Catalysis in motion: unlocking mechanistic insights with dynamic transmission electron microscopy
Hanggara
Sudrajat
 *ab and
Ari
Susanti
c
*ab and
Ari
Susanti
c
aQuantum Materials Research Group, Research Center for Quantum Physics, National Research and Innovation Agency (BRIN), South Tangerang 15314, Indonesia. E-mail: hanggara.sudrajat@brin.go.id
bCollaboration Research Center for Advanced Energy Materials, BRIN – Institut Teknologi Bandung, Bandung 40132, Indonesia
cDepartment of Chemical Engineering, State Polytechnic of Malang, Malang 65141, Indonesia
First published on 1st December 2025
Abstract
Understanding catalytic processes as they evolve under reaction conditions is essential for the rational design of efficient catalysts. Dynamic transmission electron microscopy (DTEM) has become an important tool for probing these dynamic transformations. In this contribution, we outline the development, capabilities, and future directions of DTEM in catalysis research. Key challenges, including electron beam effects and the difficulty of correlating structural dynamics with catalytic performance, are discussed. Innovations in data science, particularly machine learning for image analysis and correlative multimodal approaches, are identified as critical tools for advancing catalyst diagnostics. When combined with spectroscopy and theoretical modeling, DTEM serves as a foundational tool for building a mechanistic understanding of catalysis. As capabilities continue to evolve, DTEM is expected to provide deeper insights into existing catalytic systems and accelerate the discovery of next-generation catalysts.
1. Introduction
Catalysis is a cornerstone of modern chemical manufacturing, influencing energy conversion, environmental remediation, and materials development. The efficiency, selectivity, and durability of catalysts are fundamentally governed by their structure–property relationships, which often evolve dynamically under working conditions. Gaining insight into these transient states is therefore crucial for the rational design of next-generation, high-performance catalysts.Traditionally, our understanding of catalytic behavior has relied on ex situ characterization techniques, which provide valuable but “static” snapshots of catalyst structures. Although these methods have advanced our knowledge of structure–activity correlations, they fall short in capturing the transient atomic rearrangements and processes that govern real-time catalytic performance. To address this limitation, dynamic transmission electron microscopy (DTEM) has emerged as a powerful tool, allowing us to probe catalysts under realistic conditions with high spatial and temporal resolution.1,2 Here we use the term “dynamic TEM” in a broader context that is more relevant to the catalysis community, where “dynamic” refers to the observation of structural evolution under time-dependent external stimuli (e.g., reactive gases, heating, electrical bias, laser pulse/photoexcitation, mechanical strain, or liquid environments) including both slow processes (e.g., sintering, phase transitions) and faster catalytic events (e.g., surface restructuring, bond breaking). This usage is distinct from the definition common in the ultrafast electron microscopy community, where DTEM specifically denotes pump–probe pulsed-beam methods designed to resolve femto- to nanosecond reaction dynamics. Throughout this article, DTEM therefore refers to any time‐dependent TEM approach, with or without in situ reaction environments and simultaneous monitoring of reaction products, rather than exclusively to ultrafast pulsed‐beam techniques. By combining advanced electron optics, environmental control, and ultrafast detection technologies, DTEM enables direct visualization of structural transformations, defect evolution, and interfacial dynamics at the atomic scale in real time.
The recent evolution of DTEM builds on foundational advances in (scanning) transmission electron microscopy, (S)TEM, which integrates imaging, diffraction, and spectroscopy under applied stimuli such as temperature, electric fields, or reactive gas environments. These capabilities enable the investigation of the real-time behavior of grains, interfaces, surfaces, and defects under operating conditions with high spatial and/or temporal resolution.3 STEM, in particular, enables not only the recording of electron diffraction patterns, which can be used to establish pair distribution function (ePDF), but also the acquisition of bright-field TEM (BF-TEM), high-resolution TEM (HRTEM) phase contrast images, and annular dark-field (ADF) images based on elemental (Z) contrast (Fig. 1A). With the aid of dedicated hardware, it can further detect X-rays and inelastically scattered electrons for energy-dispersive X-ray spectrometry (EDX) and electron energy loss spectroscopy (EELS). At present, EELS and EDX have reached atomic-level precision in mapping electronic structure, elemental distributions, and bonding configurations.4 In our recent work, for example, we used STEM-EDX imaging to directly confirm the dopant site in an indium-doped SrTiO3 catalyst.5 This was achieved through real-space elemental mapping with atomic-scale resolution using a multivariate statistical analysis (MSA) protocol (Fig. 1B).
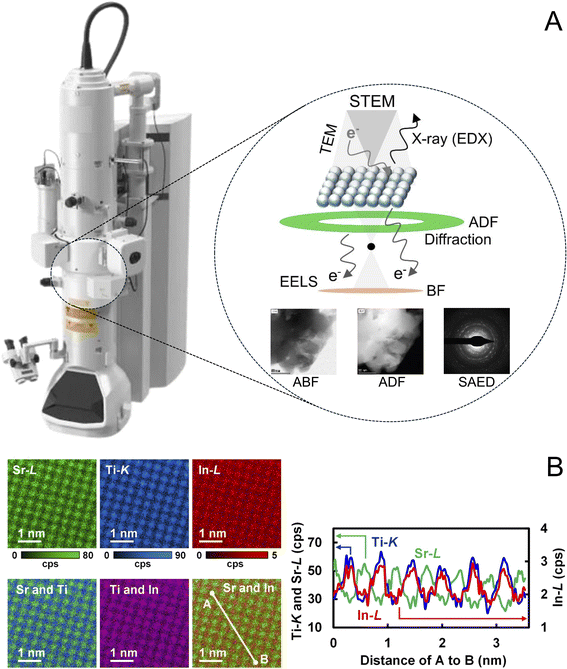 | ||
| Fig. 1 (A) Illustration of an electron microscope used to probe the local structural and chemical information of a solid sample.6 Shown are the ABF and ADF images of a poly(heptazine imide)-based catalyst and the corresponding SAED pattern, which we acquired using a Talos F200X TEM. (B) EDX maps showing Sr–L, Ti–K, and In–L maps of In–SrTiO3 integrated over 200 eV per peak and intensity profiles along lines A and B, with Sr–L, Ti–K, and In–L shown in green, blue, and red, respectively. The data are obtained with a TITAN3 G2 60–300 (FEI) microscope equipped with a silicon drift EDX detector (Super-X, FEI). Adapted with permission.5 Copyright 2021, American Institute of Physics. | ||
Given the significant scientific and technological impact of DTEM, which establishes a new paradigm for materials characterization under nonequilibrium and often extreme conditions, we are here motivated to discuss the fundamental principles, recent advances, and future directions of DTEM in catalysis research. In this highlight article, we address key challenges associated with imaging beam-sensitive materials, achieving high temporal resolution, and correlating nanoscale dynamics with macroscopic catalytic performance. We emphasize emerging multimodal and data-driven approaches, including machine learning (ML), correlative spectroscopy, and computational modeling.
2. State of the art
DTEM builds upon the core functions of conventional TEM while extending its capabilities to enable dynamic observation of materials under external stimuli. An ultrafast TEM system, for example, integrates optical components that direct laser pulses to both the electron source and the specimen, with lasers available as optional modules. This configuration accommodates multiple operational modes, including picosecond and nanosecond stroboscopy, nanosecond single-shot, nanosecond movie mode, and conventional thermionic imaging, within a single instrument.Traditional TEM techniques, while offering exceptional spatial resolution, are inherently limited by their reliance on static, high-vacuum conditions that preclude real-time imaging of chemical processes under practical environments. To overcome this limitation, DTEM incorporates temporal resolution and environmental flexibility, allowing for the observation of transient structural phenomena as they unfold during catalysis. Aberration correction further enhances spatial resolution and image clarity by compensating for imperfections in magnetic lenses, such as spherical (Cs) and chromatic (Cc) aberrations, which otherwise blur images.7,8 Using multipole elements like octapoles, aberration correctors act as diverging lenses that counter these effects, enabling sub-Angstrom point-to-point resolution and precise visualization of atomic structures, including light elements. This capability is particularly important for heterogeneous catalysis involving single-atom deposition, where atomic-level insight into surface and interface structures governs catalytic performance.
DTEM comprises several operational modalities, notably environmental TEM (ETEM) and ultrafast TEM (UTEM). ETEM, also referred to as chemical electron microscopy (CEM), enables imaging under controlled gaseous or liquid environments, thereby reproducing realistic reaction conditions within the microscope column. This is achieved through specialized environmental cells and holders that can introduce reactive species such as O2, H2, CO, or water vapor at controlled pressures and temperatures.9 These environmental cells often utilize microelectromechanical system (MEMS)-based platforms that integrate gas flow, heating, and sometimes even electrical biasing directly onto the sample chip.10 Such systems allow for operando imaging, wherein catalysts are subjected to reactive environments while undergoing continuous electron beam interrogation. Samorjai and co-workers were among the first to use ETEM to directly track reaction dynamics and atomic-scale displacements in real time.11 In their early work, they showed that under O2, Co migrates to the nanoparticle surface to form a strained CoO shell, while Pt remains in the core. Exposure to H2 reverses this process, driving Co back into the bulk and leaving a Pt-rich surface. Strain mapping confirmed lattice distortions and reversible segregation driven by Co–O bond strength and diffusion kinetics. These observations link redox-driven structural dynamics to catalytic behavior in bimetallic nanoparticles, demonstrating the value of real-time ETEM. The continued advancement of ETEM depends on the development of integrated and user-friendly instrumentation.12 For ETEM to advance from a supplementary imaging method to a primary tool in catalysis research, it must provide quantitative structure–function correlations that establish causal links between catalyst structure and performance. By integrating real-time imaging with spectroscopic analysis, ETEM correlates catalytic function to geometric and electronic characteristics, providing experimental validation for theoretical models and scaling relations. Its ability to probe catalysts under near-ambient conditions yields realistic insight into surface dynamics, deactivation, and reactivity. ETEM also complements conventional in situ techniques by rapidly delivering structural and functional information from minimal sample volumes.
On the other hand, UTEM is designed to capture ultrafast dynamics on the timescales of femtoseconds to nanoseconds. This is accomplished through the use of pulsed electron beams generated by laser-triggered photocathodes. Much like a stroboscopic camera, UTEM synchronizes these electron pulses with a secondary stimulus (e.g., a laser or voltage pulse), thereby enabling the visualization of transient states with resolution achieving sub-picosecond.4,9 While still in a less mature stage compared to ETEM, UTEM holds enormous promise for visualizing fast processes such as charge carrier dynamics, phase transitions, and atomic vibrations that are inaccessible to slower imaging techniques. Central to the success of both ETEM and UTEM approaches are advancements in hardware and detector technology. Modern direct electron detectors offer high frame rates (up to 87 kHz in specialized systems) and low readout noise, all of which are critical for capturing high-resolution images during fast events while minimizing electron dose.13 These detectors enable the acquisition of massive four-dimensional datasets (3D space + time), which require sophisticated data processing workflows and robust computational resources.3
Further improvements in temporal resolution have been made possible by optimizing the electron source. Field emission guns (FEGs), particularly Schottky-type or cold-FEGs, offer high brightness and narrow energy spreads, which are crucial for minimizing chromatic aberrations and maximizing spatial resolution under low-dose conditions.14 Monochromated sources have also been employed to improve the energy resolution of EELS measurements to a few meV, thereby enabling the detection of subtle valence state changes or vibrational modes even in beam-sensitive specimens.
Beam–sample interactions remain a critical consideration in DTEM, especially when imaging under reactive environments or when studying fragile catalysts such as single-atom systems or metal–organic frameworks (MOFs). Inelastic and elastic scattering events can lead to knock-on damage, radiolysis, and beam-induced heating, which may alter the very processes under observation. To mitigate such effects, low-dose imaging protocols, beam blanking strategies, and cryogenic techniques can be employed.3 Moreover, beam–sample interaction parameters such as convergence angle, dwell time, and scan pattern are increasingly optimized using ML approaches to reduce damage while preserving image fidelity.15
DTEM broadly encompasses in situ, time-resolved, and operando techniques. In situ TEM provides structural information under controlled environments, time-resolved TEM (TR-TEM) extends this capability by capturing transient events across relevant time scales, and operando TEM combines both structural and functional measurements under actual reaction conditions. Not all in situ TEM studies qualify as DTEM, as only those that incorporate time-dependent measurements meet this definition. Likewise, time-dependent in situ experiments are not considered operando unless reaction products are simultaneously monitored. TR-TEM, on the other hand, focuses on visualizing ultrafast dynamics that typically occur in the absence of realistic reaction environments. Therefore, although all operando TEM experiments are in situ, not all in situ studies are operando or time-resolved, and not all DTEM techniques are in situ. TR-TEM and UTEM are DTEM techniques that operate under high vacuum rather than true in situ conditions. To preserve ultrafast electron beam coherence and minimize scattering, the microscope column is maintained at ultra–high vacuum (∼10−7–10−8 Torr). Thus, these methods generally cannot accommodate gaseous or liquid environments or functional electrochemical cells, which are required for in situ and operando studies. Table 1 summarizes the conceptual relationships among in situ, time-resolved, and operando TEM techniques.
| Level/subset technique | Core focus | Key experimental capabilities | Primary data output | Typical timescale | Representative applications | Scientific purpose |
|---|---|---|---|---|---|---|
| DTEM. In this context, “dynamic” refers to time-dependent structural evolution, distinct from the pulsed-beam DTEM used for ultrafast studies | • Visualizing materials under external stimuli or working conditions | • Enables environmental control (gas, liquid, temperature, bias) and temporal imaging; may integrate complementary spectroscopy | • Sequential image series, diffraction patterns, or spectra reflecting dynamic behavior | • fs – hours depending on mode | • Any process involving structural or chemical evolution: catalysis, electrochemistry, phase transitions, beam–matter interaction studies | • Provides a unified framework for correlating dynamic structural, chemical, and functional responses of materials |
| In situ TEM. “In situ” means inside the microscope, under stimulus | • Imaging and analysis performed inside the microscope while applying controlled stimuli (e.g., heating, gas, bias, liquid) | • MEMS-based heating holders, environmental cells (ETEM), electrochemical or liquid cells | • Structural evolution snapshots; lattice changes; elemental mapping under stimulus | • s – hours. Example: heating Pt nanoparticles to 700 °C in vacuum to observe sintering | • Thermal sintering, oxidation/reduction, gas–solid reactions, bias-driven migration | • Reveals morphology and phase evolution in realistic but not necessarily active (“working”) environments |
| TR-TEM. “Time-resolved” means with explicit temporal resolution and kinetics | • Emphasizing temporal resolution; tracking events over time with high frame-rate or pulsed-beam methods | • High-speed direct-electron detectors (103–105 fps); pump–probe or pulsed electron beams (UTEM) | • Time-series movies, transient diffraction or EELS signals, kinetics of structural change | • fs – s (UTEM → fs–ns; conventional → ms–s). Example: imaging lattice contraction in a photocatalyst 10 ns after laser excitation | • Reaction kinetics, phase nucleation, charge-transfer dynamics, surface diffusion | • Quantifies rates, sequences, and lifetimes of transient states; establishes mechanistic pathways |
| Operando TEM. “Operando” means in working conditions and with real-time performance readout | • Combining real-time imaging with simultaneous measurement of catalytic or functional performance (e.g., gas composition, electrical current) | • Integration of MEMS reactors with gas analysis or electrochemical readout; synchronized imaging and signal acquisition | • Correlated datasets linking structure, chemistry, and performance metrics | • s – min (linked to catalytic turnover or electrochemical cycles). Example: imaging Ni nanoparticle restructuring while measuring H2 evolution or CO2 conversion rate in the same gas flow cell | • Catalysis under reactive gases, battery cycling, electrochemical reactions | • Establishes direct structure–function relationships; connects atomic dynamics with macroscopic activity, selectivity, and stability |
While achieving “true operando” conditions in TEM remains challenging due to vacuum constraints and spatial limitations within the microscope column, significant progress has been made through the development of custom-built MEMS-based reactors and specialized sample holders.10 The term “true operando” here refers to simultaneous measurement of structural and/or chemical information and catalytic performance under realistic reaction conditions (e.g., temperature, pressure, and reactive environment). The catalyst is actively functioning, converting reactants into products, while being imaged. In contrast, earlier studies that were described as operando sometimes merely simulated reaction conditions, such as introducing reactive gases or heating, without directly measuring catalytic activity or product formation. Such experiments should be referred to as “pseudo-operando” or “quasi-operando”, as they lack a direct correlation between observed structural changes and actual catalytic performance.
The diversity of stimuli that can be applied during DTEM has also expanded. Modern holders can incorporate heating up to 1000 °C, biasing, mechanical straining, and even light or ion irradiation, all while maintaining high spatial resolution imaging. This has enabled unprecedented studies of dynamic processes such as nanoparticle sintering, phase boundary migration, ion intercalation, surface reconstruction, and oxidation–reduction cycling.19 As the complexity of DTEM experiments increases, so too does the need for precise control and reproducibility. Automated workflows and software platforms for electron microscope alignment, image acquisition, and beam control are becoming increasingly integral to successful experimentation. We expect that self-driving DTEM capable of adapting imaging parameters in real time to evolving specimen behavior will soon be enabled through scripting and AI-based control systems. As new modalities and instrumentation continue to emerge, DTEM is poised to deepen our mechanistic understanding of industrially relevant reactions and accelerate the discovery of novel catalyst materials.
Table 2 summarizes the trade-offs in DTEM between resolution, environmental control, and chemical realism in the context of catalysis. While these methods provide deep insights into catalyst dynamics and metastable states, achieving high spatial and temporal resolution without compromising sample integrity remains a major challenge for routine use.
| Approach | Key features | Capabilities | Constraints |
|---|---|---|---|
| a Notes: A liquid-cell TEM approach integrating electrochemical control (e.g., applied potential or bias) to study electrocatalysts and electrode materials in liquid electrolytes. b An advanced TR-TEM technique combining real-space imaging with reciprocal-space diffraction as a function of time (three spatial + one temporal dimension). | |||
| ETEM-based DTEM | • Utilizes environmental TEM with differentially pumped chambers; temporal resolution typically ∼0.1–1 s | • Real-time imaging of dynamic morphological changes (e.g., sintering, reshaping) in gas-phase catalysis | • Limited to low pressures (up to ∼20 mbar); beam–gas interactions may cause artifacts; lacks true operando function link |
| Closed-cell DTEM (gas phase) | • Uses MEMS-based sealed nanoreactors with integrated heating; temporal resolution ∼0.5–2 s | • Enables higher pressures and better mimicry of reaction environments; safer gas handling | • Limited imaging resolution due to window thickness; slower temporal acquisition; restricted gas exchange rates |
| DTEM with electrochemical cells (EC-TEM)a | • Combines liquid cells and potentiostats; operates in liquid electrolyte for electrocatalysis; ∼seconds scale | • Allows monitoring of particle evolution, dissolution, and redox-driven restructuring during electrochemical reactions | • Poor atomic resolution due to liquid thickness and scattering; beam-induced radiolysis and bubble formation can distort results |
| Pump–probe ultrafast DTEM (4D-TR-TEM)b | • Uses pulsed electron beams to capture ultrafast events (∼femtoseconds to nanoseconds) | • Only technique to access ultrafast charge transfer or reaction initiation phenomena; suitable for photochemistry | • Limited to photo-driven catalysis; very low signal-to-noise; complex and expensive instrumentation |
| DTEM with concurrent spectroscopy (EELS/EDX) | • Simultaneously acquires chemical information (oxidation states, elemental maps) during time-lapsed imaging | • Correlates structure, composition, and oxidation state dynamics in real time; useful in redox-sensitive systems | • Beam-sensitive; lower temporal resolution; difficult to interpret fast changes in valence due to integration effects |
| DTEM with operando product analysis | • Combines image acquisition with catalytic conversion measurements (e.g., GC and/or MS) | • Direct link between visualized structural changes and catalyst activity/selectivity; crucial for true operando work | • Often mismatched temporal scales between structure and function measurement; challenging to miniaturize reactors |
3. Multimodal approaches
Although DTEM offers high spatial and/or temporal resolution, it lacks direct chemical or electronic information. To overcome this limitation and gain a better picture of catalyst dynamics, it is increasingly combined with complementary analytical techniques and advanced data science tools. These multimodal and data-driven approaches enable the integration of structural imaging with spectroscopy, performance monitoring, and ML, thereby maximizing the information extracted from dynamic catalyst studies.Through multimodal approaches, DTEM is frequently integrated with EELS and EDX to capture time-resolved chemical changes such as redox processes and compositional redistribution. These modalities allow us to correlate atomic-level structural evolution with changes in bonding and valence states during catalytic reactions. Near-edge structure analysis in EELS provides chemical fingerprints of oxidation states and bonding configurations, similar in sensitivity to synchrotron-based techniques like X-ray absorption spectroscopy (XAS).24–26 For example, in ceria-based systems, EELS has resolved the reversible Ce4+/Ce3+ transitions under redox conditions at nanometer resolution, showing where oxygen vacancies form and migrate.27 Furthermore, combining 4D-STEM with EELS or EDX provides insight into local strain, polarization, and chemical gradients.28 Such correlative techniques are critical for understanding complex materials like mixed-metal oxides or supported bimetallic nanoparticles, where activity depends on subtle electronic and geometric interplay. Such hybrid data acquisition is becoming increasingly streamlined with modern detector arrays and artificial intelligence (AI)-enhanced alignment algorithms that maintain temporal coherence between image and spectral frames.29
Operando TEM, where catalyst performance is monitored simultaneously with imaging, remains a frontier in the field. Most in situ TEM studies operate under simulated conditions, but advances in MEMS-based microreactors now allow for real-time gas flow, heating, and even performance measurements such as gas evolution or resistivity changes to enable operando operation.28,30 Recent systems integrate TEM holders with downstream mass spectrometry (MS), enabling time-aligned detection of catalytic products such as H2, CO2, or hydrocarbons during imaging.30 In these reaction systems, structure–function relationships can be directly established, for instance, by linking nanoparticle restructuring with spikes in activity. There is also potential for coupling ETEM with performance proxies such as electrical conductivity, diffraction signal changes, or real-time spectroscopic readouts. Such correlative signals offer intermediate steps toward fully integrated chemical analysis.28 This multimodal approach allows us to pinpoint which features: defect density, particle shape, oxidation state, are associated with increased or decreased catalytic turnover.
While multimodal characterization has emerged as a promising direction, a more systematic framework is needed to guide the integration of DTEM with complementary techniques. The selection of techniques depends on three key factors: (i) the scientific objective, (ii) the timescale of the process, and (iii) the type of chemical or structural information desired. Within this context, the first issue to address is when and why to integrate. When the goal is to correlate structural evolution with oxidation-state or bonding changes, EELS and XAS are suitable choices, as they provide sensitivity to local electronic environments. Ambient pressure X-ray photoelectron spectroscopy (AP-XPS) can also be employed as a complementary technique,31,32 as it probes surface chemical states, in contrast to XAS, which provides information about bulk electronic structure. For elemental redistribution, segregation, or diffusion phenomena, EDX or XRF offer compositional mapping across broader sample regions. For monitoring surface species and intermediates, diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), Raman, or IR spectroscopy complement DTEM by identifying functional groups and adsorbates. To directly correlate structure with catalytic performance, MS or gas chromatography (GC) can be integrated downstream for operando reaction monitoring. The optimal combination of techniques thus depends on whether the study aims to probe structure–composition, structure–bonding, or structure–function relationships.
The second issue concerns how to integrate. Multimodal workflows can generally be categorized into three levels of correlation. The first is sequential correlation, in which the same region of interest is examined on different instruments before and after DTEM measurements, offering flexible yet post hoc comparison. The second is quasi–simultaneous correlation, where DTEM and spectroscopic analyses are conducted under equivalent conditions on identical samples, yielding near-concurrent structural and chemical information. The third and most advanced level is true operando correlation, in which DTEM imaging and performance signals (e.g., MS or GC) are synchronized in real time, directly linking atomic-scale dynamics to catalytic turnover. Each level enhances the richness of information but also entails greater demands on synchronization precision and experimental complexity.
Spatial correlation between modalities can be improved by using fiducial markers, patterned supports, or ML–based image registration to align datasets from different instruments.33,34 Temporal alignment may be achieved by employing shared triggers such as gas-switch pulses, electrical bias, or laser excitation. Advanced data-fusion pipelines using dimensionality-reduction tools or ML algorithms may also enable integration of heterogeneous data (imaging, spectroscopy, and performance metrics) into unified structure–function models. A question-driven workflow should therefore precede any multimodal experiment: What structural variable needs correlation? At what temporal scale? Which complementary method provides orthogonal information? Balancing these factors helps maximize the synergy between techniques while minimizing redundancy or beam damage.
Table 3 shows a framework that correlates the scientific objective of a study to specific DTEM modes and complementary techniques, while outlining the relevant temporal regimes, practical challenges, and mitigation strategies. This framework provides a structured guide for designing multimodal DTEM experiments tailored to catalytic systems of varying complexity, from single-atom catalysts to multicomponent oxides, ensuring that each integrated method contributes distinct and orthogonal information toward elucidating dynamic structure–function relationships. For example, complementary techniques such as selected area electron diffraction (SAED) can reveal the oxidation–reduction cycles of nanoparticles (e.g., Cu ↔ Cu2O) during light-driven redox reactions, as well as phase transitions (e.g., perovskite → oxyhydroxide) that occur as a function of time or environmental conditions, while ePDF analysis quantifies local structural evolution even in amorphous or nanophase catalysts.
| Research objective | Primary DTEM mode | Complementary techniques | Correlative information | Timescale | Major experimental challenge | Recommended strategies |
|---|---|---|---|---|---|---|
| Oxidation-state and bonding dynamics | ETEM/UTEM + EELS | XAS (XANES/EXAFS), AP-XPS, NEXAFS (soft X-ray for light elements) | • Valence state mapping, coordination environment, local charge distribution | µs – s | • Beam sensitivity, low signal-to-noise ratio | • Use low-dose imaging, monochromated beam, cryogenic stabilization |
| • Correlate EELS near-edge fine structure with XAS trends | ||||||
| Surface intermediate identification | ETEM + DRIFTS/Raman/IR | AP-IR/Raman, in situ FTIR | • Vibrational fingerprint of adsorbed species, bond activation sequence | ms – min | • Temporal mismatch between imaging and spectroscopy | • Synchronize gas pulses or temperature ramps |
| • Employ dual detectors for real-time signal tracking | ||||||
| Product correlation and catalytic activity monitoring | ETEM + MS/GC | On-chip micro-MS, online GC, quadrupole MS | • Reaction rate, selectivity, turnover frequency (TOF) | s – min | • Gas flow and sampling delay, reactor miniaturization | • Use MEMS-based microreactors with differential pumping |
| • Calibrate temporal offset between imaging and product detection | ||||||
| Elemental redistribution and segregation | STEM-EDX/4D-STEM | XRF, electron probe microanalysis (EPMA) | • Elemental diffusion, alloying, phase segregation, stoichiometry drift | ms – h | • Limited spatial registration across modalities | • Employ fiducial markers and pattern registration |
| • Co-align maps using AI-based feature matching | ||||||
| Lattice strain and phase transition mapping | 4D-STEM/HRTEM | XRD, ePDF, SAED | • Strain tensor, dislocation motion, phase fraction evolution | µs – s | • Data fusion of reciprocal-space and real-space data | • Combine diffraction-based strain mapping with DFT/MD simulation for cross-validation |
| Single-atom site tracking | Aberration-corrected STEM-HAADF + EELS | XAS, EXAFS, AP-XPS | • Coordination geometry, oxidation-state fluctuation, migration pathways | ns – s | • Drift and limited temporal resolution | • Implement drift-correction algorithms |
| • Correlate with EXAFS for ensemble statistics | ||||||
| Charge-carrier and plasmonic dynamics | UTEM (pump–probe) | Ultrafast optical spectroscopy, transient absorption | • Carrier relaxation, plasmon decay, hot-electron transfer | fs – ns | • Synchronization of electron and laser pulses | • Use shared laser trigger |
| • Apply stroboscopic averaging for enhancement of SNR | ||||||
| Support interaction and interface reconstruction | ETEM/STEM-EELS | AP-XPS, XAS, low-energy ion scattering (LEIS) | • Metal–support bonding, encapsulation/exsolution behavior | s – min | • Surface contamination, thermal drift | • Combine in situ heating with real-time EELS and ex situ XPS confirmation |
| Electrochemical reaction dynamics | EC-TEM/EC-STEM | XAS, cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) | • Morphological and oxidation-state evolution under bias | ms – min | • Beam-induced radiolysis, electrolyte thickness | • Use thin liquid cells, pulsed beam illumination, and reference electrodes for calibration |
| Defect and vacancy kinetics in oxides | ETEM/STEM-EELS | XAS, positron annihilation, Raman | • Oxygen vacancy formation, migration, recombination | µs – s | • Identifying dynamic defect states in heterogeneous regions | • Combine time-resolved EELS with temperature-programmed gas switching |
| • Use ML-based pixel classification |
4. Data-driven approaches
DTEM experiments routinely produce large, multidimensional datasets (e.g., 4D or even 5D encompassing spatial, temporal, and energy dimensions),36 which are not feasible to evaluate manually. As a result, extracting statistically meaningful structural or dynamic information is challenging. ML has therefore become a valuable approach for automating data analysis and identifying subtle trends that may otherwise remain unresolved. ML models, especially convolutional neural networks (CNNs), have been used to segment nanoparticles, track atomic displacements, identify defects, and classify transient phases with high accuracy and speed.15,29 These methods dramatically reduce the labor and subjectivity involved in interpreting time-series data. For instance, ML-based denoising algorithms improve signal-to-noise ratio (SNR) in low-dose DTEM datasets, preserving features while minimizing damage-induced noise.29 Dimensionality reduction tools like principal component analysis (PCA) or nonnegative matrix factorization (NMF) are used to extract spectral signatures from EELS time-series data.37 Furthermore, ML-assisted classification can help distinguish between beam-induced changes and genuine catalytic dynamics, a critical issue in in situ studies.38The use of ML for ex situ TEM image analysis has been quite common.39 With no hardware changes, two complementary strategies can improve TEM data: (i) post-processing such as ML denoising and decomposition (early unsupervised methods for EELS/STEM, now advanced supervised deep learning (DL) such as fully convolutional neural network (FCNNs), generative adversarial networks (GANs), and sparse-coding approaches) which recover weak signals, raise SNR, enable low-dose/beam-sensitive measurements (even holography), and reveal subtle chemistry/strain; and (ii) acquisition automation such as computer-vision and few-shot/DL routines that automate alignments, aberration correction, feature detection, and active experiment steering, enabling real-time, self-aligning and autonomous setups. Today the primary bottleneck is automated analysis, where CNNs and encoder–decoder models already excel at atomic/defect detection in 2D and are extending toward 3D (by combining with clustering/anomaly detection).
Unlike ex situ TEM, ML applications for in situ, dynamic TEM are still at an early stage. Recently, Stach and co-workers investigated how CNNs can be effectively applied to segment in situ TEM images of nanoparticles while maintaining interpretability and generalization.40 Using ETEM data of Au nanoparticles as a case study, they systematically examined dataset preparation, CNN architecture, and training strategies to improve segmentation accuracy for high-resolution images. Conventional architectures such as U-Net perform well for lower-resolution data but tend to overfit or blur particle boundaries at higher resolutions. Performance was most improved by applying batch normalization and optimizing convolutional kernel size, rather than increasing network depth or complexity. Simpler, shallow CNNs can achieve comparable accuracy to deep models while being more interpretable and efficient, allowing visualization of learned filters and features. Careful data-driven model design, prioritizing regularization, simplicity, and feature understanding, can make ML segmentation both accurate and physically meaningful for large-scale TEM datasets, facilitating automated, reliable analysis in materials science.
Also using Au as a model system, the same research group then investigated the stability of supported metal nanoparticles, which is a critical factor influencing catalyst activity and lifetime.41 By integrating in situ TEM with unsupervised ML, they monitored hundreds of Au nanoparticles at 900 °C, tracking changes in number, size, and shape over time with a CNN-based analysis. Evaporation and surface diffusion dominate nanoparticle evolution. Statistical data mining enabled quantitative modeling, identified the critical particle size where Gibbs–Thomson pressure accelerates evaporation, and exposed deviations from mean-field predictions. This approach provides a generalizable framework for quantitatively assessing nanoparticle stability and transformation under reactive conditions.
Meanwhile, Chen and co-workers have developed a high-throughput workflow that pairs liquid-phase TEM with a U-Net CNN to extract quantitative, nanoscale dynamical and chemical information from noisy, low-contrast videos, something crucial for understanding and designing catalytic nanoparticles.42Fig. 2 illustrates the U-Net-based segmentation of liquid-phase TEM videos. Using physics-informed simulated TEM images for training, they demonstrate fast, robust segmentation and boundary tracking for thousands of particles and apply the method to three key problems: (i) mapping an anisotropic, nanometer-resolved interaction landscape of triangular Au nanoprisms from ≈300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pair trajectories (revealing side-by-side and tip motifs and energy wells ≈−0.4 to −0.55 kBT), which informs how shape and entropic/enthalpic factors steer assembly and active-site exposure; (ii) complete, multi-directional tracking of Au nanorod etching in FeCl3 that uncovers a three-stage, curvature-dependent etching kinetics, initial isotropic etching, a mid-regime where high-curvature (low-coordination) sites etch faster (higher local reactivity), and a late slowdown likely due to product accumulation or oxidant depletion, insight that directly links local curvature/coordination to reactivity and suggests stage-specific levers (etch/regrowth control) for shape-engineered catalysts; and (iii) “individualizing” particles inside connected assemblies to quantify assembly kinetics of concave nanocubes, where substrate adsorption/desorption makes chain growth effectively first-order (desorption-limited), a mechanistic detail relevant to controlling supported catalyst architectures. The work shows that ML–enabled, real-time boundary and trajectory extraction converts liquid-phase TEM from qualitative movies into statistically robust measurements of interaction potentials, local reactivity, and kinetic rates, tools that can directly guide catalytic design (site engineering, shape-selective synthesis, and substrate effects).
000 pair trajectories (revealing side-by-side and tip motifs and energy wells ≈−0.4 to −0.55 kBT), which informs how shape and entropic/enthalpic factors steer assembly and active-site exposure; (ii) complete, multi-directional tracking of Au nanorod etching in FeCl3 that uncovers a three-stage, curvature-dependent etching kinetics, initial isotropic etching, a mid-regime where high-curvature (low-coordination) sites etch faster (higher local reactivity), and a late slowdown likely due to product accumulation or oxidant depletion, insight that directly links local curvature/coordination to reactivity and suggests stage-specific levers (etch/regrowth control) for shape-engineered catalysts; and (iii) “individualizing” particles inside connected assemblies to quantify assembly kinetics of concave nanocubes, where substrate adsorption/desorption makes chain growth effectively first-order (desorption-limited), a mechanistic detail relevant to controlling supported catalyst architectures. The work shows that ML–enabled, real-time boundary and trajectory extraction converts liquid-phase TEM from qualitative movies into statistically robust measurements of interaction potentials, local reactivity, and kinetic rates, tools that can directly guide catalytic design (site engineering, shape-selective synthesis, and substrate effects).
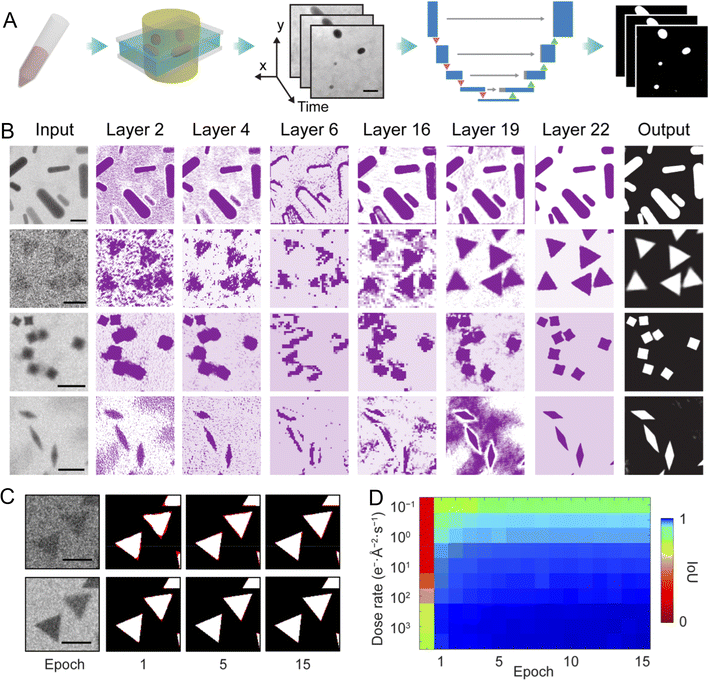 | ||
| Fig. 2 U-Net-based image segmentation for liquid-phase TEM videos. (A) Workflow showing how time-lapse TEM images are segmented using a U-Net model. (B) Representative data flow through the network: simulated TEM images of nanorods, triangular nanoprisms, concave nanocubes, and bipyramids are used as training inputs, pass through successive convolution layers (middle columns), and yield the final segmentation output. (C) Convergence of model predictions toward ground truth across training epochs, where red regions indicate deviation; examples shown for training datasets generated at 1 e− Å−2 s−1 (top) and 10 e− Å−2 s−1 (bottom). (D) Intersection-over-union (IoU) values plotted over training epochs for models trained at ten different dose rates, demonstrating improved segmentation accuracy with continued training. Scale bars: 20 nm in (A); 20 nm, 100 nm, 200 nm, and 100 nm (top to bottom) in (B); and 100 nm in (C). Adapted with permission.42 Copyright 2020, American Chemical Society. | ||
Finite-element modeling approaches have also been applied to interpret DTEM datasets and to simulate structural and dynamic responses under experimental conditions. Recently, Vincent and Crozier used aberration-corrected operando TEM combined with in situ EELS and finite-element reactor modeling to directly link atomic-scale structural dynamics at Pt/CeO2 interfaces with quantitative CO-oxidation activity (Fig. 3).43 ∼1.5–2 nm Pt particles on CeO2(111) were imaged while CO → CO2 conversion was measured and converted to TOFs (0 → 0.80 → 1.05 CO site−1 s−1 at 144, 275, 285 °C respectively). As TOF rises, they observe (i) progressive destabilization and rapid reconfiguration (“fluxionality”) of Pt nanoparticles (loss of lattice-fringe visibility), (ii) increased creation/annihilation of oxygen vacancies at perimeter sites and blurring/outward relaxation of the top CeO2 (111) layer, and (iii) a large rise in a measured “fluxional strain” of the Ce cation sublattice (≈19% → ≈33%), all consistent with enhanced lattice-oxygen transfer during a Mars–van-Krevelen cycle. Image analysis, FT quantification, and EELS + COMSOL corrections for reactor geometry show these dynamics are catalysis-driven (not merely thermal or beam effects). DFT/experimental literature numbers are invoked to argue that lattice-O abstraction by CO is comparatively facile (∼0.4 eV) whereas O2 dissociation/back-fill is harder (∼1.1 eV). Hence, O2 reduction is proposed to occur on the highly reduced CeO2 terraces, with lattice oxygen subsequently migrating to the metal–support perimeter sites where it reacts with CO. Mechanistically, the study identifies Pt–O–Ce interfacial bonds as both anchoring sites and active participants in oxygen transfer. It further shows that active sites in oxide-supported metal catalysts are dynamic ensembles whose fluxionality and local vacancy chemistry are integral to catalytic reactivity; insights that can guide design strategies such as controlling metal size or epitaxy, engineering vacancies, and tuning support strain in oxidation catalysts.
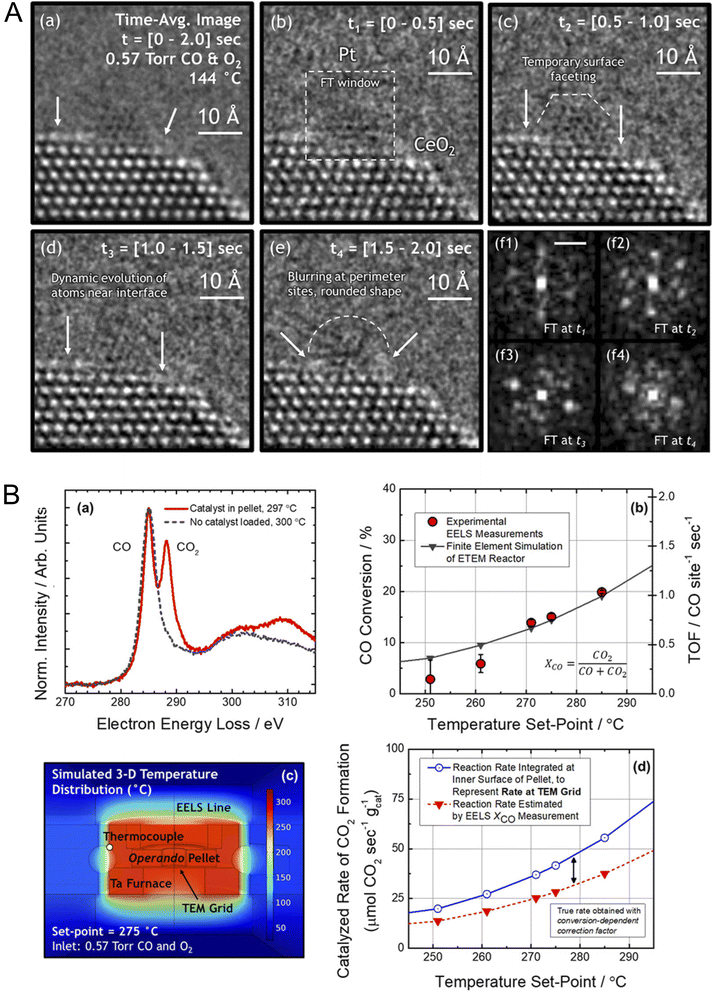 | ||
| Fig. 3 (A) Fluxional behavior of Pt/CeO2 during CO oxidation. In situ ETEM images collected at 144 °C in 0.57 Torr CO + O2 show time-averaged structure (a) and atomic-scale structural changes over 0.5 s intervals (b–e) from 0–2.0 s. (f1–f4) Corresponding Fourier transforms (FTs) from the windowed Pt region indicate evolving lattice order. Images are bandpass-filtered for clarity; FTs are derived from unfiltered, Hanning-windowed regions (modulus shown). Scale bar in (f1): 5.0 nm−1. (B) In situ detection and quantification of CO oxidation in the ETEM reactor. (a) Background-subtracted EELS spectra show CO (C π* peak at 285 eV) converting to CO2 (C π* peak at 288.3 eV), confirming the reaction occurs on Pt/CeO2. (b) CO conversion measured operando by EELS (red) plotted vs. temperature, compared with finite-element reactor simulations (gray). Error bars represent the standard deviation (SD) of five measurements. (c) Simulated temperature distribution in the reactor at a furnace setting of 275 °C. (d) Model-based analysis of product formation provides a conversion-dependent correction factor for determining the catalytic reaction rate from the measured conversion. Adapted with permission.43 Copyright 2021, Springer Nature. | ||
Chi and co-workers have also developed an unsupervised ML pipeline, automatic target generation process (ATGP) pre-conditioned Joint-NMF, that reliably extracts trace encapsulation signals from noisy STEM–EELS spectrum-images and thereby exposes previously hidden strong metal–support interaction (SMSI) features with direct relevance to catalysis.44 By combining outlier removal, local spatial filtering, log-scaling to compress support/thickness intensity differences, ATGP initialization, and a Joint-NMF decomposition, the method separates the Pd nanoparticle, the TiO2 support, a reduced TiOx surface layer, and a distinct sub-nm encapsulation (SMSI) component; it recovers subtle Ti L2,3 energy-loss near-edge structure (ELNES) onset shifts and O K/Ti fine-structure changes that indicate Ti reduction at the Pd–TiO2 interface and a one–two–monolayer, permeable encapsulation layer that would be essentially invisible to conventional background-subtraction or PCA/NMF workflows. Practically, this enables quantitative spatial mapping of encapsulation morphology and chemistry at catalytic interfaces under low-dose conditions, reduces required acquisition time (improving operando feasibility), and gives chemically interpretable endmembers that inform how encapsulation can immobilize particles, modulate active-site exposure, alter local valence/bonding, and thus tune activity/selectivity and stability, insights immediately useful for designing oxide-supported hydrogenation/oxidation catalysts and for extending to EDX, Raman, or in situ datasets where trace interfacial signals govern catalytic function.
Looking ahead, ML is expected to play an increasingly important role in guiding the design and interpretation of TEM experiments. Conventional analyses often prioritize high-contrast features, which can obscure subtle yet catalytically relevant structural motifs; current segmentation approaches likewise remain limited in resolving these fine details.6 Continued developments in computer vision are also expected to facilitate the investigation of more complex, industrially relevant catalyst systems in which multiple structural and chemical variables interact. In the longer term, autonomous operando TEM, integrating microscope control, data acquisition, real-time image processing, correlation with reaction metrics, and physical modeling, offers the potential for dynamically adaptive experiments that reduce redundancy and accelerate mechanistic understanding. As automated sampling and object classification mature, the role of the microscopist will shift from manual image collection toward experimental strategy and interpretation, guided by statistically robust datasets capable of distinguishing catalytically meaningful transformations from unrelated structural changes and thereby revealing the nanoscale origins of activity and selectivity.
5. Applications in catalysis
In catalysis research, the applications of DTEM can be broadly categorized into two areas: (i) investigating catalyst formation and (ii) elucidating catalyst function during chemical transformations. In the first area, for example, Zhang and co-workers used in situ TEM to elucidate the carburization of MoO2 nanoparticles into porous Mo2C under 20 vol.% CH4/H2 (1 bar) at 600–750 °C.45 Mo2C, typically used as a catalyst support, can also act as an active phase with catalytic properties comparable to those of noble metals due to carbon incorporation. The in situ observations revealed defect-initiated nucleation, radial and dendritic pore growth, and sintering at elevated temperatures. Complementary TEM/SAED, STEM-EDX, EELS, and XPS analyses confirmed a phase transition from monoclinic MoO2 to hexagonal Mo2C (with diffraction rings replacing spots), an increase in Mo2+ content, and complete substitution of oxygen by carbon. These transformations led to a substantial increase in BET surface area (from ∼1.11 to ∼5.00 m2 g−1) and the formation of pores with an average width of ≈1.4 nm and length of ≈9.7 nm, thereby enhancing the accessible active surface and potentially affecting TOF, mass transport, and selectivity. Mechanistically, the study revealed that (i) defects act as preferential carburization and nucleation centers; (ii) a ∼58% molar-volume contraction drives pore formation and morphological evolution; and (iii) grain boundaries impede interparticle carbonization, resulting in faster intraparticle growth (peak 2D growth >3 × 103 nm2 s−1). These findings show interface engineering and defect control as effective strategies for tailoring porosity, active-site density, and stability. Correlation of time-resolved imaging with TPSR, diffraction, and spectroscopy further verified that the observed structural evolution was genuinely gas-driven rather than beam-induced.The second area is pursued primarily to address persistent challenges in heterogeneous catalysis, most notably the identification and characterization of active surface sites under realistic, operational conditions. These sites, often at the atomic scale, are responsible for adsorption, surface reactions, and desorption of reactants and products.17,21,26,46,47 Conventional ex situ techniques fail to capture the dynamic nature of these surface processes as they occur in real time. In contrast, DTEM allows for the visualization of structural, compositional, and morphological changes in catalysts as they occur under reaction-relevant environments, including variations in temperature, pressure, and reactant concentrations. This direct imaging capability provides invaluable insights into how catalytic activity and selectivity are linked to specific surface features, such as edges, steps, defects, and exposed crystal planes.
Another key challenge addressed by DTEM is the mechanism of catalyst deactivation, which can occur via sintering, coking, poisoning, or structural reconstruction.17,21 Deactivation reduces the number of active sites, lowers catalytic efficiency, and leads to costly downtime in industrial processes. DTEM enables real-time monitoring of morphological and phase changes in catalysts during operation, thus allowing us to distinguish between different deactivation pathways. For example, the migration and agglomeration of nanoparticles into larger, less active clusters (sintering) can be directly observed under reaction conditions.17 Similarly, carbonaceous buildup (coking) and formation of inactive surface layers or subsurface voids due to diffusion effects (such as the Kirkendall effect) can be studied at high resolution.48 These insights support the development of more robust catalysts with improved long-term stability and resistance to degradation.
DTEM also plays a pivotal role in unraveling the interplay between metal catalysts and their supports, SMSI.49 This relationship greatly influences catalyst performance, particularly in terms of dispersion, stability, and resistance to sintering. For instance, the encapsulation or exsolution of metal particles from oxide supports can dynamically evolve under redox conditions and directly affect the accessibility of active sites. DTEM can potentially elucidate how SMSI can lead to reversible changes in catalyst morphology, such as the formation and dissolution of overlayer structures, and these transformations can be correlated with shifts in catalytic activity. By directly visualizing these interface phenomena, we can better tailor catalyst–support combinations and design materials with tunable activity and reactivity profiles.
By integrating DTEM with spectroscopy techniques such as DRIFT, we can then correlate morphological changes (size and shape) with chemical modifications.21 Coupling these observations with product analysis via GC or MS further transforms in situ to operando investigations, linking structural evolution directly with catalytic performance. One point to note is that TEM provides atomic-scale insights into catalyst structures, and hence its localized nature benefits significantly from complementary integral techniques.6 For instance, when analyzing crystalline or amorphous bulk phases, high-resolution (S)TEM can be paired with XRD, including Rietveld analysis and X-ray reflectometry (XRR), to gain a more averaged understanding of phase composition across a larger sample volume. Similarly, when investigating structural defects or strain, whole powder pattern modulation in XRD complements TEM findings by offering broader statistical information. For assessing surface structures, combinations with surface-sensitive spectroscopies, such as XPS, LEIS, and XRR, are applicable. For particle size distribution or coherent domain sizes, TEM data can be reinforced with integral techniques like Raman spectroscopy and Rietveld-refined XRD. Likewise, to map the spatial distribution of particles, SEM can supplement TEM, offering geometrical feature and broader field imaging. Meanwhile, EDX and EELS within TEM setups yield elemental compositions, which can be extended to bulk or surface-level compositional data through techniques like X-ray fluorescence (XRF), inductively coupled plasma optical emission spectroscopy (ICP-OES) and XPS. When going into the electronic structure of catalysts, EELS results can be correlated with findings from near-edge X-ray absorption fine structure (NEXAFS) and UV/visible spectroscopy, which offer complementary insights across broader sample regions. For phase identification, electron diffraction and convergent beam electron diffraction (CBED) from TEM provide localized crystallographic information, while XRD gives ensemble-averaged data that can confirm phase assignments. Equally important, structural relationships such as nearest-neighbor distances derived from electron diffraction pair distribution functions are supported by extended X-ray absorption fine structure (EXAFS) measurements, which offer integral views of the local atomic environment. Together, these multimodal approaches offer a more complete, statistically robust understanding of catalytic materials, bridging the gap between nanoscale insights and macroscopic catalytic function.
It should be noted, however, that analytical techniques commonly used in catalysis are not necessarily compatible with DTEM. For example, direct coupling of temperature-programmed desorption (TPD) with in situ or operando TEM is not feasible because TPD requires tightly controlled vacuum and gas dosing conditions that conflict with the gas-flow and pressure environments used during in situ imaging. Instead, the same catalyst can be characterized by TPD before and after an in situ TEM experiment to correlate surface adsorption properties with the observed structural evolution. Similarly, porosity and surface area, which are beyond the reach of TEM, can be evaluated through physisorption methods such as BET analysis.
One elegant example was provided by Yu and co-workers, who investigated the structural evolution and catalytic performance of Cu particles under operando conditions during ethylene oxidation.50Fig. 4 shows the temperature-dependent morphological evolution of Cu nanoparticles during ethylene oxidation. At low temperatures (200–300 °C), the particles maintain a stable hollow structure, while increasing the temperature to 400–700 °C induces significant structural dynamics such as collapse, fragmentation, reshaping, and migration due to redox activity. At higher temperatures (800–950 °C), the particles become more static and undergo sintering into larger, rounder shapes, with reduced mobility due to support degradation and carbonaceous deposits. Combined with online MS, three distinct catalyst regimes have been identified over a temperature range of 200–950 °C: (i) a low-temperature regime (≤500 °C) where Cu exists as Cu2O, promoting selective partial oxidation products like ethylene oxide (EO) and acetaldehyde (AcH); (ii) an intermediate-temperature regime (600–800 °C) marked by dynamic Cu0/Cu2O phase oscillations, enhancing reactivity but favoring total oxidation (CO2); and (iii) a high-temperature regime (≥900 °C) dominated by metallic Cu with a Cu2O monolayer, which continues to favor total oxidation. These dynamic transformations, influenced by interfacial strain and reduction-oxidation kinetics, challenge conventional ultra-high vacuum (UHV) findings that attributed high EO selectivity to metallic Cu surfaces. In-depth theoretical calculations using density functional theory (DFT) supported the experimental findings, showing that pristine Cu2O is more favorable for AcH production via the oxometallacycle pathway, whereas partially reduced Cu2O and strained oxide-metal interfaces favor direct EO formation with lower activation barriers. The study demonstrates that redox oscillations induce non-stoichiometric and strained oxide states that enhance selectivity toward EO before shifting toward combustion–dominant pathways at higher temperatures. The integration of operando TEM, in situ DRIFTS, and DFT allows for an unprecedented visualization of structure–performance relationships.
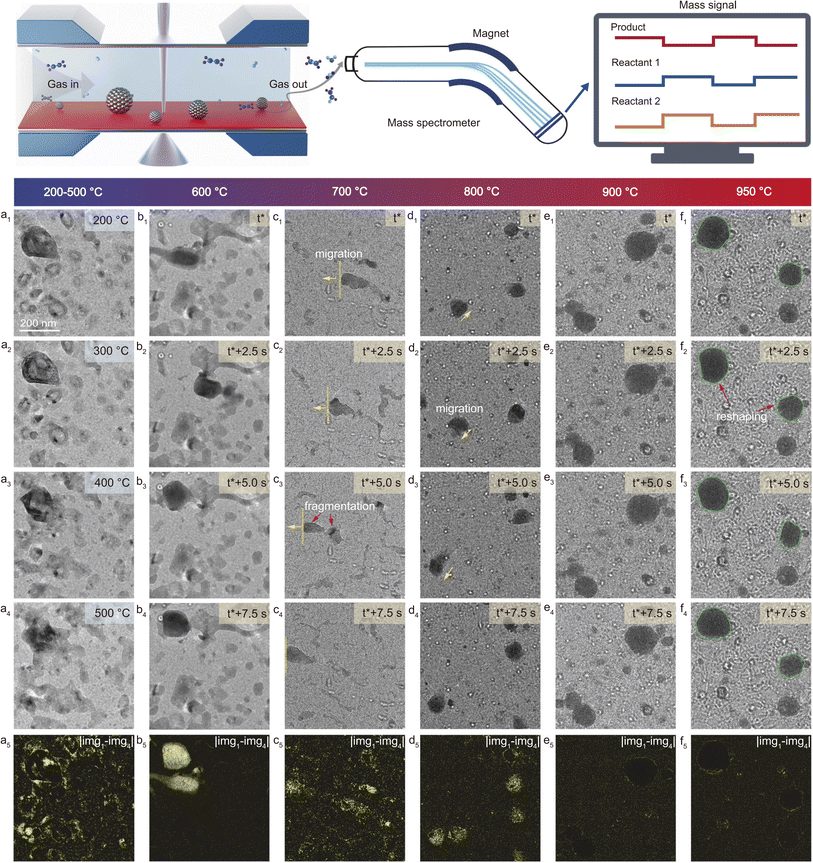 | ||
| Fig. 4 Schematic of operando technique involving TEM to investigate the temperature-dependent morphological evolution of Cu nanoparticles during ethylene oxidation. Panels (a1–f4) show in situ TEM images of Cu nanoparticles recorded at increasing temperatures under ethylene oxidation conditions (total pressure: 708 mbar; pO2 = 4.27 mbar; pC2H4 = 170.6 mbar). Each subpanel (a1–a4, b1–b4, etc.) captures sequential snapshots over time at a specific temperature, illustrating structural changes such as collapse, reshaping, migration, and sintering. Panels (a5–f5) display the difference images between the first and fourth frames at each temperature, highlighting the extent of morphological transformation. Adapted with permission.50 Copyright 2025, Springer Nature. | ||
One concern is that our primary aim in using DTEM is essentially to clarify the dynamic reaction conditions relevant to the development of catalytic systems (operando studies). The timescale considered ranges from a few milliseconds, typical for time-dependent changes limited by mass and heat transfer, to a few hours, characteristic of restructuring, surface segregation, and the lower end of sintering processes. However, conventional TEM is suited for processes on the µs–h timescale and nm–mm length scale (Fig. 5),51,52 with accessible ranges dictated by instrument capabilities. The spatiotemporal resolution also determines how reliably catalyst structure can be correlated with function. For example, in photocatalytic reactions, catalytic cycles and active-site lifetimes occur on femto- to nanosecond timescales, which lie beyond the temporal reach of most operando methods. Only ultrafast pump–probe spectroscopy provides the necessary temporal resolution to probe charge-transfer dynamics and surface species, though its application remains largely restricted to photoactive materials and photocatalytic reactions due to the nature of the “pump.”
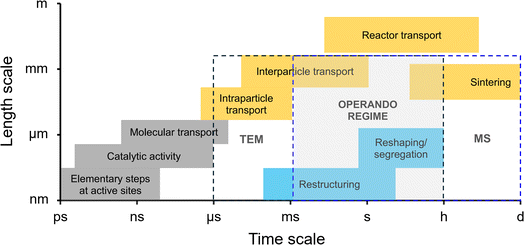 | ||
| Fig. 5 Time and length scales of catalytic processes relative to the ranges accessible to conventional TEM. Gray denotes elementary active–site processes, orange indicates solid-state catalyst dynamics, and blue represents reactant/product transport. The timescale relevant to dynamic operation spans microscopic to macroscopic domains.51,52 Techniques used for operando studies are TEM and MS. Operando measurements are feasible only within the region where TEM and MS capabilities overlap (gray box). | ||
Despite its limitations, DTEM has emerged as a transformative tool in catalysis, enabling direct observation of the structural dynamics of catalytic materials under relevant conditions. By revealing how catalysts respond to environmental stimuli at the atomic level, DTEM helps bridge the knowledge gap between static structural characterization and actual catalytic function. Its applications span various catalyst systems, including supported metal nanoparticles, single-atom catalysts, and oxide-based materials, each of which presents distinct structural and dynamic behaviors crucial to their performance.
5.1 Supported metal nanoparticles
Supported metal nanoparticles are among the most extensively studied catalytic systems due to their high surface-to-volume ratio and tunable properties. They are often prerequisite for achieving high catalytic efficiency, such in photocatalysis and electrocatalysis. During operation, these nanoparticles undergo structural changes such as coalescence, reshaping, and facet reorganization, all of which directly influence activity, selectivity, and stability. DTEM provides real-time insights into these phenomena, revealing mechanisms that are often inaccessible through ensemble-averaged or ex situ methods. In situ ETEM, for instance, can be used to probe the sintering behavior of metal nanoparticles under oxidative and reductive gas environments at elevated temperatures. In such conditions, sintering often proceeds through migration and coalescence rather than Ostwald ripening, as dynamic gas atmospheres enhance nanoparticle mobility on the support surface. Such observations help explain catalyst deactivation mechanisms in high-temperature applications.DTEM has also been used to observe the reversible formation of oxide shells on metal nanoparticles during redox cycles. In one study, real-time imaging revealed that Cu nanoparticles undergo dynamic redox transformations, forming and reducing Cu2O shells as the ambient gas composition is alternated between oxidizing and reducing environments.19 These observations provide atomic-scale evidence of oxidation state fluctuations and surface reconstruction, which are key factors that govern catalytic TOF and selectivity. Additionally, nanoparticle morphology can undergo facet reconstruction under reactive atmospheres, leading to changes in catalytic performance. For example, Rh and Pd nanoparticles have been shown to restructure from low-index to high-index facets under CO or O2 environments, a behavior linked to changes in adsorption energy landscapes on different surfaces.19 DTEM enables direct visualization of these transformations, thereby establishing structure–function correlations with temporal resolution.
Supports such as ceria can dynamically trap or release metal atoms, depending on their redox state and local oxygen vacancy concentration. In one study, single Pt atoms were found to dynamically coordinate with oxygen atoms at CeO2 surfaces during oxidative treatment, while reductive conditions promoted their migration and potential clustering.55 These findings suggest that redox cycling of the support not only affects catalytic activity but also the structural stability of the catalyst, warranting further investigation using DTEM. Moreover, dynamic coordination changes of single-atom catalysts can also be monitored using time-resolved EELS, which can detect subtle shifts in the local electronic structure and oxidation state during reaction. This multimodal approach, combining imaging and spectroscopy, has proven especially valuable in revealing the nature of the active site in single-atom catalysts, which is often a moving target during catalysis.56
Beyond ceria, other oxides such as V2O5, TiO2, and perovskites have been studied using DTEM to monitor phase transitions, cation diffusion, and morphological evolution under reducing or oxidizing environments. For example, the migration of transition metal cations within the oxide lattice during redox cycling can be tracked using high-resolution imaging and EELS, providing mechanistic insights into phenomena such as lattice oxygen participation in catalysis (the Mars–van Krevelen mechanism).58
In bimetallic catalysts, for example, DTEM has been used to visualize the redistribution of atomic species during thermal or reactive annealing, shedding light on dealloying and segregation phenomena that affect catalyst stability and activity.4 Core–shell structures, which are widely used to tune selectivity and suppress deactivation, have been investigated using in situ TEM to study shell growth, dissolution, and reconstruction under various gas compositions. In one case, the shell of a Pt@Au catalyst was observed to undergo reversible faceting and alloying with the core under reducing conditions, a process that dramatically altered the catalyst's CO oxidation behavior.4 Heterostructured oxides, such as layered perovskites or mixed-metal oxides, present additional challenges due to their complex crystal chemistry. TEM operated in a dynamic manner enables the observation of interlayer diffusion, local amorphization, and vacancy clustering, all of which contribute to reactivity. In these systems, combining TEM with in situ spectroscopy is particularly valuable, as changes in local chemistry and bonding are closely coupled to structural rearrangements.
As also pointed out earlier here, in most cases, DTEM is used for two primary purposes: (i) to monitor the formation of the active center during catalyst preparation and (ii) to observe catalytic reactions occurring at the active center. The latter is typically more complex and hence challenging to achieve. To address the challenge of probing complex catalytic processes, Marks and co-workers recently employed single-molecule atomic-resolution time-resolved electron microscopy (SMART-EM) to elucidate the mechanisms of single-site heterogeneous catalysis (SSHCs).59 SMART-EM enables real-time, atomic-scale visualization of dynamic catalytic processes that traditional ensemble techniques such as EXAFS, XANES, XPS, and even conventional electron microscopy cannot resolve. By applying SMART-EM to a carbon-supported MoO2 catalyst system used for H2 production via alcohol dehydrogenation, they were able to directly observe and record catalytically significant intermediates, such as Mo–alkoxide complexes, hemiacetals, and aldehyde-derived oligomers, on the catalyst surface (Fig. 6). SMART-EM uniquely captured stochastic conformational changes and transient states of individual molecules, such as the real-time conversion of a hemiacetal intermediate (4EtI) back to its alkoxide form (2EtI), an event that revealed kinetic details and molecular dynamics at millisecond resolution. These observations enabled the determination of key thermodynamic and kinetic parameters like activation energies and conformer lifetimes, based on the Nyquist frequency and the ergodic principle of statistical mechanics. Notably, SMART-EM enabled the direct identification of four key reaction intermediates that had remained elusive in bulk measurements: revealing a reaction pathway involving alcohol O–H and C–H activation, hydride transfer, H2 elimination, and aldehyde oligomerization. Integration with theoretical modeling and spectroscopic data validated the proposed catalytic cycle and demonstrated the capability of SMART-EM to visualize surface-bound chemistry with unprecedented clarity.
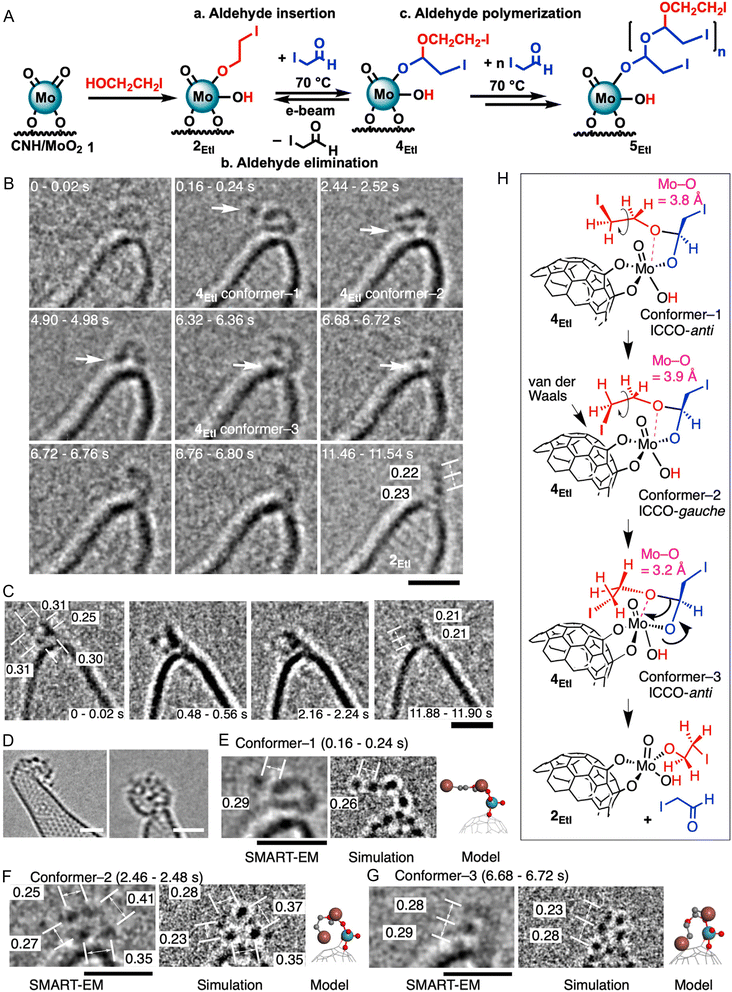 | ||
| Fig. 6 Conformation and reaction analysis of 4EtI in the SSHC 1 system. (A) Proposed aldehyde polymerization pathway. (B and C) SMART-EM images showing aldehyde elimination from 4EtI to 2EtI at 50 fps under electron dose rates (EDRs) of 6.2 × 106 (B) and 4.13 × 107 e− nm−2 s−1 (C), displayed as 20–80 ms per frame; white arrows indicate iodine. (D) Images of aldehyde oligomers (5EtI) on the catalyst, recorded at 50 fps and EDRs of 3.4–4.9 × 107 e− nm−2 s−1. (E–G) Structural models of 4EtI conformers 1–3. (H) Proposed transformation pathway via sequential C–C bond rotations; Mo–O distances are highlighted in pink. The anti-conformation dominates in 2EtI, with iodine in conformers 2 and 3 interacting with CNH. Scale bars: 1 nm; image-simulation deviation <0.05 Å. Adapted with permission.59 Copyright 2025, Elsevier. | ||
One important point is that although DTEM excels at resolving atomic-scale transformations, its true significance in catalysis lies in correlating these structural dynamics with key performance metrics such as activity, selectivity, and stability. DTEM elucidates how nanoscale phenomena, including particle sintering, oxidation-state oscillations, and single-atom migration, translate into measurable changes in catalytic output. By bridging structural observation and functional behavior, DTEM provides a mechanistic foundation for understanding active-site evolution, deactivation pathways, and metal–support interactions under realistic reaction conditions. Unlike ensemble-averaged spectroscopic methods, DTEM uniquely captures the spatiotemporal evolution of individual active centers, revealing how transient oxidation states, interfacial strain, and particle morphology modulate reactivity and selectivity. Integrating these atomic-level observations with macroscopic performance metrics such as turnover frequency, conversion, and selectivity establishes a direct causal link between local dynamics and overall catalytic behavior. These insights enable rational catalyst design strategies aimed at stabilizing active sites, optimizing support interactions, and mitigating deactivation, ultimately translating into enhanced catalytic performance under operando conditions. Table 4 maps the structural dynamics observable by DTEM to their corresponding catalytic performance metrics across representative catalyst classes. Each category highlights key atomistic processes, mechanistic implications, and practical validation methods, providing a quantitative framework for interpreting DTEM observations in catalysis research.
| Catalyst class/reaction context | Dynamic structural phenomena observable by DTEM | Linked catalytic metrics | Typical mechanistic correlation and functional insight revealed | Recommended correlative validation/complementary measurements |
|---|---|---|---|---|
| Supported metal nanoparticles (e.g., Pt, Cu, Ni, Co) | • Particle reshaping, facet reconstruction, surface atom diffusion, sintering, and dynamic oxide shell formation under reactive gases | • TOF, conversion rate, selectivity, and deactivation rate | • Changes in morphology or crystal facets alter adsorption energies (e.g., CO or H2), modifying activation barriers and selectivity | • Operando gas analysis (MS/GC), EELS for oxidation state mapping, ex situ particle-size statistics, DFT for facet-dependent energetics |
| • Dynamic oxide formation can transiently generate active oxygen species or self-heal surfaces | ||||
| Single-atom catalysts (SACs, e.g., Pt1/CeO2, Pd1/Fe2O3) | • Atom migration, trapping at vacancies, reversible clustering, and re-dispersion under redox or thermal cycles | • Intrinsic TOF per site, selectivity, stability, and activation energy | • Coordinative environment directly affects chemisorption and charge transfer | • TR-EELS or XAS for oxidation-state tracking, ML-assisted trajectory analysis of atom motion, isotopic switching to correlate with product formation |
| • Dynamic clustering reduces active single-site density but may form transient ensembles beneficial for multielectron reactions | ||||
| Bimetallic and alloy catalysts (e.g., Pt–Ni, Pd–Au, Cu–Zn) | • Surface segregation, atomic ordering/disordering, reversible alloy formation, core–shell restructuring | • Rate constants, temperature-dependent selectivity, alloy stability window | • Surface composition dynamically tunes ensemble and electronic effects, altering adsorption strength and activation barriers | • Time-resolved EDX mapping, operando XAS for coordination tracking, gas-phase product analysis vs. temperature ramps, DFT surface–model correlation |
| • Segregation often correlates with selectivity drift at elevated temperature | ||||
| Metal oxide catalysts (e.g., CeO2, TiO2, MnOx, Co3O4) | • Oxygen vacancy formation/migration, lattice relaxation, redox cycling, phase transformation (oxide ↔ suboxide) | • Redox kinetics, oxygen exchange rate, and selectivity in oxidation reactions | • Vacancy concentration and diffusion control Mars–van Krevelen reactivity | • In situ EELS for oxygen K-edge shifts, O 1s XAS for vacancy quantification, oxygen-isotope labeling combined with MS, temperature-programmed reaction monitoring |
| • Strain-coupled vacancy dynamics modulate oxygen mobility and thus rate-determining steps | ||||
| Carbide/nitride catalysts (e.g., Mo2C, VN, WC) | • Surface oxidation/reduction, carbon/nitrogen leaching, structural reconstruction under reaction conditions | • Activity in hydrogenation/dehydrogenation, stability under cycling | • Surface termination changes (M–C/N–O) alter d-band occupancy and reactivity | • Operando DRIFTS for adsorbate monitoring, EELS edge analysis for C/N bonding, gas switching (H2vs. CO2) cycles to probe reversibility |
| • Surface depletion leads to deactivation | ||||
| Catalysts prone to carbon formation (e.g., Ni/Al2O3, Fe, Co) | • Nucleation and growth of carbon filaments, amorphous carbon encapsulation, particle detachment | • Deactivation rate, coke yield, selectivity to CO/CO2 | • Filamentous carbon indicates catalytic growth; amorphous carbon signals poisoning | • Operando MS (CO/CO2/H2 balance), post-reaction Raman for carbon speciation, image correlation of same region over time, temperature-programmed oxidation |
| • DTEM reveals location and rate of carbon deposition, clarifying deactivation pathways | ||||
| Electrocatalysts and liquid-cell systems (e.g., NiFe LDH, Pt/C) | • Surface reconstruction under bias, dissolution/re-deposition, nanobubble formation, morphological coarsening | • Current density, overpotential, faradaic efficiency, and long-term stability | • Applied potential induces cation migration or amorphization | • Synchronized chronoamperometry or EIS, bubble tracking analysis, STEM-EELS for oxidation states, ex situ XPS validation |
| • Reconstructed oxyhydroxide phases often serve as real active sites | ||||
| • Gas evolution morphology impacts local mass transfer and efficiency | ||||
| Photocatalysts/ultrafast processes (e.g., TiO2, plasmonic Au, CdS) | • Transient lattice distortion, charge-carrier-induced expansion/contraction, surface plasmon oscillation, electron–phonon coupling | • Quantum yield, charge separation lifetime, photoreaction rate | • Structural relaxation dynamics determine charge-carrier recombination | • Ultrafast TEM (pump–probe), transient absorption spectroscopy (TA), synchronized photocurrent detection, DFT-based dynamic charge modeling |
| • Hot-electron transfer correlates with transient lattice expansion amplitude | ||||
| Core–shell and multicomponent catalysts (e.g., Cu@ZnO, Pt@SiO2) | • Interface diffusion, shell cracking, alloy intermixing, support encapsulation/exsolution | • Composite activity, selectivity, and thermal stability | • Interface evolution changes electron density and strain at active sites | • Element-specific EDX/EELS mapping over time, operando XAS for interface coordination, cross-section imaging before/after reaction |
| • Exsolution can generate new interfacial sites improving turnover | ||||
| Perovskite and mixed-metal oxides (e.g., La1-xSrxCoO3, BaTiO3) | • Dynamic surface reconstruction, A-/B-site cation segregation, defect ordering, amorphization under bias or temperature | • O2 evolution rate, overpotential, conductivity, and phase stability | • Surface amorphization creates active oxyhydroxide layers; cation segregation correlates with performance decay and instability | • Simultaneous electrochemical readout, time-resolved SAED for phase evolution, operando EELS for cation valence states |
| Molecular or cluster catalysts (e.g., Ru(II) complexes, Cophthalocyanines) | • Formation and decomposition of surface-bound intermediates, ligand reorganization, cluster coalescence | • Product selectivity, intermediate lifetime, TOF | • DTEM visualizes transient intermediate geometries, clarifying elementary reaction steps and selectivity control | • SMART-EM or liquid-cell TEM, correlative IR/DRIFTS or Raman spectroscopy, kinetic modeling of observed pathways |
6. Challenges
Despite its powerful capabilities, DTEM faces several challenges that must be addressed to enable broader adoption and reliable interpretation. These include beam-induced artifacts, limitations in simultaneously achieving high spatial and temporal resolution, difficulties in correlating structural observations with catalytic performance, and reproducibility issues arising from sample variability and complexity. In this section, we discuss these challenges and outline potential mitigation strategies.6.1 Electron beam effects
One of the most prominent limitations of DTEM is the effect of the electron beam on the sample. Beam–sample interactions can lead to radiolysis, knock-on damage, heating, and beam-induced chemical reactions, particularly when imaging beam-sensitive catalysts such as defect-engineered metal oxides or organic–inorganic hybrids.65–67 In ETEM or liquid cell TEM, the interaction of the beam with the gas or liquid medium generates radicals, solvated electrons, and other reactive species, which can further alter the chemistry of the system.68 This is especially problematic in catalysis studies, where distinguishing intrinsic catalytic dynamics from beam-induced effects is essential. Beam-induced sintering or redox changes may obscure the true catalytic mechanisms. Strategies to mitigate beam effects include the use of low-dose imaging protocols, beam blanking between acquisitions, reduced accelerating voltage, and cryogenic methods to limit damage.65,67,69 Careful experimental design is essential. This includes comparing illuminated and non-illuminated regions, acquiring reference datasets under low beam exposure, and using dose-rate models to guide imaging parameters.65 Quantitative dose mapping and dose–response measurements are necessary to verify that observed structural changes are reaction-driven rather than beam-induced.6.2 Limits of spatial and temporal resolution
DTEM is fundamentally constrained by trade-offs between spatial and temporal resolution. High spatial resolution requires a stable, focused electron beam with sufficient SNR, while capturing fast dynamics necessitates high frame rates and short exposure times. Achieving both simultaneously is difficult due to camera limitations, drift, and beam damage sensitivity. ETEM setups are generally limited to time resolutions on the order of milliseconds to seconds, constrained by frame rates and detector speed.70 Meanwhile, UTEM can access femtosecond–nanosecond regimes using pulsed beams, but often at the cost of reduced spatial resolution and SNR due to fewer electrons per pulse.70–72 While UTEM presents challenges such as lower SNRs due to reduced electron dose per pulse and the need for complex synchronization, its ability to resolve ultrafast events makes it a powerful tool for advancing the understanding of catalytic mechanisms. Advances in direct electron detectors and drift-correction techniques, including ML-based denoising, have helped address these challenges.29,73 Nonetheless, long-duration experiments often suffer from cumulative drift, while fast processes may still be missed. Stage stability, vibration isolation, and temperature control all remain vital for maintaining spatial precision during time-lapse imaging.656.3 Correlating observations with catalytic performance
Establishing quantitative correlations between observed structural dynamics and actual catalytic performance is another challenge. While atomic-scale imaging can reveal sintering, defect migration, or oxidation state changes, it is often unclear how these features map onto metrics like TOF or selectivity. This is partly because DTEM operates under non-industrial pressures (typically <10 Torr) and often in simplified geometries. These do not always represent real catalytic conditions.28,74 Furthermore, product analysis is rarely integrated with the microscope, making it difficult to track reaction outputs during imaging. Recent developments in microreactor-based holders and hybrid ETEM–MS platforms have begun to address this, enabling more realistic gas environments and operando analysis.28,30 However, alignment of structural, chemical, and activity data in space and time remains complex, particularly in heterogeneous samples. It is important to design experiments that include internal performance proxies, such as support conductivity changes, evolving diffraction patterns, or gas composition monitoring, alongside structural imaging to infer functional behavior.746.4 Reproducibility and sample variability
Reproducibility in DTEM experiments remains a long-standing concern, arising from variations in sample preparation, mounting, and holder design. Inconsistent FIB-thinning, drop-casting, or deposition protocols can introduce strain, contamination, or oxidation, affecting observed dynamics.75 Additionally, the small sampling volume of TEM makes it difficult to generalize findings to bulk materials or powder-bed catalysts. The use of MEMS-based chips offers improved consistency, but even these require careful calibration, including confirmation that electrical and thermal contacts are functioning correctly.74,75 Variability in chip design and sample geometry also complicates comparison across different platforms or research groups. Standardization efforts, including shared protocols, metadata reporting, and reference materials, are now gaining traction in the electron microscopy community. Cross-platform benchmarking needs to be realized, especially for in situ devices and low-dose methodologies.76 The emergence of centralized databases for storing raw and processed TEM data, complete with dose metrics and acquisition conditions, is another promising development. These databases support reanalysis and ML training, improving both transparency and generalizability of findings.76,77Table 5 summarizes key challenges when using DTEM, and how to address them. Beam damage remains a fundamental obstacle, particularly for sensitive nanomaterials, as prolonged electron exposure can alter or destroy the sample. Limited temporal resolution further hampers our ability to observe fast catalytic events, making it difficult to capture transient intermediate states. Environmental constraints, such as maintaining realistic gas or liquid reaction conditions within the TEM, limit the applicability of standard setups. Also, issues like sample drift, data interpretation, and reproducibility highlight the need for advanced instrumentation and analytical methods to ensure meaningful and reliable results.
| Challenge | Description | How to address it |
|---|---|---|
| Beam damage | • High-energy electron beams can alter or destroy sensitive catalyst structures and surface-active sites | • Use low-dose imaging techniques |
| • Employ cryo-TEM or ETEM | ||
| • Optimize beam current and exposure time | ||
| Temporal resolution limitations | • Difficulty capturing fast catalytic events or transient states | • Use pulsed electron beams (e.g., UTEM) |
| • Combine with pump-probe methods | ||
| • Use high-frame-rate cameras | ||
| Environmental constraints | • Reactions often occur under gas/liquid environments not compatible with traditional TEM | • Use ETEM or liquid-cell TEM |
| • Develop specialized holders (e.g., MEMS-based cells) | ||
| Sample drift and stability | • Thermal or mechanical instability during time-resolved imaging | • Use drift correction software |
| • Improve stage and holder stability | ||
| • Minimize temperature gradients | ||
| Limited field of view | • Difficult to monitor large areas or multiple particles simultaneously | • Perform correlative imaging across multiple scales |
| • Use ML for automated, high-throughput analysis | ||
| Interpretation of dynamic data | • Complexity in analyzing and interpreting real-time changes | • Combine with complementary techniques (e.g., spectroscopy) |
| • Develop advanced image processing and data analysis tools | ||
| Reproducibility and control | • Achieving controlled and reproducible reaction conditions in situ | • Use microfabricated reactors with precise control |
| • Standardize protocols for gas flow, temperature, and pressure |
7. Experimental design principles for reliable operando TEM studies
Among in situ, time-resolved, and operando TEM approaches, the operando method is overall the most reliable for probing catalytic dynamics, as it enables simultaneous structural, chemical, and performance analysis under realistic reaction conditions, directly linking nanoscale changes to catalytic activity. In contrast, in situ TEM lacks real-time reaction feedback, and TR-TEM, although ultrafast, is restricted to high-vacuum operation. Therefore, we dedicate this section to discuss practical strategies for achieving reliable operando TEM. A key step toward improving rigor is the establishment of clear, quantitative guidelines for experiment planning. The following discussion provides guidance for designing, executing, and validating operando TEM experiments, with emphasis on beam–sample interactions, dose calibration, temporal control, environmental stability, and reproducibility.The first consideration in any operando TEM study is the control of beam–sample interactions, which depend strongly on the material system and reaction environment.78 Electron-induced damage may arise from knock-on displacement, radiolysis, or localized heating, each requiring distinct mitigation strategies. For robust metallic catalysts, acceptable dose rates typically range from 103 to 104 e− Å−2 s−1, whereas beam-sensitive oxides, sulfides, and hybrid materials should be maintained below 103 e− Å−2 s−1.79 Low-dose imaging protocols are therefore essential and may include minimizing the convergence angle (≤10 mrad), shortening dwell time, and employing sparse scanning patterns. For prolonged observations, beam blanking, temporarily deflecting the electron beam between acquisition intervals, can reduce cumulative exposure by up to 90% (corresponding to a 10% duty cycle).80 This approach is particularly useful for slow morphological evolutions such as sintering or redox cycling. For organic–inorganic hybrids, hydrated catalysts, or liquid-phase systems, cryo-TEM offers additional protection by immobilizing radicals and suppressing radiolysis. Operating below 100 K should minimize atomic mobility and stabilize volatile intermediates, extending imaging lifetimes.
Quantitative dose calibration and monitoring are important to achieving reproducibility. Each experiment should include a quantitative dose map derived from beam current, pixel size, and dwell time.79 Comparing irradiated and unirradiated regions enables separation of reaction-driven transformations from beam-induced effects. Dose–response experiments, in which identical regions are imaged at progressively increasing dose rates, can reveal threshold values beyond which structural or chemical changes become apparent.81 Plotting these changes as a function of accumulated dose allows distinguishing intrinsic reaction kinetics from beam-stimulated artifacts. Automated dose-tracking software should also facilitate real-time quantification of total electron exposure, further supporting consistent dose management across experiments.
Moreover, temporal resolution must be matched to the characteristic timescales of the studied process. Structural events such as surface diffusion, particle coalescence, and oxidation typically occur on millisecond to second timescales and can be captured using high-frame-rate cameras operating between 103 and 105 frames per second.80 Faster processes, including charge-carrier motion or lattice vibrations, demand UTEM or pump–probe configurations with femtosecond to nanosecond resolution. Temporal undersampling may obscure transient states, whereas oversampling increases data redundancy and beam exposure. Thus, the optimal frame integration time represents a balance between time fidelity and SNR. Modern ML–based denoising and compressed sensing algorithms now permit the reconstruction of intermediate frames, enabling apparent frame rates above 105 fps without additional exposure.
Accurate environmental and thermal control is equally essential for operando reliability. Modern MEMS-based nanoreactors provide precise control of temperature and pressure, often allowing operation up to 1000 °C and several hundred millibar.82 Nevertheless, local temperature gradients and gas-flow instabilities may distort kinetics. Embedding thermocouples or resistive sensors within the MEMS chip enables direct calibration of the reaction zone.83 Gas flow rates between 0.1 and 5 mL min−1 typically maintain a stable reactant environment while minimizing turbulence. Before initiating data acquisition, gas composition and temperature should be stabilized for several seconds to ensure steady-state conditions. We recommend temperature ramp rates slower than 5 °C s−1 to prevent stage drift and thermal shock to the specimen.
Equally important, ensuring validation and reproducibility is the cornerstone of reliable operando TEM. To verify that observed structural dynamics are reaction-driven, rather than beam- or environment-induced, control experiments should be conducted under inert atmospheres such as Ar or N2, at zero electrical bias, or under dark (no-illumination) conditions. Direct comparison of these control datasets with active-state measurements enables discrimination between intrinsic and extrinsic effects. Experimental reproducibility requires repeated measurements across multiple particles or regions, ideally with at least three independent replicates per condition. Consistent documentation of all operational parameters: electron energy, beam current, exposure time, temperature, pressure, and gas composition, is essential for meaningful cross-laboratory comparison. Adopting standardized metadata formats for operando datasets will further promote transparency and reproducibility.
Data analysis and documentation are also important for ensuring transparency and reproducibility. While post-processing methods such as drift correction, motion compensation, and ML-based segmentation can enhance data interpretability, all processed results should be accompanied by their corresponding raw datasets. Maintaining both data forms enables independent validation and facilitates reuse in ML applications. The implementation of metadata-rich repositories that adhere to the FAIR (Findable, Accessible, Interoperable, and Reusable) data principles is strongly encouraged for DTEM studies to ensure traceability, transparency, and long-term accessibility. DTEM generates inherently complex and multidimensional datasets, such as 4D-STEM, EELS, EDX, and time-resolved image sequences, that are difficult to reproduce or reinterpret without well-structured metadata. Adopting FAIR practices ensures that datasets are findable through persistent identifiers (e.g., DOIs), accessible via open repositories, interoperable through standardized, non-proprietary file formats, and reusable through comprehensive documentation of beam parameters, environmental conditions, and acquisition settings. In this context, FAIR data management enables reproducibility of observed dynamics, facilitates reanalysis using emerging algorithms, and supports long-term data preservation and citation across the research community.
Table 6 presents the quantitative parameters, operational benchmarks, and validation procedures recommended for the design and execution of reliable operando TEM experiments. Each parameter is linked to the corresponding experimental decision criterion and validated through measurable indicators such as lattice-fringe integrity, EELS edge evolution, or gas-flow stability. By articulating both the numerical benchmarks and the mechanistic rationale behind each guideline, this framework offers a quantitative basis for comparing experimental conditions across laboratories.
| Design aspect | Typical parameter range/benchmark | Recommended practice or decision criterion | Validation and cross-checks | Typical materials/systems | Common pitfalls | Mitigation strategy |
|---|---|---|---|---|---|---|
| a Notes:Proper control of beam dose, environmental stability, and metadata documentation ensures that observed dynamics are intrinsic to the reaction rather than experimental artifacts. b Cumulative dose is a way to calculate the electron dose delivered to a sample in TEM, representing how many electrons strike each unit area of the sample. c I is beam current (number of electrons per second) in A (ampere), t is exposure time in s (seconds), A is irradiated area on the sample in m2 (or nm2), and e is elementary charge (charge of a single electron) in 1.602 × 10−19 C. | ||||||
| Beam-dose managementa | • 102–103 e− Å−2 s−1 for beam-sensitive oxides, sulfides, or hybrid catalysts; 103–104 e− Å−2 s−1 for robust metals or carbides | • Choose dose according to damage mechanism: (i) knock-on → lower accelerating voltage (80–200 kV); (ii) radiolysis → reduce probe current; (iii) thermal → short dwell time (<10 µs pixel−1) | • Perform dose–response series to identify structural thresholds | • Oxides (CeO2, TiO2), MOFs, carbon supports | • Overexposure induces reduction, vacancy formation, or sintering | • Use low-dose, intermittent illumination, and dose-rate monitoring in real time |
| • Total cumulative dose <105 e− Å−2 per regionb | • Record cumulative dose = (I × t)/(A × e)c | • Compare unirradiated reference regions | ||||
| • Track lattice order by FFT intensity decay or EELS edge shifts | ||||||
| Beam blanking/duty cycle | • 5–15% active illumination per acquisition cycle; blanking interval = 0.5–5 s | • Apply for slow dynamics (>1 s per frame) or drift-sensitive samples | • Compare structural evolution with and without blanking | • Metal nanoparticles, oxide supports, beam-sensitive zeolites | • Excessive blanking may miss transient states | • Optimize by test sequences and dose logging |
| • Use electrostatic or electromagnetic blankers synchronized with frame capture (timing jitter < 10 ms) | • Verify frame-to-frame drift remains <1 nm | • Insufficient blanking increases dose | ||||
| Cryogenic stabilization (cryo-TEM) | • 80–100 K (liquid N2 or He). Vacuum ≤ 10−6 mbar | • Apply for hydrated, volatile, or organic-inorganic materials | • Measure temperature stability (ΔT < 1 K min−1) | • Liquid-phase catalysts, hybrid perovskites, carbon–metal interfaces | • Ice crystallization or mechanical drift during cooling | • Use pre-vacuum freezing, beam pre-conditioning, and anti-contamination shields |
| • Cool gradually (ΔT < 2 K s−1) to prevent condensation | • Confirm absence of ice rings in FFT | |||||
| • Use plunge-freezing for aqueous or polymeric samples | • Compare pre- and post-cooling morphology | |||||
| Temporal-resolution optimization | • UTEM: 10−15–10−9 s; fast-camera ETEM: 10−6–10−3 s; conventional ETEM: 10−3–100 s | • Match acquisition frequency to event kinetics | • Cross-correlate image timestamps with external triggers (laser, bias, or gas pulse) | • Ultrafast phase transitions, plasmonic dynamics, particle sintering | • Undersampling hides transient intermediates | • Apply ML denoising or compressed sensing reconstruction |
| • Ensure ≥2× event frequency (Nyquist criterion) | • Compare to kinetic data from MS or optical probes | • Oversampling increases dose | ||||
| • Use stroboscopic repetition for reversible events | ||||||
| Environmental control (gas/liquid) | • Gas flow = 0.1–5 mL min−1; pressure = 0.1–100 mbar; temperature = 300–1000 °C | • Stabilize gas composition and temperature ≥10 s before acquisition. Use laminar flow geometry and differential pumping for higher pressures | • Validate via inline mass-flow meters, pressure sensors, and thermocouples near the reaction zone. Record steady-state spectra (e.g., EELS edge ratio) | • Catalytic oxidation/reduction, hydrogenation, or carbon reforming reactions | • Pressure spikes or nonuniform gas distribution induce local contrast artifacts | • Pre-stabilize and verify flow with calibration runs |
| Thermal management | • Heating ramp ≤ 5 °C s−1; steady drift < 1 nm min−1 | • Use closed-loop MEMS heaters with temperature feedback | • Compare on-chip resistance temperature with thermocouple calibration | • Metal-oxide or alloy catalysts under redox cycling | • Thermal drift or support delamination | • Use stepwise ramping and drift correction algorithms |
| • Avoid rapid ramping across phase transitions | • Confirm uniformity via temperature-dependent EELS | |||||
| Temporal synchronization (operando coupling) | • Timing precision < 10 ms (slow TR-TEM) or < 1 ns (UTEM) | • Synchronize acquisition with stimuli (gas switch, voltage pulse, laser) | • Use oscilloscope trace or TTL trigger logging | • Pump–probe UTEM, bias-driven electrochemical TEM | • Unsynchronized events cause phase smearing | • Recalibrate trigger delay and jitter |
| • Implement shared trigger clock | • Verify timing lag < 5% | |||||
| Reproducibility and statistics | • ≥3 independent regions or particles per condition; variance < 10% | • Maintain identical beam and environment conditions across replicates | • Calculate mean ± SD for morphological or spectral parameters | • Supported metal catalysts, oxide nanorods, single-atom catalysts | • Sample heterogeneity or mounting variability | • Use standardized MEMS holders and consistent sample loading |
| • Document sample thickness and orientation | • Report reproducibility index (R = SD/mean) | |||||
| Validation of reaction-driven changes | • Control under inert gas (Ar, N2) or zero-bias; replicate under identical beam conditions | • Perform beam-only, environment-only, and combined controls to isolate intrinsic reactivity | • Compare EELS/XAS oxidation-state changes and lattice parameters under active vs. control conditions | • Redox catalysts (Ni, Co, Fe oxides), semiconductor photocatalysts | • Misinterpreting beam-induced reduction or migration as catalytic dynamics | • Include reversibility checks by removing stimulus |
| Data integrity and reporting | • Metadata: accelerating voltage, beam current, pixel size, dwell time, frame rate, temperature, pressure, gas composition, calibration constants | • Follow FAIR data standards; include both raw and processed datasets | • Verify metadata consistency; share data via repositories (e.g., zenodo, materials cloud) | • All TR-TEM studies | • Missing or incomplete metadata prevents reproducibility | • Provide standardized data templates and deposit with digital object identifier (DOI) |
| • Retain calibration and acquisition logs | ||||||
8. Outlook
The ongoing development of DTEM continues to shape how we observe and understand catalytic materials. As the technique integrates with environmental control, ML, multimodal analytics, and predictive modeling, its potential for unraveling complex structure–function relationships under realistic conditions becomes even more transformative. The next generation of DTEM will center on true operando capabilities, deep integration with theory, automation, and standardization, paving the way for data-driven catalyst design and discovery.One of the most significant frontiers for DTEM is achieving true operando conditions. While ETEM has enabled control over gas composition, temperature, and light exposure, pressure limitations remain a barrier. Many catalytic reactions take place under pressures far exceeding those in conventional ETEM (typically <10–100 Torr).74 Recent advances in MEMS-based microreactors and differential pumping systems have begun to bridge this gap, allowing for higher pressures while maintaining imaging capabilities.28 Integration of microfabricated flow cells, temperature sensors, and localized heating has enabled more realistic reaction environments within the microscope.28,30 As these approaches mature, they will support dynamic gas-switching, multicomponent flow, and in-line detection of reaction products (e.g., MS), enabling researchers to link specific atomic events, such as sintering, oxidation, or phase transformations, to catalytic performance metrics in real time.30
Equally important is finding the trade-offs between magnification, frame rate, and SNR.6 Achieving high magnification provides detailed spatial resolution but limits the field of view and typically reduces SNR due to lower electron counts per pixel. Increasing the frame rate allows for capturing fast dynamic processes but also reduces the electron dose per frame, further compromising SNR. Conversely, improving SNR by increasing exposure time or electron dose can cause beam-induced artifacts, especially in catalytic materials surface-loaded with beam-sensitive species. Especially in operando TEM, where we aim to replicate realistic catalytic conditions, the electron beam becomes a variable that must be carefully controlled and quantified, as it represents a reactive environmental entity whose interactions with the sample can drive chemical and structural evolution. Therefore, optimizing these parameters requires careful balancing: choosing lower magnification or slower frame rates to improve SNR, or accepting reduced resolution or temporal fidelity to minimize damage. Carefully designed acquisition strategies and advanced detectors are required to mitigate these compromises and enhance data quality.
Future DTEM studies will increasingly rely on correlative and multimodal characterization. Integrating TEM with complementary techniques such as XAS, AP-XPS, Raman spectroscopy, or IR spectroscopy can provide orthogonal information about chemical states, surface species, and vibrational dynamics.28,74 Such correlative workflows offer the ability to triangulate dynamic events, capturing the interplay between local structure, electronic configuration, and chemical reactivity. For example, EELS can provide atomic-scale oxidation state mapping, while XAS offers averaged but time-resolved insights across the sample. These multi-technique pipelines will be critical for studying catalysts with complex heterogeneity, such as bimetallic nanoparticles, defect-engineered oxides, or single-atom catalysts. Combining TEM and XAS, for example, is a common practice to confirm the existence of single-atom sites on the surface of the host catalyst. There is increasing feasibility of sequential and spatially registered experiments, where the same region is studied using multiple techniques.
Among the various branches of catalysis, electrocatalysis is one of the most promising catalytic routes for renewable energy production, and research in this area has attracted significant attention. Powered by renewable electricity, electrocatalysts drive chemical reactions using electrons instead of fossil-derived energy inputs. Compared with thermal and photocatalysis, electrocatalysis offers direct control over reaction rates and selectivity through the applied potential, replacing heat or light as the primary driving force. In this context, DTEM plays an important role in guiding the design and discovery of electrocatalysts by directly revealing structural and chemical changes under working conditions using electrochemical liquid cells. For example, Abruña and co-workers used EC-STEM to directly observe the nanoscale evolution of Cu during underpotential deposition on single-crystal Au nanocubes.91 Electrochemical control was integrated with high-resolution TEM in a liquid environment (Fig. 7), enabling real-time visualization of growth modes under different applied potentials. Distinct morphological regimes were revealed from thin planar layers to island formation and dendritic growth, consistent with diffusion-controlled kinetics. Beam-induced effects were minimized to ensure the observed changes were electrochemically driven. Ex situ 4D-STEM further showed that the deposited Cu domains aligned with the crystallographic orientation of the Au substrate without forming fully epitaxial layers due to lattice mismatch.
 | ||
| Fig. 7 (A) Schematic of the operando EC-STEM cell designed for simultaneous electrochemical measurements and real-time imaging of structural evolution. (B, C) Quantitative analysis of area growth over time for two Cu particles marked (b) and (c) in (L). (D–O) Time evolution of EC-STEM showing Cu electrodeposition at −0.1, −0.2, and −0.3 V, respectively. Adapted with permission.91 Copyright 2022, American Chemical Society. | ||
The application of DTEM to study catalytic processes in batteries is also an important research direction. In a recent review, Zhang and co-workers highlighted that in situ TEM can reveal high-resolution morphological and structural evolution of sulfur species in lithium–sulfur batteries (LSBs), including volume expansion and phase changes.92 A dual-probe electrical biasing TEM holder enables real-time observation of lithiation and delithiation by applying a controlled voltage across a Li2O solid electrolyte. Using this setup, Zou and co-workers demonstrated that the hollow architecture of NiCo2O4 nanoflower–sulfur composites accommodates volume changes during sulfur lithiation, supporting stable cycling.93In situ TEM can also quantify Li+ diffusivity, as shown by Zheng and co-workers, who reported Li+ diffusivities of 10−17–10−16 m2 s−1 in MoS2-encapsulated sulfur.94 More broadly, the combination of in situ TEM and spectroscopy enables direct tracking of sulfur species, phase evolution, and charge-transfer behavior during operation, allowing correlation of catalytic effects with improved polysulfide conversion and electrochemical performance. Despite these advances, the instability of liquid electrolytes under electron-beam irradiation still limits observation under realistic conditions. Future efforts should focus on developing advanced liquid-cell and solid-state TEM systems that incorporate stable solid electrolytes suitable for in situ characterization.
Another key opportunity lies in coupling DTEM with computational modeling, such as DFT, molecular dynamics (MD), and reactive force field simulations. These methods can interpret observed transformations, validate mechanistic hypotheses, and predict reaction pathways under relevant conditions. For example, surface reconstructions or oxidation events observed in operando TEM can be directly compared to DFT-predicted energy landscapes, while MD simulations can help explain single-atom diffusion or clustering seen under thermal or reactive conditions. This experiment-theory synergy enables a richer mechanistic understanding of dynamic catalytic behavior.28,74 The ultimate goal is a feedback loop where DTEM observations refine computational models, which in turn suggest new conditions or materials to explore experimentally, creating a closed-loop discovery framework for catalyst design.
As DTEM experiments grow more complex and data-rich, the field is shifting toward automation and community-driven standardization. Automated alignment, drift correction, and data acquisition routines are becoming standard, while ML models are enabling real-time image recognition and adaptive imaging.77,95 Self-driving TEM platforms, where an AI agent autonomously adjusts imaging conditions, targets dynamic regions, and triggers spectroscopy, are yet to be realized in catalysis studies. Standardization is equally important. Protocols should be shared for beam dose management, acquisition conditions, and metadata documentation. Initiatives to develop open-access TEM databases are underway, which will store annotated datasets for benchmarking, ML training, and reproducibility studies.96,97 These collaborative efforts will reduce duplication, enhance reliability, and accelerate the collective progress of the DTEM and catalysis communities.
Equally important is the fundamental bottleneck of scaling laboratory findings and technologies to industrial settings. Mimicking catalytic processes under real industrial conditions is notoriously difficult. As catalytic systems scale up to industrial reactors, the operating conditions become increasingly nonideal due to reaction gradients, varying flow dynamics, and locally changing chemical potentials, all of which affect surface processes and catalytic performance along the reactor bed.6 These complexities hinder systematic study and highlight the need to investigate catalytically active particles under controlled, homogeneous, and empirically defined conditions that approximate real reactor environments. Interactions between gas mixtures or electrolytes and inorganic surfaces can trigger structural changes unrelated to catalysis, making self-consistent performance measurements essential. Reproducibility is further complicated by subtle structural variations between aliquots of the same catalyst batch, which encounter different chemical potentials and consequently develop distinct working structures, which is an issue amplified in structurally sensitive or metastable systems. These longstanding challenges underscore the importance of advancing operando techniques, particularly in TEM, even though current quantitative analyses still fall short of fully capturing the heterogeneity and complexity of industrial catalysts.
Looking forward, DTEM will move beyond isolated snapshots to lifespan-resolved and system-level investigations. Entire catalyst lifecycles, from synthesis and activation to steady-state operation and deactivation, will be studied dynamically, offering insights into sintering, leaching, restructuring, and regeneration under industrially relevant cycles. Such long-term studies demand low-dose, high-stability imaging, combined with automated data logging and post-processing tools. With these advances, we will be able to visualize durability, track the evolution of activity-limiting features, and design materials optimized for extended operation under harsh reaction conditions. Ultimately, the combination of operando imaging, predictive modeling, multimodal integration, and autonomous control will transform DTEM from a diagnostic technique into a central platform for intelligent catalyst discovery.
9. Summary
Dynamic transmission electron microscopy, DTEM, has advanced the study of catalytic systems by enabling real-time visualization of atomic-scale structural dynamics. It bridges the gap between static imaging and dynamic behavior, revealing mechanisms such as atom migration, nanoparticle reshaping, and phase transformations. In particular, operando TEM can directly link structural evolution to catalytic performance, thereby strengthening structure–function relationships. Although challenges remain, including beam-induced effects, resolution limits, and difficulties in replicating realistic reaction environments, ongoing advances in instrumentation, data processing, and correlative methods are rapidly expanding the capabilities of DTEM. Machine learning and autonomous control are streamlining data interpretation and enabling adaptive experimentation, while integration with theoretical modeling and spectroscopy is providing more comprehensive mechanistic insights. As standardization and open-access practices improve, DTEM is expected to move beyond characterization toward a predictive, design-driven platform that accelerates the discovery of advanced catalysts.Conflicts of interest
The authors declare no conflicts of interest.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this highlight article.Acknowledgements
The authors gratefully acknowledge financial support from the National Research and Innovation Agency (Grant No. 3/III.10.4/HK/2023).References
- J. E. Evans and N. D. Browning, Microscopy, 2013, 62, 147–156 CrossRef CAS.
- N. Browning, M. Bonds, G. Campbell, J. Evans, T. LaGrange, K. Jungjohann, D. Masiel, J. McKeown, S. Mehraeen and B. Reed, Curr. Opin. Solid State Mater. Sci., 2012, 16, 23–30 CrossRef CAS.
- M. A. Smeaton, P. Abellan, S. R. Spurgeon, R. R. Unocic and K. L. Jungjohann, ACS Nano, 2024, 18, 35091–35103 CrossRef CAS PubMed.
- F. M. Alcorn, P. K. Jain and R. M. van der Veen, Nat. Rev. Chem., 2023, 7, 256–272 CrossRef CAS.
- M. Kitta, N. Taguchi, H. Sudrajat and H. Onishi, Appl. Phys. Lett., 2021, 118, 153901 CrossRef CAS.
- S. W. Chee, T. Lunkenbein, R. Schlögl and B. Roldán Cuenya, Chem. Rev., 2023, 123, 13374–13418 CrossRef CAS PubMed.
- O. Krivanek, T. Lovejoy and N. Dellby, J. Microsc., 2015, 259, 165–172 CrossRef CAS PubMed.
- H. Wu, X. Zhao, D. Song, F. Tian, J. Wang, K. P. Loh and S. J. Pennycook, Ultramicroscopy, 2018, 194, 182–192 CrossRef CAS PubMed.
- S. A. Aseyev, E. A. Ryabov, B. N. Mironov and A. A. Ischenko, Crystals, 2020, 10, 452 CrossRef CAS.
- M. Boniface, M. Plodinec, R. Schlögl and T. Lunkenbein, Top. Catal., 2020, 63, 1623–1643 CrossRef CAS.
- H. L. Xin, S. Alayoglu, R. Tao, A. Genc, C.-M. Wang, L. Kovarik, E. A. Stach, L.-W. Wang, M. Salmeron and G. A. Somorjai, Nano Lett., 2014, 14, 3203–3207 CrossRef CAS PubMed.
- Y. Pu, B. He, Y. Niu, X. Liu and B. Zhang, Research, 2023, 6, 0043 CrossRef CAS PubMed.
- A. I. Ruskin, Z. Yu and N. Grigorieff, J. Struct. Biol., 2013, 184, 385–393 CrossRef PubMed.
- D. Muller, L. F. Kourkoutis, M. Murfitt, J. Song, H. Hwang, J. Silcox, N. Dellby and O. Krivanek, Science, 2008, 319, 1073–1076 CrossRef CAS PubMed.
- K. Sytwu, C. Groschner and M. C. Scott, Microsc. Microanal., 2022, 28, 1896–1904 CrossRef CAS PubMed.
- D. L. Rauret, A. G. Manjón and J. Arbiol, Matter, 2025, 8, 102139 CrossRef.
- H. W. Cheng, S. Wang, G. Chen, Z. Liu, D. Caracciolo, M. Madiou, S. Shan, J. Zhang, H. He and R. Che, Adv. Energy Mater., 2022, 12, 2202097 CrossRef CAS.
- D. Cheng, J. Hong, D. Lee, S.-Y. Lee and H. Zheng, Chem. Rev., 2025, 125, 1840–1896 CrossRef CAS.
- S. Hwang, X. Chen, G. Zhou and D. Su, Adv. Energy Mater., 2020, 10, 1902105 CrossRef CAS.
- E. Fahrenkrug, D. H. Alsem, N. Salmon and S. Maldonado, J. Electrochem. Soc., 2017, 164, H358 CrossRef CAS.
- A. Bergmann and B. Roldan Cuenya, ACS Catal., 2019, 9, 10020–10043 CrossRef CAS.
- H.-Y. Chao, K. Venkatraman, S. Moniri, Y. Jiang, X. Tang, S. Dai, W. Gao, J. Miao and M. Chi, Chem. Rev., 2023, 123, 8347–8394 CrossRef CAS.
- Y. Yang, Y.-T. Shao, J. Jin, J. Feijóo, I. Roh, S. Louisia, S. Yu, M. V. Fonseca Guzman, C. Chen and D. A. Muller, ACS Sustain. Chem. Eng., 2023, 11, 4119–4124 CrossRef CAS.
- P. Abellan, E. Gautron and J. A. LaVerne, J. Phys. Chem. C, 2023, 127, 15336–15345 CrossRef CAS.
- L. Sun, K. W. Noh, J.-G. Wen and S. J. Dillon, Langmuir, 2011, 27, 14201–14206 CrossRef CAS PubMed.
- H. Sudrajat, N. Ichikuni and H. Onishi, Chem. Phys., 2020, 531, 110648 CrossRef CAS.
- L. V. A. de Oliveira, N. R. C. Huaman, S. N. Monteiro, U. O. Costa and L. Vitorazi, J. Mater. Res. Technol., 2025, 35, 2736–2754 CrossRef.
- C. Ophus, Microsc. Microanal., 2019, 25, 563–582 CrossRef CAS PubMed.
- L. Jones, A. Varambhia, R. Beanland, D. Kepaptsoglou, I. Griffiths, A. Ishizuka, F. Azough, R. Freer, K. Ishizuka and D. Cherns, Microscopy, 2018, 67, 98–113 CrossRef.
- D. Nicholls, J. Lee, H. Amari, A. J. Stevens, B. L. Mehdi and N. D. Browning, Nanoscale, 2020, 12, 21248–21254 RSC.
- R. Toyoshima, M. Yoshida, Y. Monya, Y. Kousa, K. Suzuki, H. Abe, B. S. Mun, K. Mase, K. Amemiya and H. Kondoh, J. Phys. Chem. C, 2012, 116, 18691–18697 CrossRef CAS.
- H. Ali-Löytty, M. W. Louie, M. R. Singh, L. Li, H. G. Sanchez Casalongue, H. Ogasawara, E. J. Crumlin, Z. Liu, A. T. Bell and A. Nilsson, J. Phys. Chem. C, 2016, 120, 2247–2253 CrossRef.
- S. Mohammadian, J. Fokkema, A. V. Agronskaia, N. Liv, C. de Heus, E. van Donselaar, G. A. Blab, J. Klumperman and H. C. Gerritsen, Sci. Rep., 2019, 9, 3211 CrossRef PubMed.
- J. Van Hest, A. Agronskaia, J. Fokkema, F. Montanarella, A. Gregorio Puig, C. de Mello Donega, A. Meijerink, G. Blab and H. Gerritsen, J. Microsc., 2019, 274, 13–22 CrossRef CAS PubMed.
- G. H. Campbell, J. T. McKeown and M. K. Santala, Applied Physics Reviews, 2014, 1, 041101 CrossRef.
- T. Shimojima, A. Nakamura and K. Ishizaka, Rev. Sci. Instrum., 2023, 94, 023705 CrossRef CAS PubMed.
- P. G. Kotula, M. R. Keenan and J. R. Michael, Microsc. Microanal., 2003, 9, 1–17 CrossRef CAS PubMed.
- N. R. Lewis, Y. Jin, X. Tang, V. Shah, C. Doty, B. E. Matthews, S. Akers and S. R. Spurgeon, npj Comput. Mater., 2022, 8, 252 CrossRef CAS.
- M. Botifoll, I. Pinto-Huguet and J. Arbiol, Nanoscale Horiz., 2022, 7, 1427–1477 RSC.
- J. P. Horwath, D. N. Zakharov, R. Mégret and E. A. Stach, npj Comput. Mater., 2020, 6, 108 CrossRef.
- J. P. Horwath, P. W. Voorhees and E. A. Stach, Nano Lett., 2021, 21, 5324–5329 CrossRef CAS PubMed.
- L. Yao, Z. Ou, B. Luo, C. Xu and Q. Chen, ACS Cent. Sci., 2020, 6, 1421–1430 CrossRef CAS PubMed.
- J. L. Vincent and P. A. Crozier, Nat. Commun., 2021, 12, 5789 CrossRef CAS PubMed.
- T. Blum, J. Graves, M. J. Zachman, F. Polo-Garzon, Z. Wu, R. Kannan, X. Pan and M. Chi, Small Methods, 2021, 5, 2100035 CrossRef CAS.
- Y. Wang, Y. Niu, Y. Pu, S. Li, Y. Liu and B. Zhang, Chem. Synth., 2023, 3, 1–11 CAS.
- H. Sudrajat, Mater. Res. Express, 2018, 5, 095501 CrossRef.
- H. Sudrajat, I. Rossetti and J. C. Colmenares, J. Mater. Chem. A, 2023, 11, 24566–24590 RSC.
- B. D. Anderson and J. B. Tracy, Nanoscale, 2014, 6, 12195–12216 RSC.
- M. Xu, M. Peng, H. Tang, W. Zhou, B. Qiao and D. Ma, J. Am. Chem. Soc., 2024, 146, 2290–2307 CrossRef CAS PubMed.
- W. Yu, S. Yue, M. Yang, M. Hashimoto, P. Liu, L. Zhu, W. Xie, T. Jones, M. Willinger and X. Huang, Nat. Commun., 2025, 16, 2029 CrossRef CAS.
- K. F. Kalz, R. Kraehnert, M. Dvoyashkin, R. Dittmeyer, R. Gläser, U. Krewer, K. Reuter and J. D. Grunwaldt, ChemCatChem, 2017, 9, 17–29 CrossRef CAS.
- M. Plodinec, H. C. Nerl, R. Farra, M. G. Willinger, E. Stotz, R. Schlögl and T. Lunkenbein, Microsc. Microanal., 2020, 26, 220–228 CrossRef CAS PubMed.
- H. Sudrajat, J. Phanthuwongpakdee and J. C. Colmenares, Mater. Chem. Front., 2025, 9, 1917–1932 RSC.
- H. Sudrajat, S. A. Wella, J. Phanthuwongpakdee, D. Lisovytskiy, K. Sobczak and J. C. Colmenares, Nanoscale, 2024, 16, 14813–14830 RSC.
- Z. Zhang, J. Tian, Y. Lu, S. Yang, D. Jiang, W. Huang, Y. Li, J. Hong, A. S. Hoffman, S. R. Bare, M. H. Engelhard, A. K. Datye and Y. Wang, Nat. Commun., 2023, 14, 2664 CrossRef CAS PubMed.
- N. Cheng, L. Zhang, K. Doyle-Davis and X. Sun, Electrochem. Energy Rev., 2019, 2, 539–573 CrossRef.
- M. Zhu, K. Yin, Y. Wen, S. Song, Y. Xiong, Y. Dai and L. Sun, Nano Res., 2022, 15, 1319–1326 CrossRef CAS.
- C. Wang, X.-K. Gu, H. Yan, Y. Lin, J. Li, D. Liu, W.-X. Li and J. Lu, ACS Catal., 2017, 7, 887–891 CrossRef CAS.
- Y. Kratish, Y. Liu, J. Li, A. Das, L. O. Jones, A. Agarwal, Q. Ma, M. J. Bedzyk, G. C. Schatz, T. Nakamuro, E. Nakamura and T. J. Marks, Chem, 2025, 11, 102541 CAS.
- S. W. Chee, T. Lunkenbein, R. Schlögl and B. R. Cuenya, J. Phys.: Condens. Matter, 2021, 33, 153001 CrossRef CAS PubMed.
- B. Yuan, Y. Zhu, S. Liu, A. Shan and R. Wang, Adv. Funct. Mater., 2025, 2503871 CrossRef CAS.
- F. Zhang and W. Liu, Microstructures, 2024, 4, 2024041 CAS.
- D. Alloyeau, N. Ortiz Pena, G. Wang, C. Ricolleau and J. Nelayah, Adv. Phys.:X, 2025, 10, 2481277 Search PubMed.
- D. Su, Green Energy Environ., 2017, 2, 70–83 CrossRef.
- R. Egerton, Micron, 2019, 119, 72–87 CrossRef CAS PubMed.
- J. A. LaVerne and S. M. Pimblott, J. Phys. Chem., 1995, 99, 10540–10548 CrossRef CAS.
- N. M. Schneider, M. M. Norton, B. J. Mendel, J. M. Grogan, F. M. Ross and H. H. Bau, J. Phys. Chem. C, 2014, 118, 22373–22382 CrossRef CAS.
- T. Woodward and R. Back, Can. J. Chem., 1963, 41, 1463–1468 CrossRef CAS.
- M. Ilett, M. S'ari, H. Freeman, Z. Aslam, N. Koniuch, M. Afzali, J. Cattle, R. Hooley, T. Roncal-Herrero and S. M. Collins, Philos. Trans. R. Soc., A, 2020, 378, 20190601 CrossRef PubMed.
- J. Jinschek, Chem. Commun., 2014, 50, 2696–2706 RSC.
- T. Shimojima, A. Nakamura and K. Ishizaka, Microscopy, 2023, 72, 287–298 CrossRef CAS PubMed.
- E. Montgomery, D. Leonhardt and J. Roehling, Microsc. Today, 2021, 29, 46–54 CrossRef.
- S. Merkens, G. De Salvo and A. Chuvilin, Nano Express, 2023, 3, 045006 CrossRef.
- A. Velazco, A. Béché, D. Jannis and J. Verbeeck, Ultramicroscopy, 2022, 232, 113398 CrossRef CAS PubMed.
- S. Pennycook and D. Jesson, Ultramicroscopy, 1991, 37, 14–38 CrossRef.
- K. R. Fiedler, M. J. Olszta, K. H. Yano, C. Doty, D. Hopkins, S. Akers and S. R. Spurgeon, Microsc. Microanal., 2023, 29, 1931–1939 CrossRef.
- S. V. Kalinin, D. Mukherjee, K. Roccapriore, B. J. Blaiszik, A. Ghosh, M. A. Ziatdinov, A. Al-Najjar, C. Doty, S. Akers and N. S. Rao, npj Comput. Mater., 2023, 9, 227 CrossRef.
- H. Nevison-Andrews and A. Robertson, Chem. Phys. Rev., 2025, 6, 031302 CrossRef CAS.
- J. M. Zuo and J. C. Spence, Advanced Transmission Electron Microscopy, Springer, 2017 Search PubMed.
- M. Kannan, Transmission electron microscope—principle, Components and Applications, 2018 Search PubMed.
- J. Thomas and T. Gemming, Analytical Transmission Electron Microscopy: an Introduction for Operators, Springer Science & Business, 2014 Search PubMed.
- H. H. Pérez Garza, D. Morsink, J. Xu, M. Sholkina, Y. Pivak, M. Pen, S. van Weperen and Q. Xu, Micro Nano Lett., 2017, 12, 69–75 CrossRef.
- J. F. Creemer, S. Helveg, P. J. Kooyman, A. M. Molenbroek, H. W. Zandbergen and P. M. Sarro, J. Microelectromech. Syst., 2010, 19, 254–264 CAS.
- Y. Han, L. Wang, K. Cao, J. Zhou, Y. Zhu, Y. Hou and Y. Lu, Chem. Rev., 2023, 123, 14119–14184 CrossRef CAS.
- F. Wu and N. Yao, Nano Energy, 2015, 13, 735–756 CrossRef CAS.
- T. LaGrange, P. Cattaneo, B. Barwick, D. J. Flannigan, J. Weissenrieder and F. Carbone, Nat. Rev. Methods Primers, 2025, 5, 61 CrossRef CAS.
- Y. Lian, J. Sun and L. Jiang, Int. J. Mech. Syst. Dyn., 2023, 3, 192–212 CrossRef.
- A. Arbouet, G. M. Caruso and F. Houdellier, Adv. Imaging Electron Phys., 2018, 207, 1–72 Search PubMed.
- S. Takeda and H. Yoshida, Microscopy, 2013, 62, 193–203 CrossRef CAS PubMed.
- H. Zhao, Y. Zhu, H. Ye, Y. He, H. Li, Y. Sun, F. Yang and R. Wang, Adv. Mater., 2023, 35, 2206911 CrossRef CAS.
- Y. Yang, Y.-T. Shao, F. J. DiSalvo, D. A. Muller and H. D. Abruña, ACS Energy Lett., 2022, 7, 1292–1297 CrossRef CAS.
- Q. Gu, M. Lu, Y. Cao and B. Zhang, Renewables, 2023, 1, 601–621 CrossRef.
- Z. Cui, S.-A. He, Q. Liu and R. Zou, Dalton Trans., 2020, 49, 6876–6883 RSC.
- W. Tang, Z. Chen, B. Tian, H.-W. Lee, X. Zhao, X. Fan, Y. Fan, K. Leng, C. Peng, M.-H. Kim, M. Li, J. Su, J. Chen, H. Y. Jeong, X. Yin, Q. Zhang, W. Zhou, K. P. Loh and G. W. Zheng, J. Am. Chem. Soc., 2017, 139, 10133–10141 CrossRef CAS.
- M. Olszta, D. Hopkins, K. R. Fiedler, M. Oostrom, S. Akers and S. R. Spurgeon, Microsc. Microanal., 2022, 28, 1611–1621 CrossRef CAS.
- M. Ziatdinov, A. Ghosh, C. Y. Wong and S. V. Kalinin, Nat. Mach. Intell., 2022, 4, 1101–1112 CrossRef.
- M. M. Noack, P. H. Zwart, D. M. Ushizima, M. Fukuto, K. G. Yager, K. C. Elbert, C. B. Murray, A. Stein, G. S. Doerk and E. H. Tsai, Nat. Rev. Phys., 2021, 3, 685–697 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |
