Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From anion-centric to cation-enabled energetics: planar tetrazine frameworks with enhanced stability
Jatinder Singh a,
Richard J. Staples
a,
Richard J. Staples b and
Jean'ne M. Shreeve
b and
Jean'ne M. Shreeve *a
*a
aDepartment of Chemistry, University of Idaho, Moscow 83844-2343, ID, USA. E-mail: jshreeve@uidaho.edu; Fax: (+1) 208-885-5173
bDepartment of Chemistry, Michigan State University, East Lansing 48824, Michigan, USA
First published on 19th December 2025
Abstract
Nitrogen-rich heterocycles represent a fertile ground for novel reactions and unusual molecular conversions, which not only advance fundamental heterocyclic chemistry but also enable the design of next-generation energetic materials. Owing to their high energy densities and clean decomposition into environmentally benign nitrogen gas, such frameworks are of growing importance in energy and environmental science. We report a new class of tetrazine-derived cationic and zwitterionic frameworks that enable thermally robust, high-performance energetic salts suitable for advanced propellant and energetic applications. Unlike conventional anion-centric approaches, our design focuses on planar, high-nitrogen tetrazine cations that promote π–π stacking interactions, hydrogen bond networks, and compact layer-like packing. These structural characteristics yield thermal decomposition onsets of 163–320 °C, densities of 1.61–1.80 g cm−3, and calculated detonation velocities of 7866–9292 m s−1 with corresponding detonation pressures of 21–34 GPa. Our zwitterionic tetrazine highlights how cation engineering and zwitterionic design together expand the scope of tetrazine chemistry toward thermally robust energetic materials. This work expands the scope of tetrazine chemistry and highlights the utility of planar high-nitrogen frameworks in the design of stable energetic materials.
1 Introduction
High-nitrogen energetic materials are valued for their ability to couple strong detonation performance with environmentally benign decomposition into nitrogen gas.1–5 Incorporation of high-energy explosophores into nitrogen-rich heterocycles has therefore become a major design strategy, as these frameworks enhance energy density while allowing structural diversification through selective functionalization (Fig. 1A).6–10 However, this approach often introduces a central drawback: compounds bearing strongly energetic substituents typically suffer from poor thermal stability and increased sensitivity to external stimuli such as impact and friction.11–15 Balancing energy density with safety thus remains a fundamental challenge in practical energetic-material design.16–20 | ||
| Fig. 1 Production of new energetic materials. (A) N-heterocylics, energetic explosophores and N-rich compounds. (B) Commonly used energetic cations. (C) Energetic salts formation. | ||
Energetic salts provide a promising pathway to address this issue. By deprotonating acidic heterocycles or introducing acidic energetic substituents, salts can be generated via pairing with suitable cations or anions (Fig. 1B).21,22 Their ionic nature fosters dense crystal lattices stabilized by hydrogen bonding and other non-covalent interactions, which result in reduced mechanical sensitivity while retaining high energy content (Fig. 1C).23,24 Despite these advantages, progress in salt design has been strongly skewed toward anions. Nitro-, nitramino-, and azido-substituted heterocycles dominate the literature whereas energetic cations are comparatively rare and structurally simple, typically limited to ammonium, hydroxylammonium, guanidinium, or small amino/alkyl-substituted systems. This imbalance highlights the urgent need for innovative cation-design strategies.25–30
Within the family of nitrogen-rich heterocycles, tetrazines have emerged as particularly compelling scaffolds due to their exceptional nitrogen content, synthetic versatility, and ability to host diverse functional groups.31–35 The 1,2,4,5-tetrazine ring has been exploited to produce energetic salts with tunable properties, fused heterocyclic frameworks with enhanced aromatic stabilization, and coordination-driven polymers where metal–ligand interactions impart additional stability. A defining feature of planar tetrazine frameworks is their strong π–delocalization, which contributes both to aromatic stabilization and efficient crystal packing. This combination increases density and detonation performance while simultaneously improving thermal stability—critical attributes for safe handling and long-term storage.36–40
Recent studies have also revealed that substitution on the tetrazine ring can induce unusual chemical transformations, ranging from nucleophilic substitution cascades to acid-mediated nitration and nitramination. Such reactivity broadens the scope of accessible derivatives, including cationic, fused, or coordination-stabilized variants, some of which achieve a rare balance of high energy output with moderated sensitivities. These advances underscore the adaptability of the tetrazine core and highlight its promise as a platform for rational energetic-cation design. In this context, the present work explores the synthesis and characterization of tetrazine-based cations that integrate high nitrogen content, planar frameworks, and excellent thermal stability (Fig. 2). By shifting attention from anion-focused strategies to cation engineering, we aim to advance the design of high-performance, safer energetic salts.
2 Results and discussion
2.1 Synthesis
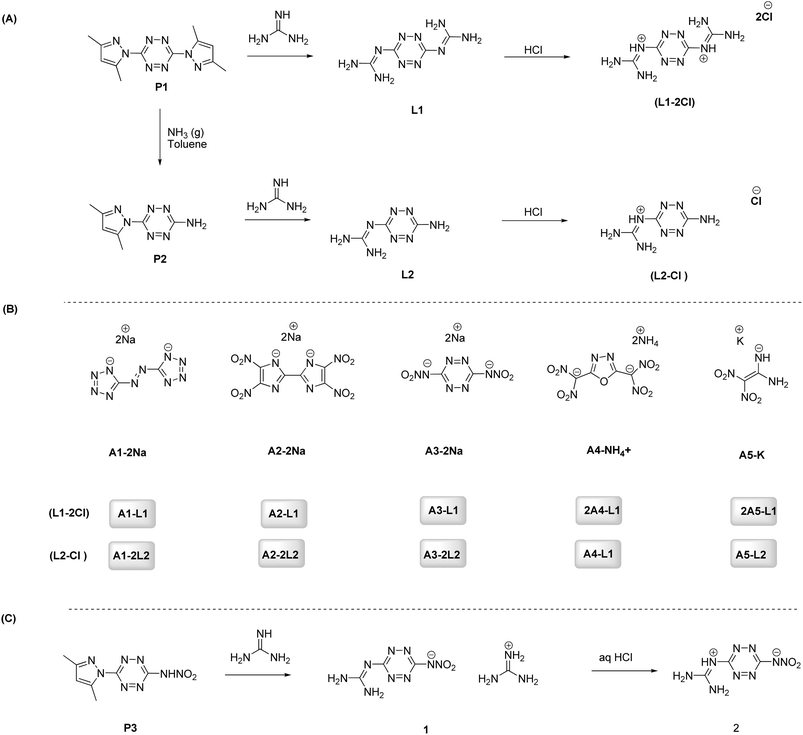
3,6-Bis(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazine (P1), 6-(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazin-3-amine (P2), and N-(6-(3,5-dimethyl-1H-pyrazol-1-yl)-1,2,4,5-tetrazin-3-yl)nitramide (P3) were synthesized following established literature procedures.41,42 The reaction of P1 and P2 with guanidine in methanol results in the formation of 2,2′-(1,2,4,5-tetrazine-3,6-diyl)diguanidine (L1) and 2-(6-amino-1,2,4,5-tetrazin-3-yl)guanidine (L2), respectively (Scheme 1A). The reaction of L1 and L2 with hydrochloric acid gives chloride salts L1-2Cl, and L2-Cl, respectively.The sodium or potassium or ammonium salts (A1–A5) were synthesized following established literature procedures (Scheme 1B).43–48 The sodium or potassium or ammonium salts of compounds A1–A5 were reacted with the hydrochlorides (L1-2Cl and L2-Cl) in aqueous solution to obtain assembled products (Scheme 1B). While the synthesis of L1 itself has been described earlier by our group, the salts and ionic derivatives presented here, which differ in counter-ion identity and solid-state packing, are unprecedented. These new salts exhibit distinct crystal-packing motifs, thermal behaviour, and mechanical-sensitivity profiles, and therefore materially expand the scope and application potential of the L1 scaffold. The reaction of P3 with guanidine in methanol results in the formation of compound 1, which was neutralized with aqueous HCl to give the zwitterionic compound 2 (Scheme 1C).
2.2 Characterization
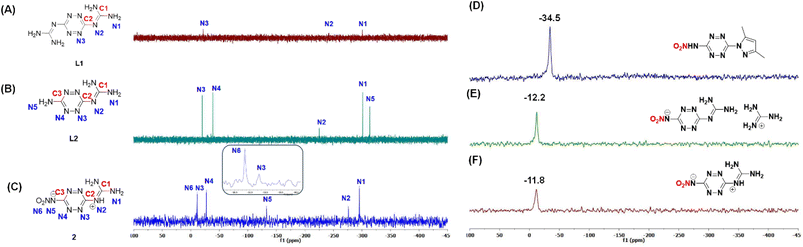
The 14N NMR spectrum of precursor P3 shows the characteristic nitramine N–NO2 resonance at −34.5 ppm for the neutral molecule (Fig. 3D). In contrast, upon deprotonation the corresponding nitramine nitrogen in compound 1 (guanidinium salt) shifts markedly upfield to −12.2 ppm (Fig. 3E), and a similar shift is observed in the zwitterionic compound 2 at −11.8 ppm (Fig. 3F). This substantial upfield migration of the nitramine signal clearly indicates that, in the zwitterionic structure, the nitramine moiety exists predominantly in its anionic (deprotonated) form.
Single crystals of compound A1–L1 suitable for X-ray analysis were obtained by slow evaporation from DMSO. It crystallizes in a monoclinic (P21/n) space group (Fig. 5A). The crystal structure supports the presence of anion and cation in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, resulting in the molecular formula C6H10N20, with a calculated crystal density of 1.715 g cm−3 at 100 K.
1 ratio, resulting in the molecular formula C6H10N20, with a calculated crystal density of 1.715 g cm−3 at 100 K.
 | ||
| Fig. 5 (A) Labeling scheme for A1–L1 (thermal ellipsoid at 50%). (B) Planar structure of A1–L1. (C) Packing diagram of A1–L1. | ||
In Fig. 5B the planar arrangement of both the anion and cation species is seen. Compound A1–L1 has planar stacking with layer spacing of 3.14 Å, which is lower than the typical 3.65–4.00 Å observed in aromatic π–π interactions. The interlayer spacing suggests that there may be strong π–π interactions between the molecules (Fig. 5C). This enhanced stacking contributes to the compact packing and may explain the relatively high density of the crystal. Single crystals of compound A3–2L2 suitable for X-ray analysis were obtained by slow evaporation from DMSO. It crystallizes in a monoclinic (I2/a) space group (Fig. 6). The crystal structure supports the presence of anion and cation in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, resulting in the molecular formula C8H14N24O4 with a density of 1.811 g cm−3 at 100 K. Each dinitramide anion is sandwiched between L2 cations through π-philic molecular recognition interactions.
2 ratio, resulting in the molecular formula C8H14N24O4 with a density of 1.811 g cm−3 at 100 K. Each dinitramide anion is sandwiched between L2 cations through π-philic molecular recognition interactions.
2.3 Physicochemical properties
High thermal stability is crucial for useful energetic materials. Thermal characteristics were determined using differential scanning calorimetry (DSC) analysis. At 5 °C min−1, the decompositions of L1 and L2 occur at 274 °C (onset) and 265 °C (onset), respectively. The energetic salts exhibit decomposition temperatures in the range of 163 °C to 320 °C (onset), respectively. To study structure–property relationships, an in-depth exploration of weak interactions was conducted via Hirshfeld surface and 2D fingerprint analyses (Fig. 7).49,50 The red regions on the Hirshfeld surfaces indicate interactions between neighboring molecules. The π–π interactions aroused by stacking of the dianion and two mono cations are visualized by plotting non-covalent interactions plots. In compound A1–L1, the nitrogen atoms of the anion form strong hydrogen bonds with the surrounding cations, with bond lengths ranging from 2.00 Å to 2.21 Å (Fig. 7M).The total amount of H-bond interactions, N–H⋯N, is 61.6% (Fig. 7J and M). The percentage of N⋯N and N⋯C interactions is 27.3%, which indicates the presence of π–π stacking between the rings. The larger value of these stabilizing interactions enhances molecular stability and insensitivity of A1–L1. For compound A3–2L22, the four O atoms from two nitroamino groups are fixed by H bonds from the surrounding cations, with lengths ranging from 1.91 Å to 2.44 Å (Fig. 7T). The total amount of H-bond interactions, O–H⋯N and N–H⋯N, is 62.7% (Fig. 7P and Q). The percentage of N⋯N and N⋯C interactions is 21.6%, which indicates the presence of π–π stacking between the rings. Compound A3–2L2 also has planar stacking with layer spacing of 3.25 Å (Fig. 7U).
For all compounds, sensitivities to impact and friction were measured by using BAM standard methods51,52 and are given in Table 1.
| MFh | Tda (°C) | ISb (J) | FSc (N) | ρd (g cm−3) | ΔHf e (kJ g−1) | Dvf (m s−1) | Pg (GPa) | |
|---|---|---|---|---|---|---|---|---|
| a Temperature (onset at 5 °C min−1) of decomposition.b Sensitivity to impact (IS).c Sensitivity to friction (FS).d Density at 25 °C using gas pycnometer.e Enthalpy of formation (calculated using isodesmic reactions with the Gaussian 03 suite of programs (revision D.01).f Velocity (calculated using EXPLO5 version 7.01.01).g Pressure (calculated using EXPLO5 version 7.01.01) (calculated using EXPLO5 version 7.01.01.).h Molecular formula.i Ref. 55. *h50 = 24 cm/2.5 kg hammer (ref. 56). *Calculated crystal density (RT). | ||||||||
| L1 | C4H8N10 | 274 | 40 | 360 | 1.61* | 2.59 | 7866 | 21.0 |
| L2 | C3H6N8 | 265 | 40 | 360 | 1.62 | 2.98 | 8020 | 22.2 |
| A1–L1 | C6H10N20 | 163 | 20 | 240 | 1.67* | 6.18 | 8970 | 30.3 |
| A1–2L2 | C8H14N26 | 195 | 25 | 360 | 1.66 | 3.64 | 8191 | 23.7 |
| A2–L1 | C10H10N18O8 | 317 | 25 | 360 | 1.74 | 2.87 | 8353 | 28.4 |
| A2–2L2 | C12H14N24O8 | 320 | 30 | 360 | 1.75 | 1.62 | 7911 | 23.6 |
| A3–L1 | C6H10N18O4 | 216 | 15 | 360 | 1.74 | 5.25 | 9292 | 34.2 |
| A3–2L2 | C8H14N24O4 | 256 | 20 | 360 | 1.76* | 3.07 | 8717 | 28.6 |
| A4–L1 | C8H10N16O9 | 186 | 15 | 360 | 1.78 | 2.65 | 8804 | 33.0 |
| A4–2L2 | C10H14N22O9 | 222 | 16 | 360 | 1.72 | 1.35 | 8015 | 24.5 |
| 2A5–L1 | C8H16N18O8 | 200 | 20 | 360 | 1.79 | 2.46 | 9036 | 32.7 |
| A5–L2 | C5H10N12O4 | 243 | 25 | 360 | 1.77 | 3.04 | 9040 | 32.2 |
| 1 | C4H10N12O2 | 263 | 30 | 360 | 1.63 | 2.13 | 8220 | 24.0 |
| 2 | C3H5N9O2 | 247 | 10 | 160 | 1.80 | 3.17 | 8967 | 31.5 |
| RDXi | C = H6N6O6 | 204 | 7.5/5.6* | 120 | 1.80 | 0.42 | 8795 | 34.9 |
These compounds show acceptably low sensitivity towards impact and friction. The sensitivities of compounds A1–L1 and A3–2L2 are further explained from both crystal structure and at the molecular level. They have a layer-like packing pattern in the crystal structure, which when subjected to external stimuli can transform the mechanical energy into relative motion between layers. At the molecular level, sensitivities of materials toward impact are very closely related to their electrostatic potentials (ESPs). The electrostatic potentials of compounds A1–L1 and A3–2L2 were calculated based on the B3LYP/6-311G(d, p) method with optimized structures. ESP minima and maxima for compounds A1–L1 and A3–2L2 are −78.9, −78.4 kcal mol−1 and +96.4, +90.9 kcal mol−1, respectively (Fig. 8).
The densities of all compounds were measured using a gas pycnometer at ambient temperature under a helium (He) atmosphere and range from 1.61 g cm−3 to 1.79 g cm−3 (Table 1). The enthalpies of formation of the new compounds were calculated using the isodesmic method with the Gaussian 03 suite of programs53 giving values for compounds in the range from 0.42 to 6.18 kJ g−1 (Table 1). All compounds have positive enthalpies of formation owing to the presence of multiple N⋯N bonds. With experimental densities and calculated enthalpies of formation, detonation properties were calculated using EXPLO5 (v7.01.01).54 The values of calculated detonation velocities and detonation pressures are given in Table 1 and fall between 7866–9292 m s−1 and 21.0–34.2 GPa, respectively, with A3-L1 showing the highest calculated detonation velocity and the highest pressure.
In addition to their favourable thermal and energetic characteristics, the poor solubility of these salts should be emphasized as a structural consequence of their molecular design. The relatively large size and high nitrogen content of the cations increase polarity and reduce compatibility with common organic solvents (Fig. 9A). At the same time, extensive intramolecular hydrogen bonding and π–π stacking interactions promote compact crystal packing, which further limits solvent penetration. These combined factors lead to materials that are markedly less soluble than many conventional energetic salts such as ammonium, hydrazinium and hydroxylammonium – which are typically very soluble in water. While reduced solubility can complicate certain processing steps, it also contributes to enhanced safety during handling and storage.
To investigate the influence of cation design on salt stoichiometry and thermal behavior, we prepared both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5-Atz based) and 1
1 (5-Atz based) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (dinitroamine based) salts using our newly developed cations with previously reported anions (Fig. 9B and C). This strategy allowed a direct comparison between different coordination modes and cation–anion ratios. In both systems, the incorporation of the new cations led to a significant increase in thermal stability relative to salts derived from earlier cationic frameworks, highlighting the stabilizing effects of strong stabilizing interactions.
2 (dinitroamine based) salts using our newly developed cations with previously reported anions (Fig. 9B and C). This strategy allowed a direct comparison between different coordination modes and cation–anion ratios. In both systems, the incorporation of the new cations led to a significant increase in thermal stability relative to salts derived from earlier cationic frameworks, highlighting the stabilizing effects of strong stabilizing interactions.
The chemical stability of energetic salts arises from a combination of intrinsic molecular features and extrinsic environmental factors; in our tetrazine-derived salts the dominant stabilizing contributions are structural rather than purely electronic.57 Intramolecular and intermolecular hydrogen bonding, together with strong π–π stacking and compact layer-like packing, restrict molecular mobility and limit access of reactive agents (solvent, moisture, oxygen) to labile sites, thereby increasing resistance to hydrolysis and other chemical degradation pathways. For instance, we have observed that ammonium-FOX slowly reverts to FOX-7 over the period of one week, whereas, the 2:1 and 1:1 salts of FOX-7 anion with L1 and L2 cations make thermally and chemically stable materials with good detonation performance.
To evaluate the applicability of energetic salts A1–L1, A3–2L2, and zwitterion 2 as solid propellants, their performance was evaluated as monopropellants (neat) and composite propellants. The specific impulse (Isp) values were calculated using EXPLO5v7.01.01 software at chamber pressure 7 MPa, expansion pressure ratio (Pc/Pe) of 70, initial T of 3300 K, ambient pressure of 0.1 MPa at equilibrium, and expansion through the nozzle. The Isp values (Table 2) of A1–L1, A3–2L2. Additionally, the Isp values of four composite propellant formulations using A1–L1, A3–2L2, 2 and RDX (i) 88% of compound and 12% Al, (ii) 78% of compound, 12% Al, and 10% HTPB. (iii) 80% compound, 20% AP (iv) 42% compound, 20% AP, 20% HTPB and 18% Al, were calculated and the results are given in Table 2. The specific impulse values (Isp) of A1–L1 are comparable to the benchmark explosive RDX.58–60 2L2 and 2 as monopropellants (neat) are 269 s, 235 s and 247 s, respectively, which is higher than AP (ammonium perchlorate) (157 s) and ADN (ammonium dinitramide) (206 s).
| Ispa,f [s] | Ispb,f [s] | Ispc,f [s] | Ispd,f [s] | Ispe,f [s] | |
|---|---|---|---|---|---|
| a Isp – Specific impulse of neat compound (monopropellant).b Isp – Specific impulse at 88% compound and 12% Al as fuel additive.c Isp – Specific impulse at 78% compound, 12% Al, and 10% HTPB as a binder.d Isp – Specific impulse at 80% compound, 20% AP.e Isp – Specific impulse at 42% compound, 20% AP, 20% HTPB and 18% Al.f Specific impulse calculated at an isobaric pressure of 70 bar and initial temperature of 3300 K using EXPLO5 V 7.01. | |||||
| A1–L1 | 269 | 262 | 251 | 268 | 236 |
| A3–2L2 | 235 | 240 | 221 | 239 | 229 |
| 2 | 247 | 253 | 239 | 252 | 232 |
| RDX | 267 | 278 | 259 | 268 | 242 |
3 Conclusions
In this work, we have designed and synthesized a family of tetrazine-based cations and their salts that integrate high nitrogen content, and facile synthetic accessibility. In addition, they exhibit remarkable thermal stabilities. Structural analyses reveal planar frameworks stabilized by π–π stacking and extensive hydrogen bonding, which contribute to high crystal densities and stability. Thermal analyses demonstrate decomposition temperatures that exceed those of conventional tetrazine derivatives and are comparable to or surpass benchmark energetic materials. The positive heats of formation, together with high detonation velocities and pressures place these tetrazine derivatives among promising candidates for energetic applications. The zwitterion further highlights the versatility of tetrazine scaffolds, combining favorable energetic characteristics with enhanced chemical stability. Overall, cation engineering and zwitterionic design expand the scope of tetrazine chemistry, providing a pathway to thermally robust and high-performing energetic materials.Conflicts of interest
The authors declare that they have no competing financial interests or personal relationships that could have influenced the work reported in this paper.Data availability
The data underlying this study are available in the published article and its online supporting information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ta08126b.CCDC 2491019, 2491020 and 2491021 contain the supplementary crystallographic data for this paper.61a–c
Acknowledgements
The diffractometer (Rigaku Synergy S) for SC-XRD was purchased with support from the National Science Foundation (MRI program) under grant no. 1919565. We are grateful to the Fluorine-19 fund for support.References
- T. M. Klapötke, Chemistry of High-Energy Materials, Walter de Gruyter, 2nd edn, 2012 Search PubMed.
- H. Gao, Q. Zhang and J. M. Shreeve, J. Mater. Chem. A, 2020, 8, 4193–4216 Search PubMed.
- Z. Xu, T. Hou, F. Yang, L. Zhang, X. Zhang, W. Liu, Q. Lang, M. Lu and Y. Xu, ACS Appl. Mater. Interfaces, 2023, 15, 41580–41589 Search PubMed.
- G. Zhang, X. Hao, Y. Zou, S. Liu, J. Wei, Z. Dong and Z. Ye, J. Mater. Chem. A, 2024, 12, 33249–33256 Search PubMed.
- D. E. Chavez, D. A. Parrish, L. Mitchell and G. H. Imler, Angew. Chem. Int. Ed., 2017, 56, 3575–3578 Search PubMed.
- T. M. Klapötke, P. C. Schmid, S. Schnell and J. Stierstorfer, Chem. Eur. J., 2015, 21, 9219–9228 Search PubMed.
- T. M. Klapötke, M. Leroux, P. C. Schmid and J. Stierstorfer, Chem. Eur. J., 2016, 11, 844–851 Search PubMed.
- P. Yin, C. He and J. M. Shreeve, Chem. Eur. J., 2016, 22, 2108–2113 Search PubMed.
- J. Singh, R. J. Staples and J. M. Shreeve, J. Mater. Chem. A, 2024, 12, 30548–30557 Search PubMed.
- J. Singh, R. J. Staples and J. M. Shreeve, J. Mater. Chem. A, 2023, 11, 12896–12901 Search PubMed.
- M. Benz, T. M. Klapotke, J. Stierstorfer and M. Voggenreiter, J. Am. Chem. Soc., 2022, 144, 6143–6147 Search PubMed.
- Q. Yu, Z. Zheng, Z. Yi, W. Yi and J. M. Shreeve, J. Am. Chem. Soc., 2025, 147, 5125–5131 Search PubMed.
- J. Singh, R. J. Staples and J. M. Shreeve, Sci. Adv., 2023, 9, eadk3754 Search PubMed.
- D. Fischer, T. M. Klapötke, J. Stierstorfer and N. Szimhardt, Chem. Eur. J., 2016, 22, 4966–4970 Search PubMed.
- X. Yu, J. Tang, C. Lei, C. Xue, G. Cheng, C. Xiao and H. Yang, J. Mater. Chem. A, 2024, 12, 19513–19520 Search PubMed.
- B. Tan, J. Su, J. Zhang, C. Tang, J. Dou, X. Yang, M. Xu, S. Zeng, W. Li, J. Luan, G. Zhang, S. Song, Q. Zhang, X. Lu, B. Wang and N. Liu, J. Mater. Chem. A, 2025, 13, 25103–25109 Search PubMed.
- X. Guo, Y. Feng, Y. Liu, Q. Liu, L. Q. Luo and H. Gao, J. Mater. Chem. A, 2025, 13, 10782–10791 Search PubMed.
- R. Lv, L. Jiang, J. Wang, S. Huang, S. Song, L. Wei, Q. Zhang and K. Wang, J. Mater. Chem. A, 2024, 12, 10050–10058 Search PubMed.
- P. Saini, J. Singh, R. J. Staples and J. M. Shreeve, J. Mater. Chem. A, 2025, 13, 17421–17428 Search PubMed.
- W. Hu, J. Tang, X. Ju, Z. Yi, H. Yang, C. Xiao and G. Cheng, ACS Cent. Sci., 2023, 9, 742–747 Search PubMed.
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Mater. Chem., 2012, 22, 20418 Search PubMed.
- S. Banik, P. Kumar, V. D. Ghule, S. Khanna, D. Allimuthu and S. Dharavath, J. Mater. Chem. A, 2022, 10, 22803–22811 Search PubMed.
- P. Bhatia, K. Pandey, B. Avasthi, P. Das, V. D. Ghule and D. Kumar, J. Org. Chem., 2023, 88, 15085–15096 Search PubMed.
- J. Singh, R. J. Staples, M. Fabin and J. M. Shreeve, J. Mater. Chem. A, 2024, 12, 17501–17509 Search PubMed.
- P. Bhatia, P. Das and D. Kumar, ACS Appl. Mater. Interfaces, 2024, 16, 64846–64857 Search PubMed.
- H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 Search PubMed.
- W. Liu, W. L. Liu and S. P. Pang, RSC Adv., 2017, 7, 3617–3627 Search PubMed.
- K. Pandey, P. Das, A. Bijlwan and D. Kumar, J. Org. Chem., 2025, 90, 7700–7711 Search PubMed.
- D. Kumar and A. J. Elias, Resonance, 2019, 24, 1253–1271 Search PubMed.
- M. Benz, T. M. Klapötke, J. Stierstorfer and M. Voggenreiter, ACS Appl. Eng. Mater., 2022, 1, 3–6 Search PubMed.
- D. E. Chavez, M. A. Hiskey and R. D. Gilardi, Org. Lett., 2004, 6, 2889–2891 Search PubMed.
- M. H. V. Huynh, M. A. Hiskey, D. E. Chavez, D. L. Naud and R. D. Gilardi, J. Am. Chem. Soc., 2005, 127, 12537–12543 Search PubMed.
- D. E. Chavez, B. C. Tappan, M. A. Hiskey, S. F. Son, H. Harry, D. Montoya and S. Hagelberg, Propellants, Explos. Pyrotech., 2005, 30, 412–417 Search PubMed.
- D. E. Chavez and M. A. Hiskey, J. Heterocycl. Chem., 1998, 35, 1329–1332 Search PubMed.
- Q. Yu, J. Singh, R. J. Staples and J. M. Shreeve, Chem. Eng. J., 2022, 431, 133235 Search PubMed.
- X. Zhang, Y. Wang, Y. Liu, Q. Zhang, L. Hu, C. He and S. Pang, ACS Appl. Mater. Interfaces, 2022, 14, 37975–37981 Search PubMed.
- D. M. Bystrov, A. N. Pivkina and L. L. Fershtat, Molecules, 2022, 27, 5891 Search PubMed.
- H. Zhang, X. Du, Y. Liu, G. Lei, P. Yin and S. Pang, ACS Omega, 2024, 9, 33557–33562 Search PubMed.
- W. Tu, L. Wen, K. Wang, Y. Liu and Z. Xie, J. Org. Chem., 2025, 90, 7766–7774 Search PubMed.
- L. L. Fershtat, FirePhysChem, 2023, 3, 78–87 Search PubMed.
- M. D. Coburn, G. A. Buntain, B. W. Harris, M. A. Hiskey, K. -Y Lee and D. G. Ott, J. Heterocycl. Chem., 1991, 28, 2049–2050 Search PubMed.
- G. F. Rudakov, T. V. Ustinova, I. B. Kozlov and V. F. Zhilin, Chem. Heterocycl. Compd., 2014, 50, 53–64 Search PubMed.
- N. Fischer, K. Hüll, T. M. Klapötke, J. Stierstorfer, G. Laus, M. Hummel, C. Froschauer, K. Wurst and H. Schottenberger, Dalt. Trans., 2012, 41, 11201–11211 Search PubMed.
- A. J. Paraskos, E. D. Cooke and K. C. Caflin, Propellants, Explos. Pyrotech., 2015, 40, 46–49 Search PubMed.
- D. E. Chavez and M. A. Hiskey, J. Energ. Mater., 1999, 17, 357–377 Search PubMed.
- H. Gao, R. Wang, B. Twamley, M. A. Hiskey and J. M. Shreeve, Chem. Commun., 2006, 4007, 4007–4009 Search PubMed.
- M. Anniyappan, M. B. Talawar, G. M. Gore, S. Venugopalan and B. R. Gandhe, J. Hazard. Mater., 2006, 137, 812–819 Search PubMed.
- Q. Yu, P. Yin, J. Zhang, C. He, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2017, 139, 8816–8819 Search PubMed.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 Search PubMed.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 Search PubMed.
- NATO, Standardization Agreement 4489 (STANAG4489), Explosives, Impact Sensitivity Tests, 1999.
- NATO, Standardization Agreement 4487 (STANAG 4487), Explosives, Friction Sensitivity Tests, 2002.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel and G. E. Scuseria, et al., Revision D.01, Gaussian Inc., Wallingford CT, 2003 Search PubMed Search PubMed.
- M. Sućeska, EXPLO5, Version 7.01.01, Brodarski Institute, 2019 Search PubMed Search PubMed.
- R. Mayer, J. Köhler and A. Homburg, Explosives, Wiley-VCH, 2007 Search PubMed.
- C. B. Storm, J. R. Stine and J. F. Kramer, Chem. Phys. Energ. Mater., 1990, 605–639 Search PubMed.
- D. Herweyer, A. A. Kitos, P. Richardson, H. Canoe, J. S. Ovens, I. Laroche, B. Jolicoeur, M. Murugesu and J. L. Brusso, Cryst. Growth Des., 2023, 23, 2576–2582 Search PubMed.
- M. Tang, X. Wang, X. Xu, Z. Zeng, C. Chen, Y. Liu, W. Huang and Y. Tang, J. Mater. Chem. A, 2024, 12, 13081–13085 Search PubMed.
- T. M. Klapötke, Chemistry of High-Energy Materials, De Gruyter, edn. 6, 2022 Search PubMed.
- D. Luca, Chemical Rocket Propulsion, Springer Aerospace Technology, 2017 Search PubMed.
- (a) CCDC 2491019: ExperimentalCrystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pm3gx.; (b) CCDC 2491020: ExperimentalCrystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pm3hy; (c) CCDC 2491021: ExperimentalCrystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pm3jz.
| This journal is © The Royal Society of Chemistry 2026 |