.37 We investigated the crystal formation at different temperatures by X-ray diffraction and took a closer look at samples with different precursor ratios with HR-STEM. We performed first principles calculations to understand the fundamental optoelectronic behavior and band alignments. Moreover, we studied the optical and photochemical properties with reflectance-, electron paramagnetic resonance and transient absorption spectroscopy to obtain a deeper understanding of charge carrier generation and lifetime in KBiS2 and heterojunctions with TiO2 as well as p-type organic semiconductor contacts.
Results and discussion
Phase formation and structural characterization
For the preparation of KBiS2 thin films, potassium ethyl xanthate (KXaEt) was recrystallized with acetone and methanol. In addition, bismuth ethyl xanthate (Bi(XaEt)3) was synthesized from KXaEt and bismuth nitrate as previously reported,37 and the synthesis is described in more detail in the Experimental section. To begin with, we investigated the thermal conversion of the potassium and bismuth xanthates as well as mixtures of both metal xanthate powders in K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi-molar ratios of 1
Bi-molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5
1, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results of the thermogravimetric analyses (Fig. 1a) provide valuable insights; the decomposition temperature and rate can distinctly influence the properties of the formed metal sulfide. While Bi(XaEt)3 shows a single sharp mass loss step at a relatively low temperature (5% mass loss) at 120 °C, KXaEt, in contrast, requires a notably higher temperature, exhibiting a sharp main mass loss with a decomposition onset at 210 °C, followed by a continuous mass loss. The main mass loss correlates with the decomposition of the organic side chains and the evaporation of the decomposition products. The subsequent smaller ongoing loss of mass can be explained by the formation of different potassium sulfide phases with decreasing sulfur content at higher temperatures, as potassium xanthate forms a mixture of several phases (the diffractogram of a KXaEt film annealed at 300 °C for 30 min is shown in the SI, Fig. S2). In contrast to potassium xanthate, the mixtures of KXaEt and Bi(XaEt)3 show no further mass loss after the characteristic 2-step decomposition.
1. The results of the thermogravimetric analyses (Fig. 1a) provide valuable insights; the decomposition temperature and rate can distinctly influence the properties of the formed metal sulfide. While Bi(XaEt)3 shows a single sharp mass loss step at a relatively low temperature (5% mass loss) at 120 °C, KXaEt, in contrast, requires a notably higher temperature, exhibiting a sharp main mass loss with a decomposition onset at 210 °C, followed by a continuous mass loss. The main mass loss correlates with the decomposition of the organic side chains and the evaporation of the decomposition products. The subsequent smaller ongoing loss of mass can be explained by the formation of different potassium sulfide phases with decreasing sulfur content at higher temperatures, as potassium xanthate forms a mixture of several phases (the diffractogram of a KXaEt film annealed at 300 °C for 30 min is shown in the SI, Fig. S2). In contrast to potassium xanthate, the mixtures of KXaEt and Bi(XaEt)3 show no further mass loss after the characteristic 2-step decomposition.
 | ||
Fig. 1 (a) TGA curves with heating rates of 10° min−1 of the individual KXaEt and Bi(XaEt)3 precursors, as well as K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi mixtures with molar ratios of 1 Bi mixtures with molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5 1, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2 1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (b) crystal structures of cubic and rhombohedral KBiS2 visualized with VESTA;41 and (c) X-ray diffractograms of different mixtures of KXaEt and Bi(XaEt)3 annealed at 300 °C for 30 min; the diffractograms are vertically shifted for better visibility with ICSD 28699 as the reference for cubic KBiS2 in red bars,25 ICSD 30775 as the reference for orthorhombic Bi2S3 in black bars42 and ICSD 143475 as the reference for rhombohedral KBiS224 in blue bars. 1; (b) crystal structures of cubic and rhombohedral KBiS2 visualized with VESTA;41 and (c) X-ray diffractograms of different mixtures of KXaEt and Bi(XaEt)3 annealed at 300 °C for 30 min; the diffractograms are vertically shifted for better visibility with ICSD 28699 as the reference for cubic KBiS2 in red bars,25 ICSD 30775 as the reference for orthorhombic Bi2S3 in black bars42 and ICSD 143475 as the reference for rhombohedral KBiS224 in blue bars. | ||
The theoretical and experimental mass losses are summarized in Table 1. The experimental mass losses of the mixtures are only slightly lower than the theoretical ones. This is most likely caused by small amounts of carbon-based residues of the alkyl chains, which remain in the solid during the thermogravimetric analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi ratios, as well as the decomposition onset (temperature at 5% mass loss)
Bi ratios, as well as the decomposition onset (temperature at 5% mass loss)
| Theoretical mass loss/% | Experimental mass loss/% | Decomposition onset temperature/°C | |
|---|---|---|---|
| a Expected mass loss between 45.6% (K2S3) and 65.6% (K2S).b The excess of KXaEt was considered with 49.6% mass loss from the experimental KXaEt measurements (300 °C). | |||
| KXaEt → KxSya | 45.6–65.6a | 45.7 (250 °C) | |
| 49.6 (300 °C) | 210.3 | ||
| 50.8 (350 °C) | |||
| Bi(XaEt)3 → Bi2S3 | 55.1 | 53.7 | 120.3 |
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 1 Bi = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
57.4 | 55.4 | 120.4 |
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 1.5 Bi = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b 1b |
55.8b | 54.6 | 120.6 |
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 2.0 Bi = 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b 1b |
54.8b | 53.7 | 121.7 |
To fabricate the thin films (see scheme Fig. S1), we first prepared the individual precursor solutions with concentrations of 0.136 mmol mL−1 in the non-halogenated solvents methanol and tetrahydrofuran (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF 1
THF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v
3, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). We subsequently mixed the precursor solutions in the volume ratios of the desired potassium to bismuth ratios.
v). We subsequently mixed the precursor solutions in the volume ratios of the desired potassium to bismuth ratios.
As previous studies used an excess of potassium for the synthesis of KBiS2,24,27 we combined different K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi ratios ranging from K
Bi ratios ranging from K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 3.5
Bi = 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (potassium-rich) to 1
1 (potassium-rich) to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75 (bismuth-rich). We dropcast the solutions onto glass substrates and annealed them at 300 °C for 30 min under an N2 atmosphere. The X-ray diffractograms of the samples with different ratios are depicted in Fig. 1c. In this experimental series, we found that a 1.5 times excess of the potassium precursor at an annealing temperature of 300 °C is optimal for the formation of cubic KBiS2, which can be seen clearly in the diffractogram of the 1.5
1.75 (bismuth-rich). We dropcast the solutions onto glass substrates and annealed them at 300 °C for 30 min under an N2 atmosphere. The X-ray diffractograms of the samples with different ratios are depicted in Fig. 1c. In this experimental series, we found that a 1.5 times excess of the potassium precursor at an annealing temperature of 300 °C is optimal for the formation of cubic KBiS2, which can be seen clearly in the diffractogram of the 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sample in Fig. 1c. The diffraction pattern shows reflections matching with the ICSD 28699 reference, with the main ones at 25.5 (111), 29.6 (200) and 42.3° 2θ (220). No additional signals from impurities or secondary phases are observable.
1 sample in Fig. 1c. The diffraction pattern shows reflections matching with the ICSD 28699 reference, with the main ones at 25.5 (111), 29.6 (200) and 42.3° 2θ (220). No additional signals from impurities or secondary phases are observable.
The conditions used in the synthesis of KBiS2 herein are remarkably less energy consuming than for the salt melt preparation found in the literature.24,27 Moreover, our route results in materials with primary crystallite sizes (estimated via the Scherrer equation based on the XRD peak broadening, see Table S1) of approx. 15 nm. This is comparable with a reported hot-injection route, which is performed at a similar temperature, and leads to nanoparticles with diameters of approx. 13 nm.26 At temperatures of 300 or 350 °C, the primary crystallite size of KBiS2, prepared with the herein described approach based on metal xanthates, leads to primary crystallite sizes of approx. 22 and 29 nm, respectively.
For a stoichiometric K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi ratio or an excess of Bi(XaEt)3, we exclusively observe the formation of orthorhombic Bi2S3 as a crystalline material (see Fig. 1c). For reference purposes, we prepared Bi2S3 films from only Bi(XaEt)3 (SI, Fig. S3 and S4). As we do not observe a distinct crystalline potassium sulfide phase in the KBS_1 sample (K
Bi ratio or an excess of Bi(XaEt)3, we exclusively observe the formation of orthorhombic Bi2S3 as a crystalline material (see Fig. 1c). For reference purposes, we prepared Bi2S3 films from only Bi(XaEt)3 (SI, Fig. S3 and S4). As we do not observe a distinct crystalline potassium sulfide phase in the KBS_1 sample (K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 1
Bi = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), we expect the potassium precursor to form an amorphous potassium sulfide species, which may reside at grain boundaries or surfaces, explaining their absence in the diffraction data. This hypothesis is further examined by transmission electron microscopy (vide infra). It is also interesting to note that upon increasing the KXaEt amount to an excess of 1.25, we can see the formation of the rhombohedral KBiS2 phase, which is corroborated by the peaks at 26.2 and 29.8° 2θ in the diffractogram, corresponding to the 012 and 104 reflections of rhombohedral KBiS2, respectively. In the samples with an increasing amount of potassium xanthate from KBS_1.25 (K
1), we expect the potassium precursor to form an amorphous potassium sulfide species, which may reside at grain boundaries or surfaces, explaining their absence in the diffraction data. This hypothesis is further examined by transmission electron microscopy (vide infra). It is also interesting to note that upon increasing the KXaEt amount to an excess of 1.25, we can see the formation of the rhombohedral KBiS2 phase, which is corroborated by the peaks at 26.2 and 29.8° 2θ in the diffractogram, corresponding to the 012 and 104 reflections of rhombohedral KBiS2, respectively. In the samples with an increasing amount of potassium xanthate from KBS_1.25 (K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 1.25
Bi = 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to KBS_1.5 (K
1) to KBS_1.5 (K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 1.5
Bi = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), we see a gradual change of the crystal structure, with only the cubic phase present in the KBS_1.5 sample (Fig. 1c).
1), we see a gradual change of the crystal structure, with only the cubic phase present in the KBS_1.5 sample (Fig. 1c).
A further increase of the potassium content (ratios from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3.5
1 to 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the precursor solution still led to the formation of cubic KBiS2 as the main phase. In addition, as we do not observe any other potassium bismuth sulfide phases like K3BiS3, we expect increasing amounts of potassium-based phases to be formed in the increasingly potassium-rich samples. This is indicated, e.g., by the emerging peaks at 17.1, 19.2, 25.2, 27.1 and 27.8° 2θ in the samples with a potassium excess of 2-fold and greater.
1) in the precursor solution still led to the formation of cubic KBiS2 as the main phase. In addition, as we do not observe any other potassium bismuth sulfide phases like K3BiS3, we expect increasing amounts of potassium-based phases to be formed in the increasingly potassium-rich samples. This is indicated, e.g., by the emerging peaks at 17.1, 19.2, 25.2, 27.1 and 27.8° 2θ in the samples with a potassium excess of 2-fold and greater.
In addition to the investigation of different K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi ratios (Fig. 1c), we varied the annealing temperature for three different K
Bi ratios (Fig. 1c), we varied the annealing temperature for three different K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi ratios to get a better insight into the phase formation. In Fig. 2a (K
Bi ratios to get a better insight into the phase formation. In Fig. 2a (K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 1
Bi = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; KBS_1), we can see mostly the orthorhombic Bi2S3 phase, which becomes more crystalline with increasing temperature. Small amounts of the KBiS2 phase are present up to 300 °C.
1; KBS_1), we can see mostly the orthorhombic Bi2S3 phase, which becomes more crystalline with increasing temperature. Small amounts of the KBiS2 phase are present up to 300 °C.
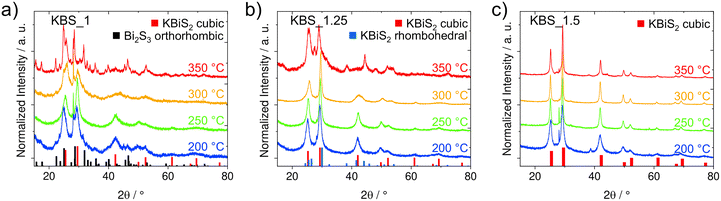 | ||
Fig. 2 X-ray diffractograms of samples prepared from the following K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi mixtures at different temperatures between 200 and 350 °C: (a) 1 Bi mixtures at different temperatures between 200 and 350 °C: (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (KBS_1), (b) 1.25 1 (KBS_1), (b) 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (KBS_1.25) and (c) 1.5 1 (KBS_1.25) and (c) 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (KBS_1.5). The diffractograms are vertically shifted for better visibility with ICSD 28699 as the reference for cubic KBiS2 in red bars,25 ICSD 30775 as the reference for orthorhombic Bi2S3 in black bars42 and ICSD 143475 as the reference for rhombohedral KBiS224 in blue bars. 1 (KBS_1.5). The diffractograms are vertically shifted for better visibility with ICSD 28699 as the reference for cubic KBiS2 in red bars,25 ICSD 30775 as the reference for orthorhombic Bi2S3 in black bars42 and ICSD 143475 as the reference for rhombohedral KBiS224 in blue bars. | ||
Although KBS_1.25 forms rhombohedral KBiS2 at 300 °C, at lower temperatures (200 and 250 °C) the cubic phase seems to be preferred (Fig. 2b). The preferential formation of the cubic phase at lower temperatures indicates that this modification is kinetically favored under our synthesis conditions. While the underlying mechanism is not yet fully understood, it may be related to differences in nucleation and growth kinetics between the cubic and rhombohedral phases. While in the TGA (performed with a heating rate of 10° min−1), the decomposition of potassium xanthate has barely started at 200 °C (cf. Fig. 1a), half an hour holding at 200 °C is sufficient to compensate for the lower temperature and obtain full conversion of the xanthates to the metal sulfides as depicted in Fig. S5. At 350 °C, the orthorhombic Bi2S3 is again the most prominent phase also in the KBS_1.25 sample series. In comparison, the 1.5-fold potassium excess in KBS_1.5 results in cubic KBiS2 at all four temperatures (Fig. 2c). With increasing temperature, the crystallinity increases as well and primary crystallite sizes from 10 ± 2 (200 °C) up to 29 ± 7 nm (350 °C) are estimated using the Scherrer equation (see also Table S1). At 200 and 250 °C, we observe a small additional sharp peak at 28.1° 2θ, which hints at a Bi2S3 secondary phase. Upon increasing the temperature to 300 °C, we only see signals corresponding to cubic KBiS2. However, above 300 °C, a secondary unidentified phase emerges with minor peaks at 27.4°, 38.3° and 44.5° 2θ. These peaks match well with the phase, which we also observe in samples with a higher potassium excess (e.g., K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 2
Bi = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; Fig. 1c), where starting at a 2-fold potassium excess, additional small peaks emerge (vide supra).
1; Fig. 1c), where starting at a 2-fold potassium excess, additional small peaks emerge (vide supra).
Next, we performed Raman spectroscopy measurements on the samples with different K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi ratios (Fig. S6, SI). The spectrum of KBS_1 matches spectra found in the literature for Bi2S3,43,44 with a broad amorphous background, which we assign to the remaining potassium sulfide. The reported bands below 100 cm−1 could not be resolved in this measurement, due to the cut-off filter at 70 cm−1. Due to the strong amorphous background, the bands between 200 and 300 cm−1 could not be resolved individually. With increasing potassium content, these bands decrease in intensity and are not present in the KBS_1.5 sample. At the same time, the broad peak around 190 cm−1 and the shoulder between 200 and 350 cm−1 shift to higher wavenumbers, indicating an exchange of the heavy bismuth with lighter potassium atoms, matching well with the formation of the potassium bismuth mixed phase.
Bi ratios (Fig. S6, SI). The spectrum of KBS_1 matches spectra found in the literature for Bi2S3,43,44 with a broad amorphous background, which we assign to the remaining potassium sulfide. The reported bands below 100 cm−1 could not be resolved in this measurement, due to the cut-off filter at 70 cm−1. Due to the strong amorphous background, the bands between 200 and 300 cm−1 could not be resolved individually. With increasing potassium content, these bands decrease in intensity and are not present in the KBS_1.5 sample. At the same time, the broad peak around 190 cm−1 and the shoulder between 200 and 350 cm−1 shift to higher wavenumbers, indicating an exchange of the heavy bismuth with lighter potassium atoms, matching well with the formation of the potassium bismuth mixed phase.
This change from the individual binary phases, present in the KBS_1 sample, to the ternary KBiS2 phases, present in the KBS_1.25 and KBS_1.5 samples, is additionally confirmed by the STEM-HAADF images, where we clearly observe the formation of large crystalline bismuth sulfide needles and amorphous potassium sulfide regions for KBS_1 (Fig. 3). HR-STEM images combined with multislice simulations confirm the orthorhombic Bi2S3 crystal phase of the needles that grow up to hundreds of nm in length (Fig. 4a and Fig. S7). With a higher potassium content, potassium and bismuth mixed regions with still amorphous potassium sulfide regions in between are revealed in KBS_1.25 (Fig. 3b). In the KBS_1.5 sample, we see that K and Bi are intermixed well in the elemental mapping (Fig. 3c). Moreover, we can still observe needle-like structures in KBS_1.25 and KBS_1.5; however, with the increased potassium content, the growth of the needles is inhibited as seen in the STEM images and elemental maps (Fig. 3 and Fig. S8).
 | ||
| Fig. 3 STEM-HAADF micrographs (left) and corresponding EDX elemental maps (right) of (a) KBS_1, (b) KBS_1.25 and (c) KBS_1.5, with K in green, Bi in blue and S in red. | ||
 | ||
| Fig. 4 HR-STEM-HAADF images of (a) KBS_1 and (b) KBS_1.5; (c) Bi2S3 oriented in the 110 plane direction with a multislice simulation and a ball-stick visualization of the reference structure in VESTA;41 (d) cubic KBiS225 oriented in the [111] direction and (e) in the [121] direction with corresponding multislice simulations45 and ball-stick visualizations. | ||
High-resolution STEM-HAADF images (Fig. 4b) further reveal the nanocrystalline morphology of the cubic KBiS2 phase in the KBS_1.5 sample. Several KBiS2 crystallites with a size of <10 nm are visible in the presented image, two of which are found to be oriented in the [111] (green box) and [121] (orange box) crystallographic orientation. A comparison with multislice simulations confirms a match to the cubic KBiS2 phase. The HR-STEM images in Fig. 4d and e additionally show the random alternation of the potassium and bismuth atoms at the metal sites in the cubic crystal lattice, appearing in different brightness due to the large difference in atomic number of the two elements. These contrast variations are closely mirrored in the corresponding multislice simulations of cubic KBiS2, assuming random occupation of K and Bi in the underlying model structure.
Optical properties and first principles simulations
We investigated the optical properties of the KBiS2 films using diffuse reflectance and transmission measurements. We note that due to the macro-porosity of all films as revealed by the SEM investigations (Fig. S9), the determined absorption coefficients of these samples (>2.104 cm−1 below approx. 600 nm; Fig. S10a) are lower than they would be for a dense film. To characterize the optical bandgaps of the materials, we used the Kubelka–Munk46 method and corresponding Tauc plots47 with the support of density functional theory simulations. The absorption spectra, Kubelka–Munk and Tauc plots are depicted in Fig. S10b and c and the corresponding reflectance spectra are shown in Fig. S11.In all three samples, we found indirect and direct transitions, which have been reported in the literature for metal sulfides before.48 The direct contributions are much more pronounced and at slightly higher energies than the indirect ones. Interestingly, in the KBS_1 sample, the indirect contribution is much stronger than the one in the pure Bi2S3 film and of marginally lower energy with 1.04 eV in the mixture compared to 1.10 eV in the pure Bi2S3 film (Fig. S12). This might be associated with the incorporation of K+ on Bi3+ sites, which can induce lattice expansion and local strain and could thereby influence the band structure, leading to a red shift in optical absorption. A similar band gap energy reduction was reported by Silva et al., who investigated the substantial influence of iron doping on the indirect bandgap of Bi2S3.49 The optical bandgaps of KBiS2 correspond well to previously reported values. For example, the direct transition of 1.80 eV for the cubic thin film is only slightly higher than the value reported in the literature (1.61 eV) and the indirect bandgap of 1.29 eV of the rhombohedral KBiS2 thin film is well comparable to the value of 1.21 eV from the literature. The experimental optical bandgaps are summarized in Table S2 with corresponding values from the literature. Due to the nanocrystalline nature of the metal sulfides resulting from xanthate precursors, the bandgaps are slightly higher.14,50 The slightly increased bandgap observed for KBiS2 may arise not only from quantum confinement effects due to the nanocrystalline domain size, but also from the presence of point defects. In particular, sulfur vacancies or antisite defects can locally modify the electronic structure and shift the absorption onset. The formation of such defects is plausible under the mild decomposition conditions of the metal xanthate precursors, where a marginally sulfur-deficient environment may occur. Consequently, both nanocrystallinity and defect-related electronic states are likely to contribute to the optical properties of the material.
Additionally, we performed density functional theory simulations using the hybrid HSE06 functional with the addition of spin–orbit coupling. The full computational methodology is provided in the Experimental section. The band structures of the rhombohedral and cubic structures are displayed in Fig. 5a and b, respectively. We find that the rhombohedral structure exhibits an indirect band gap of 1.58 eV. While our calculated band gap is ∼0.3 eV larger than the indirect gap measured in experiments, this could be due to the lack of thermal effects in our calculations or the presence of defect states and disorder in thin film samples. The direct gap is ∼0.5 eV larger than the indirect gap at an energy of 2.04 eV. This relatively strong indirect character is expected to result in reduced light absorption and weaker photovoltaic performance.
 | ||
| Fig. 5 Optoelectronic properties of KBiS2 calculated using the HSE06 + SOC functional. Electronic band structure of KBiS2 in the (a) rhombohedral and (b) cubic structures. (c) Calculated optical absorption and (d) band alignment against electron and hole contact materials. The ionization potential and electron affinity for TiO2 and spiro-OMeTAD were taken from the study of Jena et al.52 | ||
In contrast, the cubic structure displays a fundamental direct gap of 1.84 eV, in excellent agreement with the experimental value of 1.80 eV. For the cubic structure, we explicitly model a supercell with configurational disorder on the K and Bi sites and have therefore performed band unfolding to map the band structure back onto the primitive cell symmetry. We note that spin–orbit coupling plays a large role in governing the electronic properties and results in band gap renormalization of 0.23 and 0.56 eV for the cubic and rhombohedral structures, respectively.
The calculated effective masses are provided in Table 2. In the rhombohedral structure, electron and hole transport are expected to be highly anisotropic, with the effective masses in the a/b directions over 4 times smaller than that in the c direction (across the K/Bi layers). Electrons have significantly smaller effective masses of 0.21 m0, which is comparable to those of other emerging chalcogenide absorbers such as Sb2Se3 (0.35 m051). The composition disorder in cubic-structured KBiS2 provides fully isotropic transport but comes at the cost of secondary flat bands at the conduction and valence band edges. This results in both light and heavy electrons and holes contributing to transport. Similarly to the rhombohedral structure, the electron mobility is expected to be greater than the hole mobility due to the much smaller light effective masses (0.22 vs. 0.91 m0). The calculated optical absorption displayed in Fig. 5c highlights the strong onset of absorption for both compounds; however, we note that these absorption spectrum simulations do not account for indirect optical transitions (due to the absence of temperature, 0 K) and therefore only reflect the direct band gap transitions in the rhombohedral structure. Finally, we obtained the predicted bulk band alignment through ab initio slab-vacuum models. The ionization potentials (IP) of both compounds were found to be 5.5–5.6 eV, while the electron affinities (EA) are 3.7–3.9 eV (Fig. 5d). Given how close these are to the band alignments of MAPbI3 (IP = 5.5, EA = 3.9), it is expected that KBiS2 devices can make use of the same electron and hole contact materials used in perovskite photovoltaics.
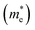 and hole
and hole 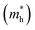 effective masses, ionization potentials (IP), and electron affinities (EA) of cubic- and rhombohedral-structured KBS obtained using hybrid density functional theory
effective masses, ionization potentials (IP), and electron affinities (EA) of cubic- and rhombohedral-structured KBS obtained using hybrid density functional theory
Photoinduced oxygen radical formation and charge photogeneration in KBiS2 films
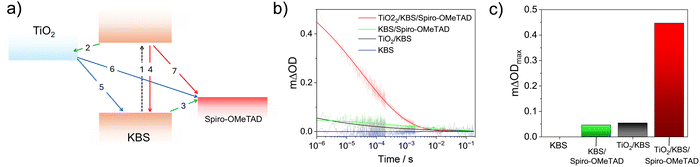
For a qualitative assessment of the oxygen radical formation upon illumination, which is an important measure for photocatalytic activity, we performed EPR measurements using DMPO as a spin trap. The samples differ only in signal-to-noise ratio, but all show EPR spectra corresponding to a DMPO–OH˙ adduct (Fig. S13, aN = 14.3 G, aH = 14.3 G).53 The slight asymmetry observed in the EPR signals and minor alteration of the line intensities suggest the presence of a second radical species, most likely DMPO–OOH˙.54,55 This indicates that KBS_1, KBS_1.25 and KBS_1.5 generate oxygen centered free radicals upon light irradiation. Based on the literature, it is plausible that the initially formed DMPO–OOH˙ adduct undergoes conversion to DMPO–OH˙ within the time frame of our experimental setup (Scheme S1).56–59 The absence of any signal without the presence of oxygen further suggests oxygen as a critical component for the formation of radicals.We next considered light-induced charge generation, separation and recombination reactions. Fig. 6a presents the charge transfer processes occurring in TiO2/KBS/spiro-OMeTAD films (KBS = KBiS2) in which the KBS film (cubic phase, KBS_1.5) forms a heterojunction with the n-type TiO2 and with the p-type spiro-OMeTAD. SEM images of such films are shown in Fig. S14 and the absorbance spectra are shown in Fig. S15. Light absorption by KBS is followed by electron transfer from KBS to TiO2 and hole transfer from KBS to the spiro-OMeTAD hole transporting layer. High-yield and long-lived charge separation across the TiO2/KBS/spiro-OMeTAD double heterojunction is a prerequisite for the successful exploitation of such layers in solar energy conversion devices (e.g., photovoltaics or photocatalysis). Herein, we used transient absorption spectroscopy60 to directly probe the hole transfer from KBS to spiro-OMeTAD (process 3, Fig. 6a) and the charge recombination kinetics (process 6, Fig. 6a). Full details of the used transient absorption spectrometer are provided in the Experimental section. We note that for the transient optical studies, we used sample KBS_1.5 owing to the higher crystallinity and less sensitive preparation of the cubic KBiS2.
We determined the kinetics of charge recombination between the photogenerated electrons in TiO2 and holes in spiro-OMeTAD (process 6, Fig. 6a) by monitoring the decay of the [spiro-OMeTAD]+ polarons at 1600 nm following pulsed excitation at 510 nm. Typical decay dynamics of different heterojunctions are presented in Fig. 6b. We note that no transient absorption signal is observed in the pristine KBS sample, indicating that electron–hole recombination dynamics in KBS occurs on timescales faster than the instrument response of our transient spectrometer (IRF = 100 ns).
With respect to the Beer–Lambert law, the change in optical density (ΔOD) is related to the concentration of photogenerated [spiro-OMeTAD]+ and is therefore a measure of the yield of hole transfer. Fig. 6c presents mΔODmax (defined as ΔOD at 1 µs) values for the samples investigated herein. Taken together, these data indicate the importance of the TiO2 electron acceptor in achieving a high yield of hole transfer. We fitted the transient absorption kinetics in Fig. 6b with a stretched exponential function:  (details are provided in the Experimental section and Table S3). Given the multiexponential nature of the kinetic traces, we define a charge recombination lifetime, τCR, as the time taken for ΔOD to reach 50% of its initial value. In this way, we estimate τCR = 41.1 µs in TiO2/KBS/spiro-OMeTAD films reported herein. It is pertinent to note that the mΔODmax and τCR values observed in our TiO2/KBS/spiro-OMeTAD films are similar in magnitude to those reported in high-performing materials such as lead halide perovskite-based films.61–63 This highlights the potential of KBS as a light harvesting material for future applications in solar energy conversion.
(details are provided in the Experimental section and Table S3). Given the multiexponential nature of the kinetic traces, we define a charge recombination lifetime, τCR, as the time taken for ΔOD to reach 50% of its initial value. In this way, we estimate τCR = 41.1 µs in TiO2/KBS/spiro-OMeTAD films reported herein. It is pertinent to note that the mΔODmax and τCR values observed in our TiO2/KBS/spiro-OMeTAD films are similar in magnitude to those reported in high-performing materials such as lead halide perovskite-based films.61–63 This highlights the potential of KBS as a light harvesting material for future applications in solar energy conversion.
Conclusion
In this study, we prepared potassium bismuth sulfide films with rhombohedral and cubic crystal phases and investigated their structural, optical and charge-generation properties. We could access both phases by adjusting the ratio of the xanthate precursors in a non-halogenated solvent mixture. Moreover, we investigated the formation of the phases and the influence of the precursor ratio, where an X-ray diffraction study revealed that cubic KBiS2 already forms at 200 °C from a metal xanthate precursor film containing a 1.5-fold potassium excess. In cubic KBiS2, K and Bi ions randomly occupy the metal sites of the cubic rocksalt structure, which we could directly observe in high resolution STEM images. We additionally discovered that with a smaller excess of potassium (K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi = 1.25
Bi = 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at an annealing temperature of 300 °C, the rhombohedral KBiS2 phase can be obtained.
1) at an annealing temperature of 300 °C, the rhombohedral KBiS2 phase can be obtained.
First principle simulations revealed a fundamental direct transition of 1.84 eV for cubic KBiS2, matching well with the experimentally determined value. Furthermore, we investigated the charge generation and lifetime properties under illumination. EPR spectroscopy confirmed oxygen centered radicals in all investigated films and with additional transient absorption spectroscopy measurements, we could disclose an excellent charge carrier lifetime of 41.1 µs for cubic KBiS2, when combined with suitable electron and hole transport layers, like TiO2 and spiro-OMeTAD. Such a charge carrier lifetime is comparable to that of the high performing lead halide perovskites and shows the great potential of this material for solar applications.
Therefore, we will exploit this low temperature KBiS2 thin film preparation method in future detailed investigations of the material as a solar absorber in photovoltaic devices and photocatalysis. In particular, we are convinced that in addition to the well-suited optical properties, the highly porous nature and the specific nanostructure of the films, as revealed by electron microscopic investigations, will be highly beneficial for the photocatalytic performance, allowing for a large available surface area and a facile diffusion of larger organic molecules towards and away from active sites.
Experimental
1H NMR (300 MHz, CDCl3): δ = 4.73 (q, 2H, OCH2), 1.52 (t, 3H, CH3) ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF solvent mixture (1
THF solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v
3 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) were prepared and mixed in the desired precursor ratios. For the thin films, about 50 µL cm−2 was pipetted on the substrate and spin coated (1500 rpm, 1500 rpm/s, 30 s) in air, resulting in films of approx. 80 nm thickness. For the thicker films (for X-ray diffraction), the same solutions were drop-coated, yielding film thicknesses of 6–10 µm. All samples were thermally annealed in the glovebox under an N2 atmosphere.
v) were prepared and mixed in the desired precursor ratios. For the thin films, about 50 µL cm−2 was pipetted on the substrate and spin coated (1500 rpm, 1500 rpm/s, 30 s) in air, resulting in films of approx. 80 nm thickness. For the thicker films (for X-ray diffraction), the same solutions were drop-coated, yielding film thicknesses of 6–10 µm. All samples were thermally annealed in the glovebox under an N2 atmosphere.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 weight ratio) and stirred overnight. The glass substrates were cleaned first with water and then with 2-propanol in an ultrasonic bath. The cleaned substrates were treated with oxygen plasma (Femto plasma etcher from Diener Electronic) for 3 min. The substrates were covered with the titania/terpineol mixture and spin coated (WS-650MZ-23NPPB) at 3000 rpm (ramp: 1000 rpm/s) for 50 s. The layers were dried at 80 °C for 15 min and annealed at 500 °C for 1 h to obtain mesoporous mp-TiO2 films with a thickness of 1 µm. For the preparation of KBiS2 films on mp-TiO2, the above-described method was used.
1.5 weight ratio) and stirred overnight. The glass substrates were cleaned first with water and then with 2-propanol in an ultrasonic bath. The cleaned substrates were treated with oxygen plasma (Femto plasma etcher from Diener Electronic) for 3 min. The substrates were covered with the titania/terpineol mixture and spin coated (WS-650MZ-23NPPB) at 3000 rpm (ramp: 1000 rpm/s) for 50 s. The layers were dried at 80 °C for 15 min and annealed at 500 °C for 1 h to obtain mesoporous mp-TiO2 films with a thickness of 1 µm. For the preparation of KBiS2 films on mp-TiO2, the above-described method was used.Characterization methods
The STEM-HAADF images were simulated with the Dr Probe Multislice simulation package (developed by Juri Barthel at Forschungszentrum Jülich/Germany).45 The full-width at half maximum of the probe was set to 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm. Crystallographic visualizations were rendered in VESTA (v. 3.5.8.).41 EDX maps were acquired and analyzed in Velox by Thermo Fisher Scientific.
nm. Crystallographic visualizations were rendered in VESTA (v. 3.5.8.).41 EDX maps were acquired and analyzed in Velox by Thermo Fisher Scientific.
Due to the strongly Z dependent scattering cross-section (∼Z2, with Z being the atomic number), the HAADF contrast is dominated by the Bi atoms in the crystal, while the other constituents (K and S) remain almost invisible. For the simulations presented in Fig. 5, a 6 nm-thick crystal was generated. For KBiS2, the Bi and K sites were randomly occupied.25 The resulting contrast variations between individual atomic columns closely resemble those observed in the experimental data.
Author contributions
M. S: conceptualization, data curation, formal analysis, synthesis and investigation, characterization methodology, project administration, visualization, writing – original draft, and writing – review & editing; M. E.: conceptualization, data curation, investigation, and writing – review & editing; D. K.: investigation, data curation, and review & editing; H. F.: conceptualization, investigation, data curation, and review & editing; D. M.: investigation, data curation, and writing – review & editing; I. H.: investigation, data curation, and writing – review & editing; T. W.: investigation, data curation, and writing – review & editing; F. W.: investigation, data curation, and writing – review & editing; J. H.: investigation, data curation, and writing – review & editing; R. W.: investigation, data curation, and writing – review & editing; A. M. G.: conceptualization, investigation, data curation, and writing – review & editing; G. G.: investigation, data curation, and writing – review & editing; G. K.: investigation, data curation, and writing – review & editing; G. T.: resources, supervision, funding acquisition, and writing – review & editing; S. A. H.: conceptualization, data curation, supervision, and review & editing; and T. R.: conceptualization, formal analysis, funding acquisition, project administration, supervision, writing – original draft, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
All evaluated data are available in the manuscript. The data supporting this article are included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5tc02554k.Additional information/data can be obtained upon reasonable request.
Acknowledgements
The authors gratefully acknowledge Graz University of Technology for financing this work through the Lead Project Porous Materials@Work for Sustainability (LP-03). S. A. H. gratefully acknowledges funding from the Engineering and Physical Sciences Research Council (EPSRC, EP/X012344/1). This work used the ARCHER2 UK National Supercomputing Service (https://www.archer2.ac.uk) via our membership of the UK's HEC Materials Chemistry Consortium, which is funded by EPSRC (EP/L000202).References
- H. Park, S. Kumar R, A. Akande, S. Sanvito and F. El-Mellouhi, Int. J. Hydrogen Energy, 2022, 47, 3358–3370 CrossRef CAS.
- Y.-Y. Sun, M. L. Agiorgousis, P. Zhang and S. Zhang, Nano Lett., 2015, 15, 581–585 CrossRef CAS PubMed.
- R. Zhou, X. Liu, S. Zhang, L. Liu, L. Wan, H. Guo, X. Yang, Z. Cheng, L. Hu, H. Niu and X. Mao, Mater. Sci. Semicond. Process., 2021, 124, 105613 CrossRef CAS.
- B. V. Gabrel'yan, A. A. Lavrentiev, I. Y. Nikiforov and V. V. Sobolev, J. Struct. Chem., 2008, 49, 788–794 CrossRef.
- L. Yu, J. Li, G. Wang, B. Peng, R. Liu, L. Shi and G. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 61116–61128 CrossRef CAS.
- Y. Bai, Z. H. J. Khoo, R. I. Made, H. Xie, C. Y. J. Lim, A. D. Handoko, V. Chellappan, J. J. Cheng, F. Wei, Y.-F. Lim and K. Hippalgaonkar, Adv. Mater., 2024, 36, e2304269 CrossRef.
- A. Sarilmaz, E. Genc, E. Aslan, A. Ozen, G. Yanalak, F. Ozel and I. H. Patir, J. Photochem. Photobiol., A, 2020, 400, 112706 CrossRef CAS.
- W. Wang, Q. Sheng, G. Zhi, Y. Zhao, R. Qu, L. Sun and S. Zhang, Appl. Surf. Sci., 2023, 639, 158251 CrossRef CAS.
- S. Kumar R, A. Akande, F. El-Mellouhi, H. Park and S. Sanvito, Phys. Rev. Mater., 2020, 4, 75401 CrossRef.
- T. Rath, J. M. Marin-Beloqui, X. Bai, A.-C. Knall, M. Sigl, F. G. Warchomicka, T. Griesser, H. Amenitsch and S. A. Haque, ACS Appl. Mater. Interfaces, 2023, 15, 41624–41633 CrossRef CAS.
- J. Lee, H. Kim, T. Lee, W. Jang, K. H. Lee and A. Soon, Chem. Mater., 2019, 31, 9148–9155 CrossRef CAS.
- T. Zhang, T. Wang, F. Meng, M. Yang and S. Kawi, J. Mater. Chem. C, 2022, 10, 5400–5424 RSC.
- C. Buchmaier, T. Rath, F. Pirolt, A.-C. Knall, P. Kaschnitz, O. Glatter, K. Wewerka, F. Hofer, B. Kunert, K. Krenn and G. Trimmel, RSC Adv., 2016, 6, 106120–106129 RSC.
- I. Zumeta-Dubé, J.-L. Ortiz-Quiñonez, D. Díaz, C. Trallero-Giner and V.-F. Ruiz-Ruiz, J. Phys. Chem. C, 2014, 118, 30244–30252 CrossRef.
- T. Fazal, B. Ismail, M. Shah, S. Iqbal, E. B. Elkaeed, N. S. Awwad and H. A. Ibrahium, Sustainability, 2022, 14, 4603 CrossRef CAS.
- S. Akhil and R. G. Balakrishna, J. Mater. Chem. A, 2022, 10, 8615–8625 RSC.
- Y. Wang, S. R. Kavanagh, I. Burgués-Ceballos, A. Walsh, D. O. Scanlon and G. Konstantatos, Nat. Photon., 2022, 16, 235–241 CrossRef CAS.
- T. Fazal, S. Iqbal, M. Shah, A. Bahadur, B. Ismail, H. S. M. Abd-Rabboh, R. Hameed, Q. Mahmood, A. Ibrar, M. S. Nasar, Y. Ehsan, A. N. Shah Saqib, Adnan and M. A. Qayyum, J. Mater. Sci.: Mater. Electron., 2022, 33, 42–53 CrossRef CAS.
- N. Suriyawong, B. Aragaw, J.-B. Shi and M.-W. Lee, J. Colloid Interface Sci., 2016, 473, 60–65 CrossRef CAS PubMed.
- S. Moon, J. Park, H. Lee, J. W. Yang, J. Yun, Y. S. Park, J. Lee, H. Im, H. W. Jang, W. Yang and J. Moon, Adv. Sci., 2023, 10, 2206286 CrossRef CAS.
- C. Chen, M. Yasugi, L. Yu, Z. Teng and T. Ohno, Appl. Catal., B, 2022, 307, 121152 CrossRef CAS.
- C. Chen, Z. Teng, M. Yasugi, H. Yang, Y. Cao, L. Yu and T. Ohno, Mater. Lett., 2022, 325, 132801 CrossRef CAS.
- T. J. McCarthy, T. A. Tanzer and M. G. Kanatzidis, J. Am. Chem. Soc., 1995, 117, 1294–1301 CrossRef CAS.
- R. McClain, C. D. Malliakas, J. Shen, J. He, C. Wolverton, G. B. González and M. G. Kanatzidis, Chem. Sci., 2021, 12, 1378–1391 RSC.
- O. Glemser and M. Filcek, Z. Anorg. Allg. Chem., 1955, 279, 321–323 CrossRef CAS.
- C. Yang, Z. Wang, Y. Wu, Y. Lv, B. Zhou and W.-H. Zhang, ACS Appl. Energy Mater., 2019, 2, 182–186 CrossRef CAS.
- J. W. Boon, Recl. Trav. Chim. Pays-Bas, 1944, 63, 32–34 CrossRef CAS.
- K. Qu, H. Bale, Z. W. Riedel, J. Park, L. Yin, A. Schleife and D. P. Shoemaker, Cryst. Growth Des., 2022, 22, 3228–3234 CrossRef CAS.
- E. Vakalopoulou, D. Knez, M. Sigl, G. Kothleitner, G. Trimmel and T. Rath, ChemNanoMat, 2023, 9, e202200414 CrossRef CAS.
- P. D. McNaughter, S. A. Saah, M. Akhtar, K. Abdulwahab, M. A. Malik, J. Raftery, J. A. M. Awudza and P. O'Brien, Dalton Trans., 2016, 45, 16345 RSC.
- M. Al-Shakban, P. D. Matthews and P. O'Brien, Chem. Commun., 2017, 53, 10058–10061 RSC.
- M. D. Khan, M. A. Malik, J. Akhtar, S. Mlowe and N. Revaprasadu, Thin Solid Films, 2017, 638, 338–344 CrossRef CAS.
- P. S. Nair, T. Radhakrishnan, N. Revaprasadu, G. Kolawole and P. O'Brien, J. Mater. Chem., 2002, 12, 2722–2725 RSC.
- T. Rath, M. Edler, W. Haas, A. Fischereder, S. Moscher, A. Schenk, R. Trattnig, M. Sezen, G. Mauthner, A. Pein, D. Meischler, K. Bartl, R. Saf, N. Bansal, S. A. Haque, F. Hofer, E. J. W. List and G. Trimmel, Adv. Energy Mater., 2011, 1, 1046–1050 CrossRef CAS.
- E. Vakalopoulou, T. Rath, M. Kräuter, A. Torvisco, R. C. Fischer, B. Kunert, R. Resel, H. Schröttner, A. M. Coclite, H. Amenitsch and G. Trimmel, ACS Appl. Nano Mater., 2022, 5, 1508–1520 CrossRef CAS.
- E. Vakalopoulou, T. Rath, F. G. Warchomicka, F. Carraro, P. Falcaro, H. Amenitsch and G. Trimmel, Mater. Adv., 2022, 3, 2884–2895 RSC.
- A. J. MacLachlan, F. T. F. O'Mahony, A. L. Sudlow, M. S. Hill, K. C. Molloy, J. Nelson and S. A. Haque, Chem. Phys. Chem., 2014, 15, 1019–1023 CrossRef CAS PubMed.
- F. T. F. O'Mahony, U. B. Cappel, N. Tokmoldin, T. Lutz, R. Lindblad, H. Rensmo and S. A. Haque, Angew. Chem. Int. Ed., 2013, 52, 12047–12051 CrossRef PubMed.
- S. Dowland, T. Lutz, A. Ward, S. P. King, A. Sudlow, M. S. Hill, K. C. Molloy and S. A. Haque, Adv. Mater., 2011, 23, 2739–2744 CrossRef CAS PubMed.
- T. Lutz, A. MacLachlan, A. Sudlow, J. Nelson, M. S. Hill, K. C. Molloy and S. A. Haque, Phys. Chem. Chem. Phys., 2012, 14, 16192–16196 RSC.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- V. Kupčík and L. Veselá-Nováková, TMPM, Tschermaks Mineral. Petrogr. Mitt., 1970, 14, 55–59 CrossRef.
- V. Darji, P. Desai, M. P. Deshpande, S. Chaki, V. Sathe, B. S. Bhatt and R. A. Dabhi, Mater. Chem. Phys., 2023, 295, 127049 CrossRef CAS.
- Y. Zhao, K. T. E. Chua, C. K. Gan, J. Zhang, B. Peng, Z. Peng and Q. Xiong, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 205330 CrossRef.
- J. Barthel, Ultramicroscopy, 2018, 193, 1–11 CrossRef CAS PubMed.
- P. Kubelka and F. Munk, Z. Tech. Phys., 1931, 12, 593–601 Search PubMed.
- P. Makuła, M. Pacia and W. Macyk, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef.
- T. Wada and T. Maeda, Phys. Status Solidi C, 2017, 14, 1600196 CrossRef.
- R. S. Silva, H. D. Mikhail, E. V. Guimarães, E. R. Gonçalves, N. F. Cano and N. O. Dantas, Molecules, 2017, 22, 1142 CrossRef PubMed.
- V. Kaltenhauser, T. Rath, W. Haas, A. Torvisco, S. K. Müller, B. Friedel, B. Kunert, R. Saf, F. Hofer and G. Trimmel, J. Mater. Chem. C, 2013, 1, 7825 RSC.
- X. Wang, A. M. Ganose, S. R. Kavanagh and A. Walsh, ACS Energy Lett., 2022, 7, 2954–2960 CrossRef CAS.
- A. K. Jena, A. Kulkarni and T. Miyasaka, Chem. Rev., 2019, 119, 3036–3103 CrossRef CAS PubMed.
- K. Makino, H. Imaishi, S. Morinishi, T. Takeuchi and Y. Fujiza, Biochem. Biophys. Res. Commun., 1986, 141, 381–386 CrossRef CAS PubMed.
- E. Finkelstein, G. M. Rosen and E. J. Rauckman, Arch. Biochem. Biophys., 1980, 200, 1–16 CrossRef CAS PubMed.
- E. Finkelstein, G. M. Rosen and E. J. Rauckman, J. Am. Chem. Soc., 1980, 102, 4994–4999 CrossRef CAS.
- F. Parrino, S. Livraghi, E. Giamello, R. Ceccato and L. Palmisano, ACS Catal., 2020, 10, 7922–7931 CrossRef CAS.
- D. T. Sawyer, M. J. Gibian, M. M. Morrison and E. T. Seo, J. Am. Chem. Soc., 1978, 100, 627–628 CrossRef CAS.
- T. Shoji, L. Li, Y. Abe, M. Ogata, Y. Ishimoto, R. Gonda, T. Mashino, M. Mochizuki, M. Uemoto and N. Miyata, Anal. Sci., 2007, 23, 219–221 CrossRef PubMed.
- S. Fuloria, V. Subramaniyan, S. Karupiah, U. Kumari, K. Sathasivam, D. U. Meenakshi, Y. S. Wu, M. Sekar, N. Chitranshi, R. Malviya, K. Sudhakar, S. Bajaj and N. K. Fuloria, Antioxidants, 2021, 10, 128 CrossRef CAS PubMed.
- R. J. E. Westbrook, T. J. Macdonald, W. Xu, L. Lanzetta, J. M. Marin-Beloqui, T. M. Clarke and S. A. Haque, J. Am. Chem. Soc., 2021, 143, 12230–12243 CrossRef CAS PubMed.
- N. Aristidou, C. Eames, M. S. Islam and S. A. Haque, J. Mater. Chem. A, 2017, 5, 25469–25475 RSC.
- T. Webb, X. Liu, R. J. Westbrook, S. Kern, M. T. Sajjad, S. Jenatsch, K. D. G. I. Jayawardena, W. H. K. Perera, I. P. Marko, S. Sathasivam, B. Li, M. Yavari, D. J. Scurr, M. R. Alexander, T. J. Macdonald, S. A. Haque, S. J. Sweeney and W. Zhang, Adv. Energy Mater., 2022, 12, 2200666 CrossRef CAS.
- J. Guo, B. Wang, D. Lu, T. Wang, T. Liu, R. Wang, X. Dong, T. Zhou, N. Zheng, Q. Fu, Z. Xie, X. Wan, G. Xing, Y. Chen and Y. Liu, Adv. Mater., 2023, 35, e2212126 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- A. V. Krukau, O. A. Vydrov, A. F. Izmaylov and G. E. Scuseria, J. Chem. Phys., 2006, 125, 224106 CrossRef PubMed.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- M. Ångqvist, W. A. Muñoz, J. M. Rahm, E. Fransson, C. Durniak, P. Rozyczko, T. H. Rod and P. Erhart, Adv. Theory Simul., 2019, 2, 1900015 CrossRef.
- A. Zunger, S.-H. Wei, L. G. Ferreira and J. E. Bernard, Phys. Rev. Lett., 1990, 65, 353–356 CrossRef CAS PubMed.
- A. M. Ganose, A. J. Jackson and D. O. Scanlon, J. Open Source Softw., 2018, 3, 717 CrossRef.
- B. Zhu, S. R. Kavanagh and D. Scanlon, J. Open Source Softw., 2024, 9, 5974 CrossRef.
- K. Brlec, D. Davies and D. Scanlon, J. Open Source Softw., 2021, 6, 3171 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |