Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering MOFs for thin-film and nanofilm nanocomposite membranes for CO2 separation
Farhang
Pazanialenjareghi
 ,
Shweta
Singh
,
Fathy
Attia
,
Shweta
Singh
,
Fathy
Attia
 ,
Venkat Sainath Reddy
Munnangi
,
Emmanuel M.
Nsengiyumva
,
Venkat Sainath Reddy
Munnangi
,
Emmanuel M.
Nsengiyumva
 ,
Yang
Jiao
and
Haiqing
Lin
,
Yang
Jiao
and
Haiqing
Lin
 *
*
Department of Chemical and Biological Engineering, University at Buffalo, The State University of New York, Buffalo, NY 14260, USA. E-mail: haiqingl@buffalo.edu
First published on 8th December 2025
Abstract
Microporous crystalline metal–organic frameworks (MOFs) have been incorporated into polymers to enhance carbon capture performance due to their well-controlled pore sizes and porosity. However, MOFs may aggregate in the polymers and form interfacial voids, resulting in reduced selectivity. Such challenges are exacerbated when they are incorporated into thin-film nanocomposite (TFN) or nanofilm nanocomposite (NFN) membranes, where the effects of interfacial interactions and nanoconfinement become more pronounced in defect-free films as thin as <100 nm. To address these issues, novel MOFs have been developed to improve their distribution in thin films and their contribution to gas separation properties, such as surface functionalization, defect engineering, amorphization, and incorporation with polymers and macrocycles. We critically assess these strategies and highlight their contributions to enhancing CO2 separation properties. Understanding the scaling and integration of these engineered MOFs in TFNs provides insights into designing next-generation membranes for molecular and ion separations.
1. Introduction
Membrane technology has emerged as one of the leading processes for carbon capture, reducing CO2 emissions into the atmosphere, due to its high energy efficiency, lack of chemical waste, compact design, and simplicity in operation and maintenance.1,2 Industrial membranes are usually made of polymers because they are low-cost and easy to process.3,4 However, their separation performance often suffers from a trade-off between permeability and selectivity, i.e., polymers with higher permeability tend to exhibit lower selectivity.5 To address this, polymers are incorporated with nanofillers featuring well-controlled nanopores and unique separation properties, synergizing the advantages of both polymers and nanofillers.6,7 Particularly, crystalline metal–organic frameworks (MOFs) formed by the self-assembly of metal ion clusters and organic ligands have attracted substantial attention,8,9 due to their great flexibility of metal ions and ligands in designing desirable micropores in achieving superior gas separation properties.10–13Significant progress has been made in developing mixed matrix freestanding films (MMFs, >10 µm) with superior gas separation properties, and several challenges have been identified. First, conventional MOF nanoparticles (NPs) can aggregate in polymers due to incompatibility, forming non-selective interfacial voids and thereby reducing gas selectivity.11,14–16 Second, polymer chains may penetrate into and around the porous structure of MOFs and become rigidified, decreasing gas separation efficiency.17 Finally and most importantly, industrial membranes often comprise selective layers as thin as <100 nm to achieve high gas permeance,18–20 and the scalable production of defect-free thin-film (<1000 nm) nanocomposite (TFN) and nanofilm (<100 nm) nanocomposite (NFN) membranes with controlled distribution of MOFs remains a significant hurdle.15,21–23
Extensive strategies have been adopted to engineer MOFs that address the aforementioned challenges, such as fine-tuning pore sizes and surfaces, enhancing gas separation properties, and achieving uniform dispersion in polymers.10,24–26 First, the surface of MOFs can be modified with functional groups (SM-MOFs), such as amines27–29 and ionic liquids (ILs),30,31 to introduce affinity towards polymers; and in situ growth of MOFs and in situ polymerization of polymers were used to enhance the dispersibility of MOF NPs.32,33 Second, defects can be introduced to MOFs, resulting in defect-engineered MOFs (DE-MOFs)34–36 and even amorphous MOFs (aMOFs).37–39 Third, MOF structures can be functionalized by polymers (polyMOFs)40,41 and macrocycles (MC-MOFs);42–44 and MOFs with unique morphologies (such as wrinkled surfaces) have been synthesized.45
Various aspects of the MMFs containing MOFs have been reviewed in the literature, such as in situ synthesis of MOFs and polymers,32 engineering interfacial compatibility,11,33 scalability and stability,46 and H2 separations.47 MMFs based on advanced MOFs such as DE-MOFs,26,35 aMOFs,48,49 and post-modified MOFs50 were also summarized. Furthermore, TFN membranes based on conventional MOFs and SM-MOFs have also been reviewed for various gas and liquid separations.15,48,51
This paper provides a comprehensive and critical review of the state-of-the-art MOF-based TFN membranes with superior CO2 separation performance, highlighting the role of the engineered MOFs in fabricating nanocomposite membranes with enhanced separation performance (Fig. 1). We first describe the gas transport model in TFN and NFN membranes and highlight the leading ones containing conventional MOF NPs with good CO2 separation properties. Second, advanced MOFs engineered to improve membrane CO2 separation properties are critically reviewed, such as SM-MOFs, DE-MOFs, aMOFs, polyMOFs, MC-MOFs, and 2D MOFs. Third, the effect of the engineered MOFs on membrane separation properties is systematically compared, and the large-scale production of TFN membranes is discussed. Finally, we provide our perspectives on the future direction of developing this exciting membrane platform for practical gas separations. Elucidating interactions between these advanced MOFs and polymers may help design nanocomposites for other applications, such as membranes for electrochemical devices, coatings, sensors, and structured materials.
2. Conventional MOF-based TFN membranes
This section introduces the rationale of incorporating conventional MOFs to enhance CO2 separation properties, as well as conventional methods for fabricating TFN membranes.2.1. Gas transport models
Gas transport through polymeric materials (like MMFs) usually follows a solution-diffusion mechanism, and gas permeability (PA) can be expressed as:| PA = SA × DA | (1) |
TFN membranes are characterized by gas permeance (QA). QA has units of GPU, where 1 GPU = 10−6 cm3(STP) cm−2 s−1 cmHg−1. The ideal permeance (QA,ideal) is determined by the selective layer and given by:
| QA,ideal = PA/l | (2) |
Various models have been developed to describe gas permeability in the MMFs. For example, the Lewis–Nielsen model is valid with the filler loading (φd) up to 64 vol%. The gas permeability of MMFs (PM) can be expressed in eqn (3):52
 | (3) |
 | (4) |
 | (5) |
Eqn (5) predicts that introducing a highly permeable discontinuous phase in a polymer can increase permeability without affecting gas selectivity.
The Maxwell model has also been widely used to estimate gas permeability in MMFs (φd < 20%). At γ >> 1, the model can be reduced to the following equation:
 | (6) |
However, as both Lewis–Nielsen and Maxwell models do not consider the morphology and distribution of MOFs and their interfacial incompatibility with polymers, they only provide general guidance and lack accuracy in quantitative prediction.52,53
2.2. Resistance model and geometric restriction in membranes
Fig. 2 displays schematic diagrams of gas permeation through thin-film composite (TFC) and TFN membranes.2,52 Gutter layers are typically used to prevent the pore penetration of the coating solution into the porous support and to provide a smooth surface for coating the selective layer.19,54–56 In the absence of the effect from the porous support, gas permeation through the membranes can be estimated using a resistance-in-series model:| 1/QA = 1/QA,ideal + (l/PA)G | (7) |
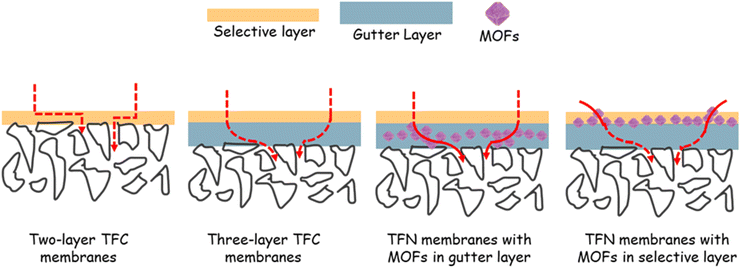 | ||
| Fig. 2 Scheme of gas permeation through multi-layer TFC and TFN membranes, including MOFs in the gutter and selective layers. | ||
However, the porous support often exerts significant geometric restriction,19,20 where gas molecules prefer to diffuse through the surface pores of the porous support, thus increasing the diffusion path. Toward this, the permeation efficiency (βA) is defined to represent the effect of the gutter layer and the surface pore size and porosity of the porous support:57,58
 | (8) |
The βA values can be estimated from computational fluid dynamics (CFD) simulations.
2.3. Typical polymers and methods to fabricate TFN membranes
Fig. 3 shows typical materials used to construct TFN membranes.2,52 Porous supports can be made of polyvinylidene fluoride (PVDF), polysulfone (PSF), and polyacrylonitrile (PAN), which have low costs and good processability. Gutter layers can be made of polydimethylsiloxane (PDMS) and poly(1-trimethylsilyl-1-propyne) (PTMSP), which exhibit high gas permeability and excellent coatability.19 Additionally, MOF nanosheets have been directly used as gutter layers,59,60 and MOFs have been incorporated into the PDMS layer to enhance its permeance.61,62 Several polymers have been widely employed for TFN membranes, including commercial poly(ethylene oxide) (PEO)-containing copolymers such as Pebax and PolyActive, amorphous PEO (aPEO), polymers of intrinsic microporosity (PIMs), and polyvinyl amine (PVAm).54,55,63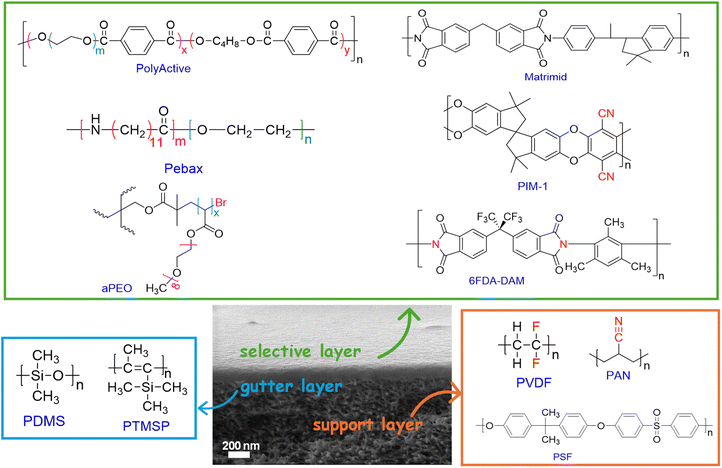 | ||
| Fig. 3 Configurations of TFN membranes, and chemical structures of typical polymers, gutter layers, and porous supports. | ||
TFN membranes can be fabricated using conventional methods, including in situ growth and polymerization, interfacial polymerization (IP), dip coating, and spinning coating (Fig. 4a).54,56,63 Novel approaches have also been demonstrated at a lab scale, including additive manufacturing (3D printing), continuous assembly of polymers (CAP), and spray coating (Fig. 4b).64–67 For instance, PIM-1 containing HKUST-1 was deposited on PAN support using a 3D printing method (Fig. 4c),68 and the layer thickness was varied between 2.5 µm and 400 nm by manipulating coating solution concentration and coating cycle. Fig. 4d presents the use of CAP to synthesize cross-linked PEO as thin as 30 nm on top of a MOF substrate, which exhibited CO2 permeance of 3000 GPU with CO2/N2 selectivity of 34.65
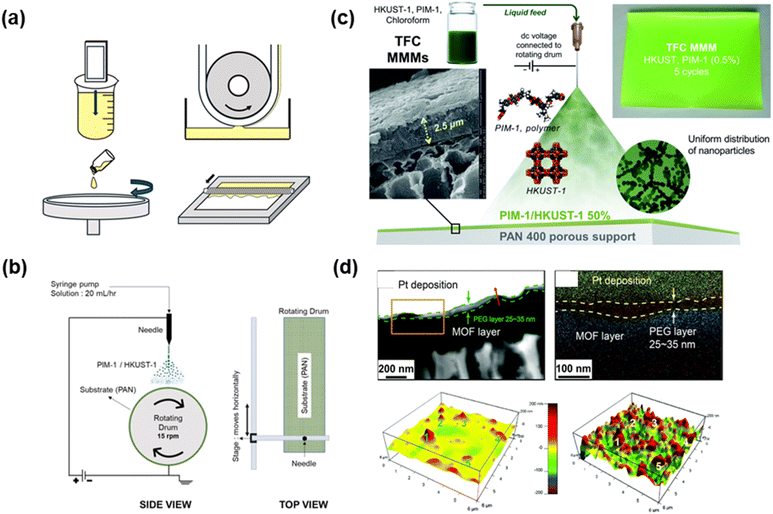 | ||
| Fig. 4 Fabricating TFN membranes. (a) Common coating methods, including dip-coating (top left), kiss-coating (top right), spin-coating (bottom left), and bar-coating (bottom right).55 Copyright 2025. Reproduced with permission from Elsevier. (b) Schematic of electrospray used for fabricating (c) membranes comprising PIM-1 and HKUST-1.68 Copyright 2021. Reproduced with permission from the Royal Society of Chemistry. (d) Cross-sectional TEM and EDX mapping (top) and 3D AFM images (bottom) of membranes prepared by CAP.65 Copyright 2018. Reproduced with permission from the Royal Society of Chemistry. | ||
2.4. TFN membranes based on conventional MOFs
Conventional TFN membranes are often prepared using conventional MOFs. Table 1 summarizes representative membranes with superior CO2/N2 or CO2/CH4 separation properties. Generally, adding MOFs increased both gas permeance and selectivity, and the effect depends on the NP surface chemistry. For example, MOF-808 exhibits large pores (4.8 and 18.4 Å) and unsaturated metal sites, providing high CO2 adsorption capacity; adding 33 mass% MOF-808 (30–60 nm) in a PVAm selective layer (140 nm) increased CO2 permeance by 100% from 1376 to 2753 GPU and CO2/N2 selectivity by 120% from 82 to 181.69 Compatibilizers can be used to mitigate interfacial incompatibility. For instance, adding 10 mass% ionic liquid (IL) into nanofilms of ZIF-8 and a polymerizable ionic liquid (PIL) increased CO2 permeance from 1056 to 1106 GPU and CO2/N2 selectivity from 22 to 27.70| Support/gutter layer | Selective layers | T (°C)/p (bar) | CO2 permeance (GPU) | Selectivity | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polymera | MOFs | Sizes (nm) | MOF (wt%) | l (nm) | CO2/N2 | CO2/CH4 | ||||
| a PA: polyamide; PBE: poly(2-[3-(2H-benzotriazol-2-yl)-4-hydroxyphenyl] ethyl methacrylate)-co-poly(oxyethylene methacrylate); PTO: poly(tetrahydrofurfuryl methacrylate)-co-poly(poly(oxyethylene methacrylate)); PAP: poly(1-allyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide-co-poly(ethylene glycol) methyl ether methacrylate); PVI-POEM: poly(vinyl imidazole)-poly(oxyethylene methacrylate); PGO: poly(glycidyl methacrylate)-co-poly(oxyethylene methacrylate). | ||||||||||
| PSF/PDMS | aPEO | UiO-66-NH2 | 45–85 | 0 | 178 | 23/2 | 1400 | 50 | 52 | |
| 10 | 200 | 23/2 | 2900 | 48 | ||||||
| PSF/PDMS | PVAm | MOF-808 | 30–60 | 0 | — | 25/2 | 1376 | 82 | 69 | |
| 33 | 145 | 25/2 | 2753 | 181 | ||||||
| PSF/PDMS | PA | ZIF-8 | 75 | — | 190 | 25/1.5 | 1600 | 35 | 71 | |
| 5 | 65 | 25/1.5 | 2740 | 104 | ||||||
| PSF/PTMSP | IL-Pebax | — | — | 300 | 35/3 | 497 | 27 | 72 | ||
| ZIF-8 | 30 | 15 | 300 | 35/3 | 751 | 25 | ||||
| ZIF-94 | 48 | 15 | 300 | 35/3 | 819 | 25 | ||||
| PSF | PBE | MOF-808 | 500–600 | 0 | — | 30/1 | 431 | 36 | 73 | |
| 40 | 350 | 30/1 | 1069 | 53 | ||||||
| PAN/PDMS@ aMOF | PIM-1 | — | — | 650 | 35/1 | 4320 | 19 | 74 | ||
| MOF-74-Ni | 20–30 | 10 | 670 | 35/1 | 5018 | 31 | ||||
| UiO-66-NH2 | 20–30 | 10 | 660 | 35/1 | 7460 | 26 | ||||
| PSF/PTMSP | PTO | UTSA-16 | 6000 | 0 | 250 | 30/1 | 737 | 38 | 17 | 75 |
| 10 | 300 | 30/1 | 1070 | 41 | 17 | |||||
| PSF/PTMSP | PAP | ZIF-8 | 60 | 0 | 600 | 25/1 | 1056 | 22 | 10 | 70 |
| 10 | 600 | 25/1 | 1017 | 33 | 13 | |||||
| PSF/PTMSP | PVI-POEM | ZIF-8 | 100–200 | 0 | 300 | 30/1 | 1086 | 40 | 19 | 76 |
| 50 | 300 | 30/1 | 4474 | 32 | 12 | |||||
| PSF/PTMSP | PGO | — | — | 600 | 25/1 | 889 | 31 | 15 | 77 | |
| MIL-140C | 0.2 × 2 µm | 10 | 600 | 25/1 | 1364 | 40 | 22 | |||
| UiO-67 | 200 | 10 | 600 | 25/1 | 1301 | 36 | 16 | |||
The morphology and size of MOF NPs significantly impact their CO2 separation properties. Furthermore, the incorporation of NPs in the nanofilms affects polymer chain dynamics, resulting in unexpected benefits, such as enhanced resistance to physical aging and increased polymer chain rigidity (and thus size-sieving ability). The details are described below.
The particle size plays a critical role in their dispersibility and compatibility within polymers. Smaller NPs exhibit better dispersibility and higher interfacial surface areas, and thus, they are often preferred. For instance, MOF NPs with ∼100 nm were added into the PAN nanofiber supports, and then PIM/PU blends were spun-coated as selective layers (∼180 nm) over a PDMS gutter layer; adding UiO-66-NH2 increased CO2 permeance from 1140 to 3690 GPU and CO2/N2 selectivity from 20 to 92.82 Interestingly, micron-sized UTSA-16 particles (∼6 µm) were dispersed in a copolymer (PTO) of ∼300 nm; adding 30 wt% UTSA-16 increased CO2 permeance from 700 to 1800 GPU and slightly decreased CO2/N2 selectivity from 38 to 32.75
Modulators can also be used to fine-tune MOF morphology. For instance, using monocarboxylic acid- and amine-based modulators can allow the growth of MOFs with different morphologies.83 Specifically, nanocubes were obtained using both acetic acid and pyridine, and nanosheets were obtained when only the aminated modulator was used. Similarly, using benzoic acid and pyridine resulted in the formation of nanorods and nanoplates, respectively.
Zr-based MOFs with different morphologies have been investigated. For example, UiO-67 and MIL-140 have the same building blocks, while UiO-67 has a 3D shape with larger cages (12 and 23 Å), and MIL-140 has a rod shape with 9 Å pore sizes.77 Adding 10 mass% MIL in PGO increased CO2 permeance from 889 to 1364 GPU, CO2/N2 selectivity from 31 to 40, and CO2/CH4 selectivity from 15 to 22. By contrast, adding 10 mass% UiO increased CO2/N2 selectivity to 35 but barely affected CO2/CH4 selectivity. The discrepancy was attributed to the polymer's easier infiltration into the UiO-67 with its more open structures, which blocked gas permeation.
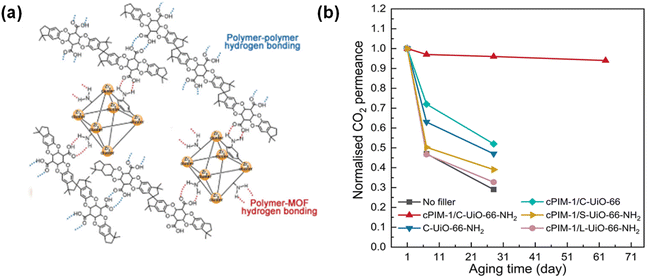 | ||
| Fig. 5 TFN membranes with conventional MOF NPs for CO2/N2 separation. UiO-66-NH2 to enhance resistance to physical aging in cPIM-1, including (a) scheme of H-bonds and (b) effect of the filler type on the normalized CO2 permeance.84 Copyright 2023. Reproduced with permission from John Wiley & Sons, Inc. | ||
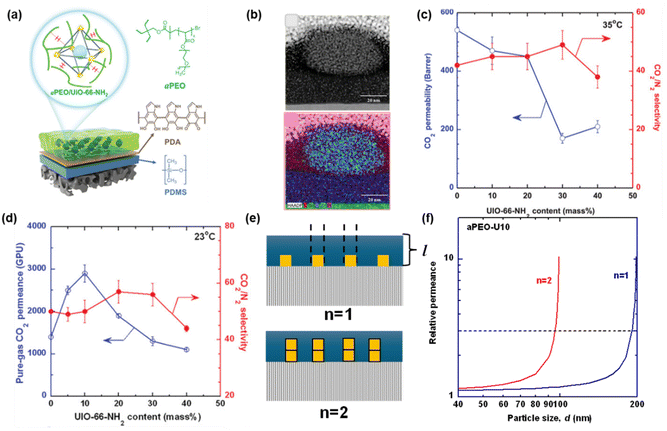 | ||
| Fig. 6 Effect of nanoconfinement and NP distribution in TFN membranes based on UiO-66-NH2 and aPEO. (a) Membrane configuration. (b) TEM images of nanofilms showing good interfacial compatibility. Comparison of CO2/N2 separation properties between (c) thick films (200 µm) and (d) nanofilms (∼120 nm). (e) Schematic of the selective layer. (f) Permeance ratio (defined as QExp/QaPEO) as a function of d and n values with 10 mass% UiO-66-NH2. Copyright 2024.52 Reproduced with permission from John Wiley & Sons, Inc. | ||
To estimate the effect of the NPs on the gas permeance of the membrane, the NPs were assumed to be uniform cubes with a length of d (nm) and an infinite gas permeance (Fig. 6e). The experimental gas permeance of the membrane (QExp) can be estimated using the resistance in parallel model, and the following equation was derived:
 | (9) |
3. TFN membranes based on engineered MOFs
MOFs have been molecularly engineered to improve their compatibility with polymers, distribution in nanofilms, and gas separation properties. This section focuses on recently emerged MOFs, including SM-MOFs, DE-MOFs, aMOFs, polyMOFs, 2D MOFs, and others with unique morphologies.3.1. TFN membranes based on SM-MOFs
The surface of MOFs can be modified using a covalent or coordinative approach,50,87,88 as summarized in Table 2. For example, polymethylmethacrylate (PMMA), polyurethanes, and polyimides were used to modify the surface, improving interfacial compatibility and MOF dispersibility, as well as enhancing gas separation performance.89–95 Specifically, UiO-66-NH2 reacted with the dianhydride end groups of 6FDA-Durene oligomers, reducing the MOF agglomeration;93 ZIF-8 NPs were modified with a shell of poly(1,3-dioxolanne) methacrylate (PDXLMA) and fabricated into TFN membranes, leading to CO2 permeance of 3969 GPU and CO2/N2 selectivity of 28.96 Additionally, unsaturated metal ions/clusters can be coordinated via chemicals containing functional groups like amines. For example, tetraethylenepentamine (TEPA) was used to modify Mg-MOF-74, where TEPA coordinated with the unsaturated Mg2+ centers, enhancing their dispersibility.97| Membr-anes | Support/gutter layer | Selective layers | T (°C)/p (bar) | CO2 permeance (GPU) | Selectivity | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymers | MOFs | Size (nm) | MOF (wt%) | l (nm) | CO2/N2 | CO2/CH4 | |||||
| NFN | PAN | PEGMEA-PEODMS | c-UiO-66 MA | 40–50 | 0 | 100 | 25/1 | 1450 | 25 | 98 | |
| 40 | 100 | 3076 | 26 | ||||||||
| PSf/PTMSP | PGO | — | — | 80 | 25/1 | 1393 | 23 | 10 | 99 | ||
| UiO-66 | 50–150 | 20 | 100 | 1555 | 35 | 14 | |||||
| UiO-66-Br | 50–150 | 20 | 70 | 1703 | 37 | 14 | |||||
| UiO-66-NO2 | 50–150 | 20 | 80 | 1816 | 37 | 14 | |||||
| TFN | PAN | ZIF-8/PDXLAMA1h | 444 | 350 | 25/1 | 6035 | 21 | 96 | |||
| ZIF-8/PDXLAMA2h | 991 | 630 | 3969 | 28 | |||||||
| PSF/PTMSP | Pebax | — | 0 | 600 | 35/3 | 181 | 43 | 19 | 100 | ||
| UiO-66-NH2 | 4 | 5 | 700 | 277 | 45 | ||||||
| UiO-66-NO2 | 6 | 5 | 700 | 155 | 51 | ||||||
| PAN/PDMS | PI | NH2-ZIF-8 | 82 | 0 | 300 | 30/1 | 304 | 22 | 101 | ||
| 20 | 140 | 778 | 34 | ||||||||
Fig. 7a shows that UiO-66 MA NPs were copolymerized in situ with poly(ethylene glycol) methyl ether methacrylate (PEGMEMA) and dimethylsiloxane-ethylene oxide copolymer (PEODMS), forming a covalently crosslinked MOF–polymer network.98 This strategy enabled high MOF loadings (40 wt%) without aggregation within 100 nm-thick selective layers (Fig. 7b). Adding 40% filler achieved CO2 permeance of 3067 GPU while retaining CO2/N2 selectivity of 26. By contrast, UiO-66-NH2 was directly dispersed in the polymer to form membranes (h-UiO-66-MA@P), which exhibited CO2/N2 selectivity decreasing with increasing MOF loading, highlighting the effectiveness of the covalent bonding between the polymer and MOFs.
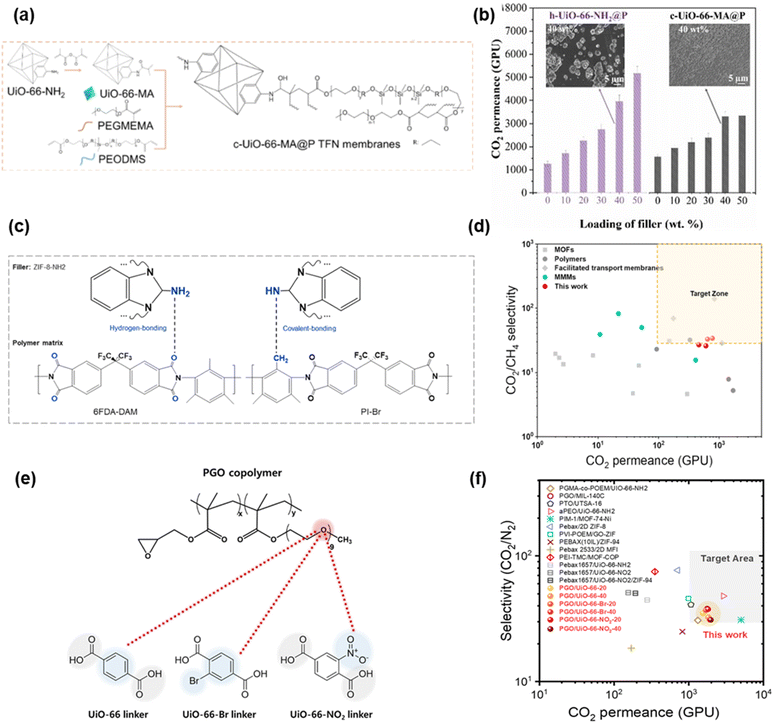 | ||
| Fig. 7 TFN membranes based on SM-MOFs. (a) Scheme of in situ copolymerization of UiO-66-MA and PEGMEMA, and (b) effect of MOF loadings on CO2 permeance.98 Copyright 2024. Reproduced with permission from John Wiley & Sons, Inc. (c) Scheme of bonding between amine ZIF-8 and PIs and (d) gas separation properties.101 Copyright 2023. Reproduced with permission from Elsevier. (e) Scheme of interaction between the polymer and UiO ligands and (f) gas separation properties.99 Copyright 2025. Reproduced with permission from the Royal Society of Chemistry. | ||
Fig. 7c illustrates the affinity between amine-functionalized ZIFs and PIs, enabling the fabrication of TFN membranes with a thickness of ∼200 nm.101 Adding 20 wt% NH2-ZIFs increased CO2 permeance by 156% to 778 GPU with CO2/N2 selectivity by 55% to 34. In another study, functionalized UiO-66 NPs (50–150 nm) were used to fabricate 100 nm-thick NFN membranes (Fig. 5e).99 UiO-66-Br and UiO-66-NO2 provided higher CO2 sorption capacity and better interfacial compatibility than UiO-66, and adding 40 wt% UiO-66-Br or UiO-66-NO2 increased CO2 permeance from 1393 to 1900 GPU and CO2/N2 selectivity from 23 to 37 (Fig. 5f).
3.2. TFN membranes based on DE-MOFs
DE-MOFs can increase surface area, tune pore volume and structure, and provide metal and ligand changes.35,102–104 They are often prepared by de novo and post-synthesis treatment.80,105 In the de novo approach, defects are generated during MOF formation by regulating synthesis conditions, such as ligand composition and solution temperature. Particularly, using two or more ligands in a starting solution introduces competition between ligands to coordinate with metal ions, resulting in defects due to unmatched crystal structures.106,107 For example, adding a thermally labile ligand to the MOFs before thermal treatment at the decomposition temperature induced structures with missing ligands;105 amino benzoic acid was added to terephthalic acid, resulting in defective sites and introducing amino groups with affinity towards CO2.105 This method is also influenced by other factors, such as mixed metal sources, solvent types, and other synthesis methods (like microwave).80,107For the method of post-synthesis treatment, MOFs are post-modified by exchanging ligands or metal centers, a process also known as post-synthesis exchange (PSE).50,87,88 The coordination of metal–ligand is kinetically unstable, allowing for breakage or reformation within a MOF lattice.50,88Fig. 8a and b illustrates the metal or ligand exchange by adding a new metal or ligand, respectively. For instance, a bimetallic Zr/Ti-based MOF was synthesized with high CO2 adsorption capacity, and more stable MOFs were prepared by complete metal exchange of Zn2+ with Cu2+.108,109 The ligand exchange can be achieved by exposure to a ligand-concentrated solution or a ligand vapor.50 For example, ZIF-8 was exposed to various halogenated imidazoles in the vapor phase.110 Additionally, defects can be introduced in MOFs through etching using chemicals (such as acids or bases) or plasma treatment.50,88
 | ||
| Fig. 8 DE-MOFs. (a) UiO-66 modified with metal exchange.111,112 Copyright 2016. Reproduced with permission from the Royal Society of Chemistry. (b) ZIF-8 modified by vapor-phase ligand exchange.110 Copyright 2020. Reproduced with permission from American Association for the Advancement of Science. (c) CFM images showing the defective structures of single crystals of HKUST-1 (top row) and MOF-5 (bottom row);106 Copyright 2017. Reproduced with permission from Elsevier. (d) HRTEM of MOFs without defects (top row), with missing linker defects (middle row), and with missing cluster defects (bottom row).107 Copyright 2020. Reproduced with permission from the Royal Society of Chemistry. | ||
Defects in DE-MOFs can be characterized using various techniques, including topological, structural, and quantitative studies.35,105–107 Scanning and transmission electron microscopy (SEM and TEM) can be used to monitor changes in MOF topologies. Structural defects can be validated using powder and single-crystal X-ray diffraction (PXRD and SXRD), BET surface area and pore volume, thermogravimetric analysis (TGA), nuclear magnetic resonance (NMR), and positron annihilation lifetime spectroscopy (PALS) (Fig. 8c and d). Various 3D tools can also be used, such as confocal fluorescence microscopy (CFM), fluorescence lifetime imaging (FLIM), and scanning electron diffraction (SED). X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) have also been used to measure defects quantitatively.106,107
DE-MOFs have gained significant interest in designing TFN membranes, as summarized in Table 3. For instance, hollow UiO NPs (H-UiO) were synthesized using an anisotropic acid etching method and then embedded in Pebax-2533 to fabricate membranes for CO2 separation.113 The H-UiO NPs were characterized using potentiometric acid–base titration, XRD, BET, and 1H NMR to confirm the crystalline structure and missing linkers. For example, NMR results showed that acid etching decreased the molar ratio of acetate to terephthalate, indicating the presence of missing linkers and an increased content of defect-terminal hydroxyl groups. Adding 6 mass% H-UiO-66 in 6 µm films enhanced CO2 permeance by 240% from 150 to 520 GPU and CO2/N2 selectivity by 130% from 19 to 44. By contrast, adding 6 mass% conventional UiO-66 increased CO2 permeance only to 380 GPU and CO2/N2 selectivity to 34.113
| Membranes | Support/gutter layer | Selective layers | T (°C)/p (bar) | CO2 (H2) permeance (GPU) | Selectivity | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymers | MOFs | Size (nm) | MOF (wt%) | l (nm) | CO2/N2 | CO2/CH4 | H2/CO2 | |||||
| NFN | PAN | Cu(SIF)6(pyz)3@PEG | 5–10 | 80 | 50 | 25/1 | (3643) | 76 | 114 | |||
| Si/ZnO | Pebax | HKUST-1 | 15 | 0 | 100 | 25/1 | (543) | 13 | 115 | |||
| — | 100 | (8460) | 41 | |||||||||
| TFN | α-Al2O3 | Pebax 2533 | — | — | 6000 | 25/1 | 150 | 19 | 113 | |||
| H-UiO-66 | 6 | 800 | 1876 | 41 | ||||||||
| PES | Pebax | — | — | 1870 | 25/1 | 150 | 18 | 116 | ||||
| UiO-66 | 60 | 2 | 640 | 656 | 50 | |||||||
| PSF | Pebax | — | — | 220 | 25/1 | 394 | 25 | 117 | ||||
| ASM-202 | 78 | 10 | 267 | 936 | 52 | |||||||
| AAO | ZIF-62 | — | — | 2000 | 25/1 | 551 | 28 | 30 | 118 | |||
| PSF | Pebax | 2D ZIF-8 | 3.5 nm × 1.6 µm | 0 | — | 35/2 | 161 | 44 | 119 | |||
| 10 | 780 | 710 | 77 | |||||||||
| PAN | 6FDA-DAM | NTU-82 | 5–6 | 0 | 600 | 25/3 | 250 | 19 | 120 | |||
| 15 | 800 | 1190 | 20 | |||||||||
Fig. 9a displays a hybrid filler of CNC@UiO-66 with unsaturated metal sites, which interact with CO2, resulting in increased separation performance.116 For instance, adding 1 wt% fillers in TFN membranes (Fig. 9b) increased CO2 permeance from 150 to 644 GPU and CO2/N2 selectivity from 18 to 44, surpassing the upper bound (Fig. 9c).
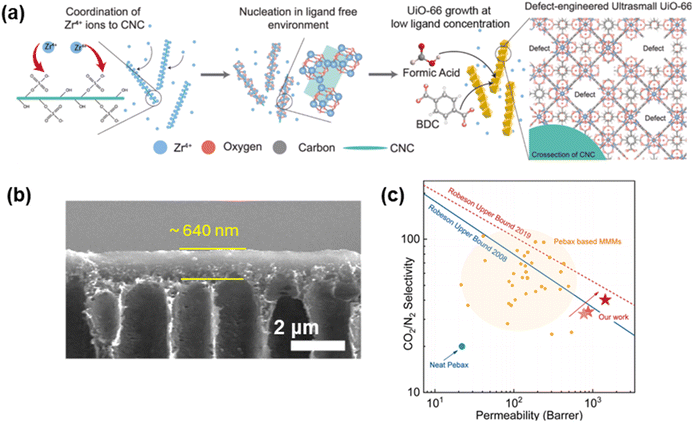 | ||
| Fig. 9 TFN membranes with DE-MOFs. (a) Synthesis of DE-UiOs by acid modulation, (b) cross-section images of membranes containing 2 wt% DE-UiOs, and (c) gas separation properties.116 Copyright 2025. Reproduced with permission from Elsevier. | ||
DE-MOFs can also be surface-functionalized to further improve CO2/N2 separation properties.121,122 For instance, defective UiO-66-NH2 was synthesized by the modulation with trifluoroacetic acid (TFA), which was then modified with an IL, [bmim][Tf2N].121 The modification improved their dispersibility and compatibility with PIM-1 and affinity towards CO2, enhancing CO2 solubility and CO2/N2 solubility selectivity. Similar approaches have been used to improve CO2 separation properties in MMFs.123–127
3.3. TFN membranes based on aMOFs
Compared to conventional MOFs, aMOFs preserve the same connection between the metal nodes and ligands with potential porosity,128–130 but they have short-range orders, instead of long-range orders, resulting in diffusive peaks instead of sharp peaks in XRD patterns.129,130 The aMOFs can be obtained from MOFs using several methods, such as pressure-, heat-, and mechanical milling-induced amorphization (Fig. 10a).39,131–133 Specifically, if a MOF exhibits a melting temperature (Tm) lower than its decomposition temperature (Td), the MOF can be melted and rapidly cooled to form glassy MOFs (g-MOFs), a sub-category of aMOFs.38,131 | ||
| Fig. 10 TFN membranes based on aMOFs. (a) Melt-quenching process to prepare aMOFs from MOFs;134 Copyright 2024. Reproduced with permission from Springer Nature. (b) Schematic of membranes based on amorphous ASM-202 and (c) enhanced CO2/N2 separation properties.117 Copyright 2024. Reproduced with permission from Elsevier. | ||
The aMOFs can be used as fillers for gas separation. For instance, ASM-202 NPs of 80 nm were embedded in Pebax (∼250 nm) (Fig. 10b and c);117 incorporating 10 wt% ASM-202 increased CO2 permeance from 394 to 936 GPU and CO2/N2 selectivity from 25 to 52, which can be partially ascribed to its amorphous structure. Additionally, ASM-202 provided more open metal sites and N-doped structures with affinity towards CO2.
The g-MOFs attract attention due to their absence of grain boundaries and good filler/polymer compatibility.48,118,135–142 Notably, g-MOFs can only be obtained from a limited number of MOFs because amorphization usually needs high temperatures above 350–400 °C, while only a few types of ZIFs (like ZIF-4 and ZIF-6) can be stable at such temperatures.48,142 However, g-MOFs usually exhibit lower porosity than MOFs and therefore lower gas permeability. To address this issue, glassy ZIF-62 (g-ZIF-62) was prepared by in situ thermal treatment at 420 °C in PIM-1.135 The thermal treatment removed interfacial voids of g-ZIF-62/PIM-1 and partially cross-linked the polymer, enhancing the free volume and gas permeability. For instance, introducing 30 wt% g-ZIF-62 increased CO2 permeability from 4654 to 5914 Barrer and CO2/CH4 selectivity from 18 to 66 at 25 °C.
The aMOFs can be blended with conventional MOFs, forming a crystal-glass composite membrane (CGCM).48,142–146 The MOF has a Td higher than the Tm of the g-MOF, and thus, its crystalline structure can be preserved during the melting process, while avoiding the formation of voids between the filler and polymer. Additionally, microporous polymers with low melting temperatures (180 °C) were used to prepare CGCMs, instead of g-MOFs.141 Crystalline MIL-101 NPs with a high surface area were dispersed into the Zn–P-dmbIm (a coordination polymer) with a relatively low surface area. MIL-101 preserved its crystalline structure during the heating process, and adding 10 wt% MIL-101 dramatically increased CO2 permeability from 850 to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Barrer and CO2/N2 selectivity from 3 to 62.
000 Barrer and CO2/N2 selectivity from 3 to 62.
Glassy ZIF-62 membranes were also prepared using a large-molecule solvent as the structure-directing agent (SDA), resulting in large pores preserved in ZIFs with different topologies.137 Consequently, the g-MOF NPs exhibited high porosity and formed continuous channels, resulting in CO2 permeance of 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GPU and good CO2/N2 selectivity of 15.137
000 GPU and good CO2/N2 selectivity of 15.137
3.4. TFN membranes based on polyMOFs
PolyMOFs have been synthesized using polymers as ligands in the MOFs (Fig. 11a).40,42,147–149 They combine the crystalline structure of MOFs and the amorphous behavior of polymers. PolyMOFs have been explored for gas separations.150,151 However, they often had large particles, making them unfavorable for membrane applications. | ||
| Fig. 11 TFN membranes based on polyMOFs. (a) Schematic of synthesis and SEM image of polyUiO.40 Copyright 2020. Reproduced with permission from American Chemical Society. (b) Synthesis of MOF, polyMOFs, and cPIM-based polyMOFs,150 (c) CO2/N2 separation properties, and (d) comparison with the leading membranes.150 Copyright 2023. Reproduced with permission from Springer Nature. | ||
To address this issue, polyMOFs were synthesized by in situ growth of UiO-66 with cPIM-1 with functional moieties similar to the ligand (Fig. 11b).150 Increasing the cPIM-1 content decreased the crystallinity, ultramicropore size, and CO2-sorption-derived surface area but decreased microporosity and N2-sorption-derived surface area, which was beneficial for CO2/N2 separation. The obtained polyMOF NPs exhibited good dispersibility in coating solutions and were then added to PIM-1 with good compatibility. TFC membranes with a selective layer (∼3 µm) containing 20 wt% polyMOFs were fabricated at a large scale (9–400 cm2), and they exhibited CO2 permeance of 4800 GPU and CO2/N2 selectivity of 21 (Fig. 11c and d).
3.5. TFN membranes based on MC-MOFs
MC-MOFs were developed by incorporating macrocycle molecules in the frameworks, including crown ethers (CEs), cyclodextrins, calixarenes, and pillararenes (Fig. 12a).43,44,152 Due to their limited rotational degrees, these macrocyclic hosts possess well-defined cavities for functionalization. As such, MC-MOFs exhibit crystalline structure, high surface area, and functional cavities.44 | ||
| Fig. 12 TFN membranes based on MC-MOFs. (a) Example of MC-MOFs.44 Copyright 2025. Reproduced with permission from Elsevier. (b) Scheme of surface modification of MOFs with carboxylic CEs and (c) separation performance;155 Copyright 2023. Reproduced with permission from John Wiley & Sons, Inc. | ||
Macrocycles can be incorporated in MOFs using covalent grafting to the surface and non-covalent blending for pore tuning, and they may be directly used as a ligand in synthesizing MOFs.44 For instance, CEs can be trapped inside the frameworks during the MOF synthesis.153 Additionally, carboxylic- and nitrobenzo-functionalized CEs were used to modify the MOF surface, which increased dispersibility and separation performance.154,155 Notably, due to steric hindrance, macrocycles tend to exhibit low reactivity for functionalization.44
Fig. 12b shows that MOFs were surface-modified using CEs to enhance dispersibility and stability within the polymers.155 CEs have cyclic cavities, which are hydrophobic inside and hydrophilic outside, and they have been investigated for ion separations. Specifically, carboxylic-based 21-Crown-7-Ether (21CE-COOH) was used to modify the exterior surface of a series of MOFs with a coordination bond between the –COOH group of CE and metal ions. The surface modification enhanced MOF-solvent interaction and prevented inter-particle agglomeration, improving dispersibility in the polymer. CE-MOFs were also incorporated in polyimides (Fig. 11c); adding CE-functionalized MOFs increased CO2 adsorption capacity to 1.75 × 10−2 mmol g−1 (resulting in CO2/N2 selectivity of 70), while adding MOF and a mixture of MOF and CE achieved CO2 adsorption capacity of 0.75 × 10−2 and 1.0 × 10−2 mmol g−1, respectively.
Nitrobenzo-based CE was used to modify the surface of Azo-UiO-66 NPs, enhancing their compatibility with Pebax-1657 and increasing SO2/N2 selectivity from 486 to 643.154 Additionally, CEs were encapsulated in the dynamic cage of ZIF-7, narrowing the pore size and increasing N2/CH4 selectivity from 2 to 7.156
3.6. TFN membranes based on 2D MOFs
TFN membranes using ultrathin 2D nanosheet MOFs have been explored for CO2 separations,119,120,157–160 because 2D MOFs with high aspect ratios provide better interactions/interfaces with polymers, mitigating interfacial voids.120 For example, bimetallic MOF-74 nanosheets with open metal sites were synthesized (Fig. 13a) and blended with PVAm and fabricated into TFN membranes with a 300 nm selective layer (Fig. 13b); the membrane exhibited an outstanding H2 permeance of 1000 GPU and H2/CO2 selectivity of 800, far exceeding Robeson's upper bound (Fig. 12c).160 | ||
| Fig. 13 TFN membranes based on 2D MOFs. (a) Preparation of bi-metallic MOF-74; (b) cross-section view, and (c) superior H2/CO2 separation properties.160 Copyright 2024. Reproduced with permission from Elsevier. (d) Preparation of UiO nanosheets by etching, (e) cross-section image, and (f) superior CO2/N2 separation performance.161 Copyright 2023. Reproduced with permission from John Wiley & Sons, Inc. | ||
UiO nanosheets were also synthesized from bulk crystals using an anisotropic etching method (Fig. 13d), and they were fabricated into Pebax-based TFN membranes with the selective layer of 900 nm (Fig. 13e);161 adding 4 wt% nanosheets increased CO2 permeance by 86% from 747 to 1650 GPU and CO2/N2 selectivity by 105% from 16 to 33 (Fig. 13f).
NTU-82 nanosheets containing Hf4+ metal cluster were synthesized using a capping agent (formic acid), leading to H-bonds between the nanosheets with 6FDA-DAM and PIM-1.120 Adding 15 mass% nanosheets in the 6FDA-DAM selective layer (800 nm) increased CO2 permeance from 250 to 1190 GPU while retaining CO2/N2 selectivity of 20, while adding 15 mass% nanosheets in PIM-1 (1500 nm) increased CO2 permeance from 480 to 2520 GPU while retaining CO2/N2 selectivity of ≈13.
3.7. TFN membranes based on other engineered MOFs
Advanced MOFs with unique morphologies have been made, such as interconnected channels, wrinkled surfaces, and interwoven networks. Fig. 14a illustrates a solid-solvent processing method to prepare a nanolayer of TFN membranes containing 80 vol% MOFs.114 The MOFs were dispersed at a molecular level in the polymer because the polymer can dissolve the metal salt. The selective layer had a filler-dominant structure with interconnected channels for gas transport (Fig. 14b), resulting in high separation performance (Fig. 14c).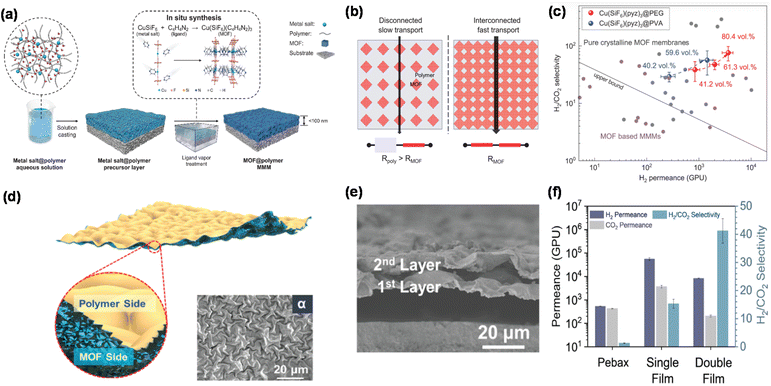 | ||
| Fig. 14 TFN membranes based on other engineered MOFs. (a) Schematic of membranes comprising 80 vol% MOFs in the polymer, (b) interconnected pathways, and (c) superior H2/CO2 separation properties.114 Copyright 2023. Reproduced with permission from the American Association for the Advancement of Science. (d) Scheme of double-sided wrinkled TFN membranes (inset shows the α pattern of the membrane), (e) cross-section view, and (f) gas separation performance of Pebax, single, and double wrinkled films.45 Copyright 2023. Reproduced with permission from the American Association for the Advancement of Science. | ||
Wrinkled MOF films with various Turing (wrinkled) patterns were synthesized by changing the reagent concentrations before coating with a Pebax layer (Fig. 14d).45 The obtained membranes exhibited H2 permanence of 8460 GPU and H2/CO2 of 41 (Fig. 14e and f).
Interwoven MOF-gel polymer networks were also used to prepare NFN membranes with selective layers as thin as 50 nm.162 Their molecular weaving strategy resulted in flexible 3D UiO-66 gel networks incorporating PEI and GA, which provided H-bonding and coordination with MOF gels. The obtained membranes achieved H2 permanence of 845 GPU and H2/CO2 selectivity of 17, and their fabrication was successfully scaled up to a large area (>160 cm2).
4. Discussions
4.1. Comparison of MOFs for their improvement in separation properties
Table 4 summarizes the formation mechanisms, structure characteristics, and enhancement in gas separation for conventional and engineered MOFs. Each engineered MOF represents an effective way to enhance membrane separation properties, and its effects depend on its unique structure and interactions with the polymer matrix.| MOFs | Synthesis mechanism | Procedures | Effects on gas separation properties |
|---|---|---|---|
| Conventional MOFs | Mixing metal clusters and organic ligands in a solvent | Coordination bonds between metal and ligand | Temperature, modulating agent, and solvent affect NP sizes and morphologies |
| SM-MOFs | Modifying the surface of MOFs with coordination or covalent bonds | Using chemicals after MOF formation | Enhanced polymer-MOF compatibility and functionalities |
| DE-MOFs | Adjusting metal–ligand coordination bonds | Mixed ligands or metal ions, or post-exchange with ligands or metal ions | Missing linkers or metal centers; increased porosity and surface area |
| aMOFs | Transition from crystalline to amorphous MOFs | Melt-quenching; pressure and mechanical milling | Mitigated grain boundaries and filler-polymer interfacial voids |
| polyMOFs | Interaction between metal centers and ligand-like polymers | Adding polymeric co-ligands to the parent solutions | Enhanced MOF dispersibility and functionalities |
| MC-MOFs | Coordination with open metal centers on MOF surface | Using macrocycles as the co-ligands or surface-modifying agents | Creating additional selective gates and enhancing MOF dispersibility |
| 2D MOFs | Top-down or bottom-up approaches | Stacking MOF layers along the vertical direction via weak interaction forces | Enlarged surface area; enhanced open metal sites; shorter transport pathway |
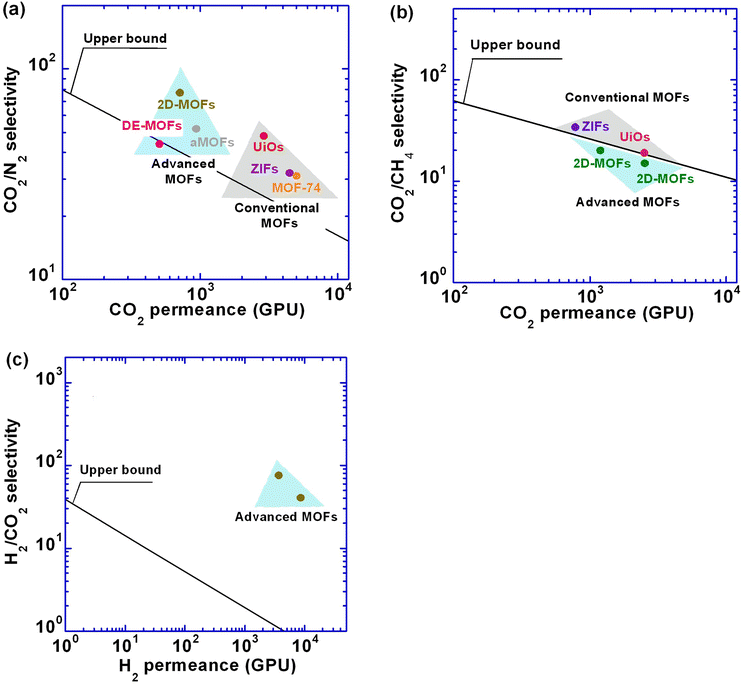
Fig. 15 and Table 5 highlight the use of advanced MOFs to enhance the separation properties of CO2/N2, CO2/CH4, and H2/CO2. The enhancement of gas permeance and selectivity by adding MOFs can also be characterized by permeance enhancement (βP, %) and selectivity enhancement (βS, %), which are defined as the increase relative to those of the pristine polymeric membranes. Furthermore, the overall enhancement in the separation performance is given by a filler enhancement index (Findex):163
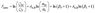 | (10) |
| Separations | Category | Samples | β P (%) | β S (%) | F index (%) | Ref. |
|---|---|---|---|---|---|---|
| CO2/N2 | MOFs | aPEO + UiO | 107 | −4 | 0.61 | 165 |
| POEM + ZIF | 312 | −20 | 0.77 | 76 | ||
| PIM + MOF-74 | 16 | 63 | 1.6 | 74 | ||
| Engineered MOFs | Pebax + DE-MOFs | 265 | 132 | 3.7 | 113 | |
| Pebax + aMOFs | 137 | 108 | 3.0 | 117 | ||
| Pebax + 2D MOF | 341 | 75 | 3.1 | 119 | ||
| CO2/CH4 | MOFs | PI + ZIF | 156 | 55 | 2.1 | 101 |
| PIM + UiO | −45 | 46 | 0.39 | 85 | ||
| Engineered MOFs | 6FDA-DAM + 2D | 376 | 5 | 1.7 | 120 | |
| PIM + 2D MOF | 425 | 25 | 2.3 | |||
| H2/CO2 | Engineered MOFs | Cu(SIF)6(pyz)3@PEG | — | — | — | 114 |
| Pebax + HKUST | 1460 | 215 | 5.4 | 115 |
4.2. Scale-up of TFN membranes
Though rarely, large-scale TFN membranes have been successfully produced. For example, NFN membranes based on interwoven MOF-gel polymer networks (50 nm) were prepared at 160 cm2 using a blade casting technique;162 membranes based on cPIM-1 and polyUiO (∼3 µm) were fabricated at 400 cm2 using a scalable bar-coating method;150 membranes based on aPEO and UiO-66-NH2 were fabricated at ∼100 cm2 using an automatic coating machine;52 membranes based on PVAm and rigid ZIF-8 were fabricated at 3100 cm2 with a blade casting method.166 Notably, NFN membranes with MOF layers of 50–130 nm were also successfully synthesized at 2400 cm2 for gas separations.167,168 Nevertheless, we envision that if MOF NPs can be dispersed in polymer coating solutions, TFN membranes can be fabricated using the roll-to-roll process developed for polymeric membranes.5. Conclusion and perspectives
The platform of TFN membranes represents a fruitful marriage of polymeric membranes and MOFs with an enormous library of chemistry, pore size, porosity, and morphology. Many membranes present CO2 separation properties above Robeson's upper bound and surpassing state-of-the-art polymeric membranes. We expect that TFN and NFN membranes will continue to make great strides in the coming decades, and the following challenges should be addressed to bring this exciting materials platform to practical use. First, more studies should focus on the scalable fabrication of TFN membranes consistently using roll-to-roll processes (as opposed to thick freestanding films), similar to the challenges faced by many nanomaterials for practical applications.169 The aggregation and distribution of the MOFs in thin films should be controlled to optimize gas separation properties. For example, the location of the NPs on the surface or at the bottom of the selective layers exerts a dramatic influence on gas permeance.Second, there is an imperative need to understand the difference between the bulk films and nanofilms for nanocomposites, which is critical to designing NFN membranes.85,170,171 The polymer chain dynamics of the nanofilms can be influenced by their thickness, particularly in the presence of MOF NPs with diameters comparable to the selective layer thickness, which affects polymer chain conformations and packing density in the particle/polymer interface, impacting molecular separation properties.170 For instance, decreasing the thickness from 60 µm to 35 nm dramatically decreased gas permeability for 6FDA-DAM (by 72%) and PIM-1 (by 87%) due to the nanoconfinement-induced microstructure change and physical aging.172 Despite a rich literature focusing on polymer dynamics of nanocomposites containing NPs (like silica and Au),173 there are very few studies on MOF-based TFNs. Additionally, the nano-confinement in the thin films may also influence the NP aggregation and distribution.
Third, while the pore sizes and porosity of MOFs have been extensively explored to improve gas diffusivity and selectivity, the potential affinity between the MOFs and targeted gases has been rarely investigated.174 Particularly, MOFs can have open metal sites (OMS), such as MOF-74 and HKUST-1, which have high metal site density and strong interactions with various gas molecules.175,176 We also expect that new MOFs can be designed with modeling, simulation, and machine learning for membrane gas separations,177–179 similar to other scientific fields that have been involved in and developed through the use of artificial intelligence (AI).180–182
Finally, for MOFs to be incorporated into nanofilms of <100 nm in NFN membranes, they should be less than 100 nm in diameter and preferably less than 50 nm. Therefore, it is crucial to be able to synthesize nano-sized MOFs with high yields on a large scale at a low cost.
Conflicts of interest
The authors declare no competing financial interests.Data availability
Data are available upon request to the corresponding author.Acknowledgements
This work received financial support from the U.S. Department of Energy Small Business Technology Transfer Program (DE-SC0020730).References
- W. J. Koros and C. Zhang, Nat. Mater., 2017, 16, 289–297 CrossRef CAS.
- M. Galizia, W. S. Chi, Z. P. Smith, T. C. Merkel, R. W. Baker and B. D. Freeman, Macromolecules, 2017, 50, 7809–7843 CrossRef CAS.
- M. Sanderu, E. M. Sandru, W. F. Ingram, J. Deng, P. M. Stenstad, L. Deng and R. J. Spontak, Science, 2022, 376, 90–94 CrossRef.
- Y. Han and W. Ho, J. Membr. Sci., 2021, 628, 119244 CrossRef CAS.
- H. Park, J. Kamcev, L. M. Robeson, M. Elimelech and B. D. Freeman, Science, 2017, 356, eaab0530 CrossRef PubMed.
- Y. Hua, S. Park and H.-K. Jeong, J. Environ. Chem. Eng., 2024, 12, 113753 CrossRef CAS.
- L. Hu, K. Chen, W. I. Lee, K. Kisslinger, C. Rumsey, S. Fan, V. T. Bui, N. Esmaeili, T. Tran, Y. Ding, M. Trebbin, C.-Y. Nam, M. T. Swihart and H. Lin, Adv. Mater., 2023, 35, 2301007 CrossRef CAS.
- M. S. Denny, J. C. Moreton, L. Benz and S. M. Cohen, Nat. Rev. Mater., 2016, 1, 1–17 Search PubMed.
- G. Cai, P. Yan, L. Zhang, H. Zhou and H. Jiang, Chem. Rev., 2021, 121, 12278–12326 CrossRef CAS.
- Q. Qian, P. A. Asinger, M. J. Lee, G. Han, K. Mizrahi Rodriguez, S. Lin, F. M. Benedetti, A. X. Wu, W. S. Chi and Z. P. Smith, Chem. Rev., 2020, 120, 8161–8266 CrossRef CAS.
- Q. Zhao, S. Lian, R. Li, Z. Yu, Q. Liu, G.-L. Zang and C. Song, Chem. Eng. J., 2022, 443, 136290 CrossRef CAS.
- K.-G. Liu, F. Bigdeli, A. Panjehpour, S. Hwa Jhung, H. A. J. Al Lawati and A. Morsali, Coord. Chem. Rev., 2023, 496, 215413 CrossRef CAS.
- L. Hu, K. Clark, T. Alebrahim and H. Lin, J. Membr. Sci., 2022, 644, 120140 CrossRef CAS.
- M. Liu, M. D. Nothling, P. A. Webley, Q. Fu and G. G. Qiao, Acc. Chem. Res., 2019, 52, 1905–1914 CrossRef CAS.
- D. Zhao, F. Feng, L. Shen, Z. Huang, Q. Zhao, H. Lin and T.-S. Chung, Chem. Eng. J., 2023, 454, 140447 CrossRef CAS.
- Y. Duan, L. Li, Z. Shen, J. Cheng and K. He, Membranes, 2023, 13, 1–31 CrossRef PubMed.
- Y. Jia, K. Wong, C. Z. Liang, J. Wu, T.-S. Chung and S. Zhang, Prog. Mater. Sci., 2024, 146, 101324 CrossRef CAS.
- Z. Yang, P. F. Sun, X. Li, B. Gan, L. Wang, X. Song, H. D. Park and C. Y. Tang, Environ. Sci. Technol., 2020, 54, 15563–15583 CrossRef CAS.
- G. Zhang and H. Lin, Green Energy Environ., 2024, 9, 1220–1238 CrossRef CAS.
- J. Wu, F. Hillman, C.-Z. Liang, Y. Jia and S. Zhang, J. Mater. Chem. A, 2023, 11, 17452–17478 RSC.
- F. Pazani, M. Shariatifar, M. Salehi Maleh, T. Alebrahim and H. Lin, Sep. Purif. Technol., 2023, 308, 122876 CrossRef CAS.
- Z. Dai, J. Deng, L. Ansaloni, S. Janakiram and L. Deng, J. Membr. Sci., 2019, 578, 61–68 CrossRef CAS.
- A. Raza, M. Askari, C. Z. Liang, N. Peng, S. Farrukh, A. Hussain and T.-S. Chung, J. Membr. Sci., 2021, 625, 119124 CrossRef CAS.
- Y. Cheng, S. J. Datta, S. Zhou, J. Jia, O. Shekhah and M. Eddaoudi, Chem. Soc. Rev., 2022, 51, 8300–8350 RSC.
- M. R. A. Hamid, Y. Qian, R. Wei, Z. Li, Y. Pan, Z. Lai and H.-K. Jeong, J. Membr. Sci., 2021, 640, 119802 CrossRef.
- W. Su, Y. Xiang, Y. Dai, Y. Wang, S. Zhong and J. Li, Chem. Commun., 2024, 60, 7124–7135 RSC.
- B. Ghalei, K. Sakurai, Y. Kinoshita, K. Wakimoto, A. P. Isfahani, Q. Song, K. Doitomi, S. Furukawa, H. Hirao and H. Kusuda, Nat. Energy, 2017, 2, 1–9 Search PubMed.
- N. Tien-Binh, D. Rodrigue and S. Kaliaguine, J. Membr. Sci., 2018, 548, 429–438 CrossRef CAS.
- Y. Song, S. Zheng, Y. Ji, B. He and M. Wang, Chem. Eng. J., 2025, 518, 164526 CrossRef CAS.
- M. Zeeshan, H. C. Gulbalkan, O. Durak, Z. P. Haslak, U. Unal, S. Keskin and A. Uzun, Adv. Funct. Mater., 2022, 32, 2204149 CrossRef CAS.
- S. Hussain, A. Ali, S. Foorginezhad, Y. Chen and X. Ji, Sep. Purif. Technol., 2024, 360, 130997 CrossRef.
- L. Zhang, Y. He and Y. Fu, Chem. Commun., 2025, 61, 2878–2890 RSC.
- S. Yu, C. Li, S. Zhao, M. Chai, J. Hou and R. Lin, Nanoscale, 2024, 16, 7716–7733 RSC.
- M. Taddei, Coord. Chem. Rev., 2017, 343, 1–24 CrossRef CAS.
- S. Li, W. Han, Q. F. An, K. T. Yong and M. J. Yin, Adv. Funct. Mater., 2023, 33, 2303447 CrossRef CAS.
- X. Qiu and R. Wang, Coord. Chem. Rev., 2025, 526, 216356 CrossRef CAS.
- M. Kim, Y. Lee and H. R. Moon, Acc. Chem. Res., 2024, 57, 2347–2357 CrossRef CAS.
- T. D. Bennett and S. Horike, Nat. Rev. Mater., 2018, 3, 431–440 CrossRef.
- Z. Yu, L. Tang, N. Ma, S. Horike and W. Chen, Coord. Chem. Rev., 2022, 469, 214646 CrossRef CAS.
- M. Kalaj, K. C. Bentz, S. Ayala Jr, J. M. Palomba, K. S. Barcus, Y. Katayama and S. M. Cohen, Chem. Rev., 2020, 120, 8267–8302 CrossRef CAS.
- T. Kitao, Y. Zhang, S. Kitagawa, B. Wang and T. Uemura, Chem. Soc. Rev., 2017, 46, 3108–3133 RSC.
- S. Kumar, B. Mohan, B. Musikavanhu, X. Wang, R. Muhammad, X. Yang and P. Ren, Coord. Chem. Rev., 2025, 524, 216286 CrossRef CAS.
- Y. Liang, E. Li, K. Wang, Z. Guan, H. He, L. Zhang, H. Zhou, F. Huang and Y. Fang, Chem. Soc. Rev., 2022, 51, 8378–8405 RSC.
- S. Cheng, Y. Liu and Y. Sun, Coord. Chem. Rev., 2025, 534, 216559 CrossRef CAS.
- X. Luo, M. Zhang, Y. Hu, Y. Xu, H. Zhou, Z. Xu, Y. Hao, S. Chen, S. Chen and Y. Luo, Science, 2024, 385, 647–651 CrossRef CAS.
- M. Shan, X. Geng, I. Imaz, A. Broto-Ribas, B. Ortín-Rubio, D. Maspoch, L. Ansaloni, T. A. Peters, A. Tena and M. E. Boerrigter, J. Membr. Sci., 2024, 691, 122258 CrossRef CAS.
- Q. Wu, X. He, C. Cui, B. Qi and J. Wei, Sep. Purif. Technol., 2025, 354, 128946 CrossRef CAS.
- Y. Zhang, B. H. Yin, L. Huang, L. Ding, S. Lei, S. G. Telfer, J. Caro and H. Wang, Prog. Mater. Sci., 2025, 151, 101432 CrossRef CAS.
- S. Sun, Y. Zhang, M. Wang, Y. Zhao, J. Peng, S. Cong and H. Pang, Ind. Eng. Chem. Res., 2025, 64, 14771–14788 CrossRef CAS.
- T. Chen and D. Zhao, Coord. Chem. Rev., 2023, 491, 215259 CrossRef CAS.
- Q. Ma, J. Li, Y. Li and J. Choi, J. Membr. Sci., 2024, 690, 122201 CrossRef CAS.
- G. Zhang, C. J. Shah, W. I. Lee, K. Kisslinger, N. Esmaeili, V. T. Bui, L. Zhu, C. Y. Nam and H. Lin, Adv. Funct. Mater., 2024, 34, 2404785 CrossRef CAS.
- T. Alebrahim, L. Huang, H. K. Welgama, N. Esmaeili, E. Deng, S. Cheng, D. Acharya, C. M. Doherty, A. J. Hill, C. Rumsey, M. Trebbin, T. R. Cook and H. Lin, ACS Appl. Mater. Interfaces, 2024, 16, 11116–11124 CrossRef CAS.
- C. Liang, T.-S. Chung and J.-Y. Lai, Prog. Polym. Sci., 2019, 97, 101141 CrossRef CAS.
- M. Yu, A. B. Foster, S. E. Kentish, C. A. Scholes and P. M. Budd, J. Membr. Sci., 2025, 722, 123844 CrossRef CAS.
- J. Zhao, G. He, G. Liu, F. Pan, H. Wu, W. Jin and Z. Jiang, Prog. Polym. Sci., 2018, 80, 125–152 CrossRef CAS.
- L. Zhu, M. Yavari, W. Jia, E. P. Furlani and H. Lin, Ind. Eng. Chem. Res., 2017, 56, 351–358 CrossRef CAS.
- M. Kattula, K. Ponnuru, L. Zhu, W. Jia, H. Lin and E. P. Furlani, Sci. Rep., 2015, 5, 15016 CrossRef.
- M. Liu, K. Xie, M. D. Nothling, P. A. Gurr, S. S. L. Tan, Q. Fu, P. A. Webley and G. G. Qiao, ACS Nano, 2018, 12, 11591–11599 CrossRef CAS.
- Y. Ma, N. Liu, Y. Zhang, Z. Xu, J. Chen, H. Wu and J. Meng, J. Environ. Chem. Eng., 2025, 13, 116087 CrossRef CAS.
- Y. Zhang, L. Xu, X. Hu, B. Chen, X. Chen, Q. Li, H. Mao and Z. Zhao, Chem. Eng. J., 2025, 510, 161702 CrossRef CAS.
- M. Liu, K. Xie, M. D. Nothling, L. Zu, S. Zhao, D. J. E. Harvie, Q. Fu, P. A. Webley and G. G. Qiao, ACS Cent. Sci., 2021, 7, 671–680 CrossRef CAS.
- Z. Dai, L. Ansaloni and L. Deng, Green Energy Environ., 2016, 1, 102–128 CrossRef.
- P. Fu, H. Li, J. Gong, Z. Fan, A. T. Smith, K. Shen, T. O. Khalfalla, H. Huang, X. Qian, J. R. McCutcheon and L. Sun, Prog. Polym. Sci., 2022, 126, 101506 CrossRef CAS.
- K. Xie, Q. Fu, C. Xu, H. Lu, Q. Zhao, R. Curtain, D. Gu, P. A. Webley and G. G. Qiao, Energy Environ. Sci., 2018, 11, 544–550 RSC.
- G. Zhao, W. Han, L. Dong, H. Fan, Z. Qu, J. Gu and H. Meng, Green Energy Environ., 2022, 7, 1143–1160 CrossRef CAS.
- X. Qian, M. Ostwal, A. Asatekin, G. M. Geise, Z. P. Smith, W. A. Phillip, R. P. Lively and J. R. McCutcheon, J. Membr. Sci., 2022, 645, 120041 CrossRef CAS.
- S. K. Elsaidi, M. Ostwal, L. Zhu, A. Sekizkardes, M. H. Mohamed, M. Gipple, J. R. McCutcheon and D. Hopkinson, RSC Adv., 2021, 11, 25658–25663 RSC.
- C. Ge, M. Sheng, Y. Yuan, F. Shi, Y. Yang, S. Zhao, J. Wang and Z. Wang, Carbon Capture Sci. Technol., 2024, 10, 100156 CAS.
- M. Kang, H. Min, S. Kim and J. Kim, J. Membr. Sci., 2024, 698, 122611 CrossRef CAS.
- N. Li, Z. Wang, M. Wang, M. Gao, H. Wu, S. Zhao and J. Wang, J. Membr. Sci., 2021, 624, 119095 CrossRef CAS.
- L. Martínez-Izquierdo, C. Téllez and J. Coronas, J. Mater. Chem. A, 2022, 10, 18822–18833 RSC.
- H. Min, M.-B. Kim, Y.-S. Bae, P. K. Thallapally, J. H. Lee and J. Kim, Membranes, 2023, 13, 287 CrossRef CAS PubMed.
- M. Liu, M. D. Nothling, P. A. Webley, J. Jin, Q. Fu and G. G. Qiao, Chem. Eng. J., 2020, 396, 125328 CrossRef CAS.
- H. Min, M. Kang, Y.-S. Bae, R. Blom, C. A. Grande and J. Kim, J. Membr. Sci., 2023, 669, 121295 CrossRef CAS.
- C. Lee, M. Kang, K. Kim and J. Kim, J. Membr. Sci., 2022, 642, 119913 CrossRef CAS.
- M. Kang, T. Kim, H. Han, H. Min, Y. Bae and J. Kim, J. Membr. Sci., 2022, 659, 120788 CrossRef CAS.
- D. Jiang, C. Huang, J. Zhu, P. Wang, Z. Liu and D. Fang, Coord. Chem. Rev., 2021, 444, 214064 CrossRef CAS.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H. Zhou, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed.
- A. Kirchon, L. Feng, H. F. Drake, E. A. Joseph and H. Zhou, Chem. Soc. Rev., 2018, 47, 8611–8638 RSC.
- Z. Hu, I. Castano, S. Wang, Y. Wang, Y. Peng, Y. Qian, C. Chi, X. Wang and D. Zhao, Cryst. Growth Des., 2016, 16, 2295–2301 CrossRef CAS.
- S. Fan, J. Wang, L. Liao, J. Feng, B. Li and S. Zhang, Chem. Eng. J., 2023, 468, 143645 CrossRef CAS.
- M.-H. Pham, G.-T. Vuong, F.-G. Fontaine and T.-O. Do, Cryst. Growth Des., 2012, 12, 3091–3095 CrossRef CAS.
- B. Qiu, M. Yu, J. M. Luque-Alled, S. Ding, A. B. Foster, P. M. Budd, X. Fan and P. Gorgojo, Angew. Chem., Int. Ed., 2024, 63, e202316356 CrossRef CAS.
- G. Chen, H. Zhu, G. Liu, G. Liu and W. Jin, Angew. Chem., Int. Ed., 2025, 64, e202418649 CrossRef CAS PubMed.
- G. Zhang, V. Bui, Y. Yin, E. H. R. Tsai, C. Y. Nam and H. Lin, ACS Appl. Mater. Interfaces, 2023, 15, 35543–35551 CrossRef CAS PubMed.
- J. Lee, J. Lee, J. Y. Kim and M. Kim, Chem. Soc. Rev., 2023, 52, 6379–6416 RSC.
- Z. Yin, S. Wan, J. Yang, M. Kurmoo and M.-H. Zeng, Coord. Chem. Rev., 2019, 378, 500–512 CrossRef CAS.
- H. Molavi, A. Shojaei and S. A. Mousavi, J. Mater. Chem. A, 2018, 6, 2775–2791 RSC.
- B. J. Yao, L. G. Ding, F. Li, J. T. Li, Q. J. Fu, Y. Ban, A. Guo and Y. B. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 38919–38930 CrossRef CAS PubMed.
- X. Jiang, S. Li, S. He, Y. Bai and L. Shao, J. Mater. Chem. A, 2018, 6, 15064–15073 RSC.
- H. Molavi and A. Shojaei, ACS Appl. Mater. Interfaces, 2019, 11, 9448–9461 CrossRef CAS.
- Q. Qian, A. X. Wu, W. S. Chi, P. A. Asinger, S. Lin, A. Hypsher and Z. P. Smith, ACS Appl. Mater. Interfaces, 2019, 11, 31257–31269 CrossRef CAS.
- H. Wang, S. He, X. Qin, C. Li and T. Li, J. Am. Chem. Soc., 2018, 140, 17203–17210 CrossRef CAS.
- Y. Zhang, X. Feng, H. Li, Y. Chen, J. Zhao, S. Wang, L. Wang and B. Wang, Angew. Chem., Int. Ed., 2015, 54, 4259–4263 CrossRef CAS.
- H. Han, J. M. P. Scofield, P. A. Gurr, P. A. Webley and G. G. Qiao, Adv. Mater. Interfaces, 2024, 11, 2400113 CrossRef CAS.
- X. Su, L. Bromberg, V. Martis, F. Simeon, A. Huq and T. A. Hatton, ACS Appl. Mater. Interfaces, 2017, 9, 11299–11306 CrossRef CAS.
- S. Fan, C. Liang, F. Feng, K. Wong, K. Wang, S. Jia, N. Bhuwania, S. Zhang and S. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202421028 CrossRef CAS.
- S. Min, M. Kang, Y.-J. Jeon, E.-Y. Kim, J. H. Kim and J.-H. Kim, J. Mater. Chem. A, 2025, 27356–27366 RSC.
- L. Martínez-Izquierdo, C. García-Comas, S. Dai, M. Navarro, A. Tissot, C. Serre, C. Téllez and J. Coronas, ACS Appl. Mater. Interfaces, 2024, 16, 4024–4034 CrossRef.
- S. Song, M. Zhao, Z. Guo, Y. Ren, J. Wang, X. Liang, Y. Pu, S. Wang, H. Ma and X. Wang, J. Membr. Sci., 2023, 669, 121340 CrossRef CAS.
- S. Dissegna, K. Epp, W. R. Heinz, G. Kieslich and R. A. Fischer, Adv. Mater., 2018, 30, e1704501 CrossRef PubMed.
- D. Fan, A. Ozcan, O. Shekhah, R. Semino, M. Eddaoudi and G. Maurin, J. Membr. Sci. Lett., 2022, 2, 100029 CrossRef.
- Z. Fang, B. Bueken, D. E. De Vos and R. A. Fischer, Angew. Chem., Int. Ed., 2015, 54, 7234–7254 CrossRef CAS.
- G. Choi, M. Mandal, H. Jung, J. Panda, Y. Kwon, K. Zhang, E. Vivek, M. Shon, K. Ravi, K. Baek, H. Kwon, J. Yeo and K. Cho, J. Mater. Sci. Technol., 2024, 201, 95–118 CrossRef CAS.
- J. Ren, M. Ledwaba, N. M. Musyoka, H. W. Langmi, M. Mathe, S. Liao and W. Pang, Coord. Chem. Rev., 2017, 349, 169–197 CrossRef CAS.
- W. Xiang, Y. Zhang, Y. Chen, C.-j. Liu and X. Tu, J. Mater. Chem. A, 2020, 8, 21526–21546 RSC.
- X. Song, T. K. Kim, H. Kim, D. Kim, S. Jeong, H. R. Moon and M. S. Lah, Chem. Mater., 2012, 24, 3065–3073 CrossRef CAS.
- D. Sun, W. Liu, M. Qiu, Y. Zhang and Z. Li, Chem. Commun., 2015, 51, 2056–2059 RSC.
- W. Wu, J. Su, M. Jia, Z. Li, G. Liu and W. Li, Sci. Adv., 2020, 6, eaax7270 CrossRef CAS PubMed.
- Y. Bai, Y. Dou, L. Xie, W. Rutledge, J. Li and H. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC.
- M. Kim, J. F. Cahill, H. Fei, K. A. Prather and S. M. Cohen, J. Am. Chem. Soc., 2012, 134, 18082–18088 CrossRef CAS.
- J. Yan, Y. Sun, T. Ji, M. Wu, S. Meng, W. Dong, Y. Liu, K. Yu, W. Hu, B. Sun, P. Lu, Y. Li, H. Hu and Y. Liu, AIChE J., 2024, 70, 1–11 Search PubMed.
- G. Chen, C. Chen, Y. Guo, Z. Chu, Y. Pan, G. Liu, G. Liu, Y. Han, W. Jin and N. Xu, Science, 2023, 381, 1350–1356 CrossRef CAS PubMed.
- C. Jiao, X. Song, X. Zhang, L. Sun and H. Jiang, ACS Appl. Mater. Interfaces, 2021, 13, 18380–18388 CrossRef CAS.
- X. Wang, S. F. Seyedpour, S. Hrapovic, U. D. Hemraz, M. Mozafari, M. Soroush, M. A. Islam, A. Mollahosseini, M. Sadrzadeh and J.-Y. Cho, Clean. Eng. Tech., 2025, 27, 100999 CrossRef.
- X. Zhang, X. Bai, Y. Wang, F. Hu, X. Lu and J. Li, J. Membr. Sci., 2024, 692, 122290 CrossRef CAS.
- S. Xie, X. Tan, Z. Xue, P. Geysens, H. Pan, W. Guo, Z. Zhou, X. Zhang, I. F. J. Vankelecom and J. Fransaer, Angew. Chem., Int. Ed., 2024, 63, e202401817 CrossRef CAS.
- S. Jeong, J. Jeong, S. Lim, W. Kim, H. Kwon and J. Kim, Chem. Eng. J., 2024, 481, 148294 CrossRef CAS.
- J. Wan, M. Nian, C. Yang, K. Ge, J. Liu, Z. Chen, J. Duan and W. Jin, J. Membr. Sci., 2022, 642, 119991 CrossRef CAS.
- Z. Liu, H. Zhao, B. Hua, Y. Wang, T. Lu, M. Guo, G. Dong, J. Zhu and Y. Zhang, ACS Appl. Mater. Interfaces, 2025, 17, 6867–6877 CrossRef CAS.
- J. Zhang, X. Sun, J. Xin, L. Wang, Y. Fan, J. Zheng, S. Li, J. Xu, N. Li and S. Zhang, ACS Sustain. Chem. Eng., 2025, 13, 2368–2379 CrossRef CAS.
- Y. Cui, X. Cui, G. Yang, P. Yu, C. Wang, Z. Kang, H. Guo and D. Xia, J. Membr. Sci., 2024, 689, 122174 CrossRef CAS.
- J. J. Teesdale, M. Lee, R. Lu and Z. P. Smith, J. Am. Chem. Soc., 2023, 145, 830–840 CrossRef CAS.
- C. Wang, Y. Sun, L. Li, R. Krishna, T. Ji, S. Chen, J. Yan and Y. Liu, Angew. Chem., Int. Ed., 2022, 61, e202203663 CrossRef CAS.
- J. Yan, Y. Sun, T. Ji, C. Zhang, L. Liu and Y. Liu, J. Membr. Sci., 2022, 653, 120496 CrossRef CAS.
- T. Lee, A. Ozcan, I. Park, D. Fan, J. Jang, P. G. M. Mileo, S. Yoo, J. Roh, J. Kang, B. Lee, Y. Cho, R. Semino, H. Kim, G. Maurin and H. Park, Adv. Funct. Mater., 2021, 31, 2103973 CrossRef CAS.
- T. D. Bennett and A. K. Cheetham, Acc. Chem. Res., 2014, 47, 1555–1562 CrossRef CAS PubMed.
- J. Kang, X. Yang, Q. Hu, Z. Cai, L. M. Liu and L. Guo, Chem. Rev., 2023, 123, 8859–8941 CrossRef CAS.
- Z. Lin, J. J. Richardson, J. Zhou and F. Caruso, Nat. Rev. Mater., 2023, 7, 273–286 CAS.
- J. Fonseca, T. Gong, L. Jiao and H. Jiang, J. Mater. Chem. A, 2021, 9, 10562–10611 RSC.
- J. M. Tuffnell, C. W. Ashling, J. Hou, S. Li, L. Longley, M. L. Rios Gomez and T. D. Bennett, Chem. Commun., 2019, 55, 8705–8715 RSC.
- H. Mahdavi, F. Zadehahmadi, M. Arzani, L. Melag, A. L. Sutton, M. M. Sadiq, Z. Xie, M. R. Hill and B. D. Freeman, Adv. Mater., 2025, e05579 CrossRef CAS.
- M. Kim, H.-S. Lee, D.-H. Seo, S. J. Cho, E.-c. Jeon and H. R. Moon, Nat. Commun., 2024, 15, 1174 CrossRef CAS PubMed.
- Y. Feng, W. Yan, Z. Kang, X. Zou, W. Fan, Y. Jiang, L. Fan, R. Wang and D. Sun, Chem. Eng. J., 2023, 465, 142873 CrossRef CAS.
- D. Li, Z. Yang, L. Yang, C. Ma, M. Ye, Y. Sun, Z. Qiao and A. Chen, J. Membr. Sci., 2024, 695, 122492 CrossRef CAS.
- S. Li, C. Ma, J. Hou, S. Yu, A. Chen, J. Du, P. A. Chater, D. S. Keeble, Z. Qiao, C. Zhong, D. A. Keen, Y. Liu and T. D. Bennett, Nat. Commun., 2025, 16, 1622 CrossRef CAS PubMed.
- Y. Wang, H. Jin, Q. Ma, K. Mo, H. Mao, A. Feldhoff, X. Cao, Y. Li, F. Pan and Z. Jiang, Angew. Chem., Int. Ed., 2020, 59, 4365–4369 CrossRef CAS PubMed.
- H. Xia, H. Jin, Y. Zhang, H. Song, J. Hu, Y. Huang and Y. Li, J. Membr. Sci., 2022, 655, 120611 CrossRef CAS.
- Y. Zhang, Y. Wang, H. Xia, P. Gao, Y. Cao, H. Jin and Y. Li, Chem. Commun., 2022, 58, 9548–9551 RSC.
- N. Li, C. Ma, Z. Wang, D. Li, Z. Qiao and C. Zhong, J. Membr. Sci., 2025, 715, 123453 CrossRef CAS.
- M. Y. B. Zulkifli, K. Su, R. Chen, J. Hou and V. Chen, Adv. Membr., 2022, 2, 100036 CrossRef.
- A. M. Chester, C. Castillo-Blas, L. Wondraczek, D. A. Keen and T. D. Bennett, Chem. Eur. J., 2022, 28, e202200345 CrossRef CAS PubMed.
- Z. Li, Y. Wang, J. Zhang, S. Cheng and Y. Sun, Membranes, 2024, 14, 1–18 Search PubMed.
- R. Lin, M. Chai, Y. Zhou, V. Chen, T. D. Bennett and J. Hou, Chem. Soc. Rev., 2023, 52, 4149–4172 RSC.
- N. Ma and S. Horike, Chem. Rev., 2022, 122, 4163–4203 CrossRef CAS PubMed.
- B. Hosseini Monjezi, K. Kutonova, M. Tsotsalas, S. Henke and A. Knebel, Angew Chem. Int. Ed. Engl., 2021, 60, 15153–15164 CrossRef CAS PubMed.
- S. Yang, V. V. Karve, A. Justin, I. Kochetygov, J. Espín, M. Asgari, O. Trukhina, D. T. Sun, L. Peng and W. L. Queen, Coord. Chem. Rev., 2021, 427, 213525 CrossRef CAS.
- L. Hu, W.-I. Lee, S. Roy, A. Subramanian, K. Kisslinger, L. Zhu, S. Fan, S. Hwang, V. T. Bui, T. Tran, G. Zhang, Y. Ding, P. M. Ajayan, C.-Y. Nam and H. Lin, Nat. Commun., 2024, 15, 5688 CrossRef CAS PubMed.
- T. Lee, B. Lee, S. Yoo, H. Lee, W. Wu, Z. P. Smith and H. Park, Nat. Commun., 2023, 14, 8330 CrossRef PubMed.
- P. Su, S. Chen, L. Chen and W. Li, J. Membr. Sci., 2024, 691, 122246 CrossRef CAS.
- H. Zhang, R. Zou and Y. Zhao, Coord. Chem. Rev., 2015, 292, 74–90 CrossRef CAS.
- T. Xu, B. Wu, W. Li, Y. Li, Y. Zhu, F. Sheng, Q. Li, L. Ge, X. Li, H. Wang and T. Xu, Sci. Adv., 2024, 10, eadn0944 CrossRef CAS PubMed.
- Q. Xin, J. Dong, W. Shao, X. Ding, N. Gao, L. Zhang, H. Jin, H. Chen and Y. Zhang, J. Membr. Sci., 2025, 714, 123431 CrossRef CAS.
- X. Yang, Q. Zhang, Y. Liu, M. Nian, M. Xie, S. Xie, Q. Yang, S. Wang, H. Wei, J. Duan, S. Dong and H. Xing, Angew. Chem., Int. Ed., 2023, 62, e202303280 CrossRef CAS.
- Z. Li, T. Li, W. Zheng, X. Li, J. Zhu, M. Yu, X. Jiang, X. Wu, G. He and J. Zhang, J. Membr. Sci., 2025, 717, 123657 CrossRef CAS.
- Z. Qin, J. Wei, Y. Wu, M. Deng, L. Yao, L. Yang, W. Jiang, J. Zheng, Z. Liu and Z. Dai, Results Eng., 2024, 24, 103184 CrossRef CAS.
- A. Sabetghadam, X. Liu, S. Gottmer, L. Chu, J. Gascon and F. Kapteijn, J. Membr. Sci., 2019, 570, 226–235 CrossRef.
- Y. Cheng, X. Wang, C. Jia, Y. Wang, L. Zhai, Q. Wang and D. Zhao, J. Membr. Sci., 2017, 539, 213–223 CrossRef CAS.
- C. Zhu, Y. Peng, K. Li, L. Liu and W. Yang, J. Membr. Sci., 2024, 709, 123140 CrossRef CAS.
- Y. Sun, J. Yan, Y. Gao, T. Ji, S. Chen, C. Wang, P. Lu, Y. Li and Y. Liu, Angew. Chem., Int. Ed., 2023, 62, e202216697 CrossRef CAS PubMed.
- Q. Wang, Y. Tang, L. Liu, C. Liu, K. Zhang, X. Tian, X. Feng, R. Zhang, Y. Yu and T. Gu, J. Membr. Sci., 2025, 726, 124067 CrossRef CAS.
- C. Chuah, K. Goh, Y. Yang, H. Gong, W. Li, H. E. Karahan, M. D. Guiver, R. Wang and T. Bae, Chem. Rev., 2018, 118, 8655–8769 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- Y. Feng, Q. Chen, M. Jiang and J. Yao, Ind. Eng. Chem. Res., 2019, 58, 17646–17659 CrossRef CAS.
- Z. Gu, X. Zhang, R. Liang, F. Wang, D. Wang, A. Chen and Z. Qiao, ACS Appl. Polym. Mater., 2025, 7, 11890–11900 CrossRef CAS.
- Y. Liang, Z. Zhang, A. Chen, C. Yu, Y. Sun, J. Du, Z. Qiao, Z. Wang, M. D. Guiver and C. Zhong, Angew. Chem., Int. Ed., 2024, 63, e202404058 CrossRef CAS.
- C. Yu, X. Cen, Z. Zhang, Y. Sun, W. Xue, Z. Qiao, M. D. Guiver and C. Zhong, Adv. Mater., 2023, 35, 2307013 CrossRef CAS.
- C. A. Mirkin, S. H. Petrosko, N. Artzi, K. Aydin, A. Biaggne, C. J. Brinker, K. E. Bujold, Y. C. Cao, R. R. Chan, C. Chen, P.-C. Chen, X. Chen, O. J. G. L. Chevalier, C. H. J. Choi, R. M. Crooks, V. P. Dravid, J. S. Du, S. B. Ebrahimi, H. Fan, O. K. Farha, C. A. Figg, T. D. Fink, C. M. Forsyth, H. Fuchs, F. M. Geiger, N. C. Gianneschi, K. J. Gibson, D. S. Ginger, S. Guo, J. S. Hanes, L. Hao, J. Huang, B. M. Hunter, F. Huo, J. Hwang, R. Jin, S. O. Kelley, T. J. Kempa, Y. Kim, S. Kudruk, S. Kumari, K. M. Landy, K.-B. Lee, N. J. Leon, J. Li, Y. Li, Z. Li, B. Liu, G. Liu, X. Liu, L. M. Liz-Marzán, J. H. Lorch, T. Luo, R. J. Macfarlane, J. E. Millstone, M. Mrksich, C. J. Murphy, R. R. Naik, A. E. Nel, C. Oetheimer, J. K. Hedlund Orbeck, S.-J. Park, B. E. Partridge, N. A. Peppas, M. L. Personick, A. Raj, N. Ramani, M. B. Ross, S. B. Ross, E. H. Sargent, T. Sengupta, G. C. Schatz, D. S. Seferos, T. Seideman, S. E. Seo, B. Shen, W. Shim, D. Shin, U. Simon, A. J. Sinegra, P. T. Smith, A. M. Spokoyny, P. J. Stang, A. H. Stegh, J. F. Stoddart, D. F. Swearer, W. Tan, M. H. Teplensky, C. S. Thaxton, D. R. Walt, M. X. Wang, Z. Wang, W. D. Wei, P. S. Weiss, P. H. Winegar, Y. Xia, Y. Xie, X. Xu, P. Yang, Y. Yang, Z. Ye, K. R. Yoon, C. Zhang, H. Zhang, K. Zhang, L. Zhang, X. Zhang, Y. Zhang, Z. Zheng, W. Zhou, S. Zhu and W. Zhu, ACS Nano, 2025, 19, 31933–31968 CrossRef CAS.
- C.-C. Lin, E. Parrish and R. J. Composto, Macromolecules, 2016, 49, 5755–5772 CrossRef CAS.
- J. Huang, J. Zhou and M. Liu, JACS Au, 2022, 2, 280–291 CrossRef CAS PubMed.
- T. Lee, J. Jang, B. Lee, W.-N. Wu, Z. P. Smith and H. B. Park, Macromolecules, 2024, 57, 11242–11250 CrossRef CAS.
- S. K. Kumar, B. C. Benicewicz, R. A. Vaia and K. I. Winey, Macromolecules, 2017, 50, 714–731 CrossRef CAS.
- Y. Chen, W. Lu, M. Schroder and S. Yang, Acc. Chem. Res., 2023, 56, 2569–2581 CrossRef CAS PubMed.
- Ü. Kökçam-Demir, A. Goldman, L. Esrafili, M. Gharib, A. Morsali, O. Weingart and C. Janiak, Chem. Soc. Rev., 2020, 49, 2751–2798 RSC.
- J. E. Bachman, Z. P. Smith, T. Li, T. Xu and J. R. Long, Nat. Mater., 2016, 15, 845–849 CrossRef CAS PubMed.
- H. Demir, H. Daglar, H. C. Gulbalkan, G. O. Aksu and S. Keskin, Coord. Chem. Rev., 2023, 484, 215112 CrossRef CAS.
- X. Cheng, Y. Liao, Z. Lei, J. Li, X. Fan and X. Xiao, J. Membr. Sci., 2023, 672, 121430 CrossRef CAS.
- Z. Zhang, X. Cao, C. Geng, Y. Sun, Y. He, Z. Qiao and C. Zhong, J. Membr. Sci., 2022, 650, 120399 CrossRef CAS.
- Z. Zheng, O. Zhang, C. Borgs, J. T. Chayes and O. M. Yaghi, J. Am. Chem. Soc., 2023, 145, 18048–18062 CrossRef CAS.
- Z. Zheng, Z. Rong, N. Rampal, C. Borgs, J. T. Chayes and O. M. Yaghi, Angew. Chem., Int. Ed., 2023, 62, e202311983 CrossRef CAS.
- A. Ozcan, F. X. Coudert, S. M. J. Rogge, G. Heydenrych, D. Fan, A. P. Sarikas, S. Keskin, G. Maurin, G. E. Froudakis, S. Wuttke and I. Erucar, J. Am. Chem. Soc., 2025, 147, 23367–23380 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |