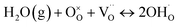Phase diagrams and chemical expansion upon hydration of proton conductors BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)n (0 ≤ x ≤ 0.8; 0 ≤ n ≤ 0.1)
Lozane
Hamze
,
Olivier
Joubert
and
Eric
Quarez
 *
*
Nantes Université, CNRS, Institut des Matériaux de Nantes Jean Rouxel, IMN, F-44000 Nantes, France. E-mail: eric.quarez@cnrs-imn.fr
First published on 2nd October 2025
Abstract
Proton-conducting ceramics are promising candidates for applications in sustainable energy technologies, with BaZrxCe0.8−xY0.1Yb0.1O2.9 (BZCYYb) perovskites standing out as excellent proton conductors. However, comprehensive structural studies of these materials, particularly concerning their hydrated and dehydrated states, as well as the effects of composition (x) and temperature, are still limited. This study explores the crystal structures of BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)n and BaZrxCe0.8−xY0.1Yb0.1O2.9 (0 ≤ x ≤ 0.8) using neutron and X-ray diffraction techniques. The chemical expansion due to water incorporation is quantified by measuring unit cell volumes via X-ray diffraction, showing strong agreement with mass loss data from thermogravimetric analysis (TGA). Phase diagrams for both hydrated and dehydrated phases are developed, revealing a decrease in symmetry and a reduction in phase transition temperatures as x increases. Hydration notably affects octahedral tilting, as evidenced by comparisons of hydrated and dehydrated structures at room temperature (RT). Furthermore, a bond valence sum (BVS)-based approach is proposed, offering improved predictions of octahedral tilting and structural stability compared to the traditional Goldschmidt tolerance factor. These structural insights, particularly the influence of hydration, are essential for advancing our understanding of these materials and providing a solid foundation for linking their structure to their properties.
1. Introduction
Since the discovery in 2010 of the proton-conducting perovskite BaZr0.1Ce0.7Y0.1Yb0.1O2.9 (BZCYYb1711), which exhibits both high protonic conductivity and enhanced chemical stability, this material has emerged as a benchmark electrolyte for protonic ceramic fuel cells (PCFCs) and protonic ceramic electrolysis cells (PCECs).1 To date, more than 230 publications, according to the Web of Science, have investigated this compound and related compositions within the BaZrxCe0.8−xY0.1Yb0.1O2.9 family.2–9The high proton conductivity of these oxides stems from their ability to incorporate protons via a hydration reaction. In humid atmospheres, water molecules react with oxygen vacancies and lattice oxygen to form hydroxyl groups, according to the reaction:
This results in hydrated compositions that can be written as BaZrxCe0.8−xY0.1Yb0.1O2.9−n□0.1−n(OH)2n (0 ≤ n ≤ 0.1), which reflects the consumption of n lattice oxygens and the formation of 2n hydroxyl groups. An alternative formulation, BaZrxCe0.8−xY0.1Yb0.1O2.9□0.1−n(H2O)n (0 ≤ n ≤ 0.1), more clearly illustrates water incorporation without implying lattice oxygen removal. For simplicity, the latter notation, BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)n, omitting explicit reference to the oxygen vacancy, will be used consistently throughout this manuscript.
These materials adopt the perovskite ABO3 structure, with Ba occupying the A-site and Zr, Ce, Y, and Yb substituting on the B-site. The mismatch in ionic radii between A- and B-site cations induces structural distortions via cooperative tilting of the BO6 octahedra, resulting in symmetry lowering from the ideal cubic structure. Due to the low X-ray scattering factor of oxygen, subtle superlattice reflections associated with octahedral tilting are often undetectable by X-ray diffraction. In contrast, neutron diffraction is highly effective in detecting such symmetry changes due to its sensitivity to both cations and oxygen.
We recently reported on an intermediate composition, BaZr0.4Ce0.4Y0.1Yb0.1O2.9 (BZCYYb4411), which exhibits enhanced chemical stability as a result of its higher Zr/Ce ratio.10,11 That study explored three sintering strategies, two of which successfully reduced the sintering temperature from 1600 °C to 1400 °C, enabling one-step synthesis and sintering while preserving high ionic conductivity.
In an earlier neutron diffraction study, we investigated BaZr0.1Ce0.7Y0.1Yb0.1O2.9 and identified a tetragonal I4/mcm structure at RT, with a transition to the cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase around 650 °C.12 At the time, the material was assumed to be in a dry state, as no deliberate hydration was performed. However, subsequent thermogravimetric analysis (TGA) and additional diffraction studies revealed that these materials readily absorb moisture during synthesis or sintering, resulting in hydrated states.
m phase around 650 °C.12 At the time, the material was assumed to be in a dry state, as no deliberate hydration was performed. However, subsequent thermogravimetric analysis (TGA) and additional diffraction studies revealed that these materials readily absorb moisture during synthesis or sintering, resulting in hydrated states.
Given the strong link between hydration state and proton conductivity, we conducted a systematic investigation of the BaZrxCe0.8−xY0.1Yb0.1O2.9 series, with explicit consideration of hydration effects. The specific objectives of this study are:
• To determine the crystal structures of both fully hydrated BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 at RT via neutron diffraction and as a function of temperature via X-ray diffraction, as well as the fully dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 compounds (0 ≤ x ≤ 0.8) under the same conditions, using neutron data as a structural reference;
• To correlate mass changes upon dehydration, as measured by TGA, with unit cell volume changes observed by X-ray diffraction, thereby isolating the chemical expansion effect associated with water uptake;
• To determine the thermal expansion coefficients (TECs) of both hydrated and dehydrated samples, revealing the effects of hydration and the Zr/Ce ratio on TEC values;
• To compare the RT structures of hydrated and dehydrated materials to assess the structural impact of hydration;
• To construct phase diagrams for both hydrated and dehydrated members of the BaZrxCe0.8−xY0.1Yb0.1O2.9 series, identifying key phase transitions;
• Finally, to introduce a new predictive approach based on bond valence sum (BVS) calculations, which incorporates the effects of oxygen vacancies and provides more accurate insights into octahedral tilting and phase stability than the traditional Goldschmidt tolerance factor.
2. Experimental
2.1 Powder synthesis
Nine compositions of BaZrxCe0.8−xY0.1Yb0.1O2.9 (0 ≤ x ≤ 0.8) were synthesized via the glycine–nitrate process (GNP), following procedures reported previously.10,12 Stoichiometric amounts of the corresponding metal nitrates were employed. The as-synthesized nanoparticles were collected, manually ground in an agate mortar, and subsequently pressed into pellets. To prevent interaction with the crucible and to compensate for possible Ba evaporation, powders of identical composition were used as both a bedding layer and a covering medium during sintering. The pellets were sintered in air at 1600 °C for 1 h, cooled to room temperature, and polished to remove any superficial surface layer. For thermogravimetric analysis (TGA), X-ray diffraction, and neutron diffraction measurements, the pellets were ground into fine powders and stored in closed glass vials under ambient conditions until use. Under these conditions, the samples reacted with atmospheric moisture, indicative of hydrated phase formation, which was subsequently confirmed to be fully hydrated by TGA and neutron diffraction.2.2 Characterization techniques
Two types of experimental series were conducted:
• All nine compositions were examined under nitrogen (U-grade, Air Liquide; pH2O < 10−3 atm) from RT to 1000 °C, with one scan recorded every 100 °C. The first pattern corresponds to the fully hydrated phase at RT.
• Seven compositions (x = 0, 0.1, 0.2, 0.3, 0.4, 0.6, and 0.8) were studied under dynamic vacuum (∼1–5 × 10−5 mbar), from 1000 °C down to RT, with one scan recorded every 100 °C. These conditions are standard for achieving water-free states and for preventing rehydration during cooling. The final pattern corresponds to the fully dehydrated phase at RT.
For both neutron and X-ray powder diffraction, Rietveld refinements were performed using Jana2006 software.13 More accurate standard deviations of refined parameters were obtained using the Bérar and Lelann corrections.14 Error bars for structural parameters were plotted considering standard deviations, obtained after the Bérar correction, multiplied by a factor of 3.
3. Results and discussion
3.1 Evidence for water incorporation
Fig. 1 presents the TGA curve of the as-prepared BZCYYb1711 sample (x = 0.1), conducted from RT to 1000 °C under a nitrogen atmosphere. A mass loss begins near 300 °C and ends around 900 °C, resulting in a total weight reduction of ∼0.6%. This thermal behavior, typical of proton-conducting ceramic phases, is attributed to the release of incorporated water.15–19 In the compound BaZr0.1Ce0.7Y0.1Yb0.1O2.9, full hydration corresponds to complete occupation of oxygen vacancies by oxygen from incorporated water, yielding BaZr0.1Ce0.7Y0.1Yb0.1O2.9(H2O)0.1. The theoretical mass loss associated with full dehydration is 0.56%, which is in close agreement with the measured ∼0.6%, confirming that the as-prepared BZCYYb1711 is fully hydrated. In Fig. 1, the mass loss is also expressed as the number of incorporated water molecules per formula unit, denoted n, with n = 0.1 representing the fully hydrated state.Further evidence for water incorporation is provided by X-ray thermodiffraction, performed on as-prepared BaZrxCe0.8−xY0.1Yb0.1O2.9 samples with x = 0.2, 0.4, 0.5, and 0.6 under a nitrogen atmosphere (Fig. 1). During the first heat treatment, data were collected every 100 °C from RT to 1000 °C (red markers). After cooling to RT, a second heat treatment was conducted under identical conditions (black markers). The pseudo-cubic subcell volume is plotted as a function of temperature. At low temperatures, the linear increase in cell volume reflects the thermal expansion of the hydrated phase, from which the thermal expansion coefficient (TEC) can be extracted (see Section 3.3.3).
Deviations from linearity, observed at ∼500 °C for x = 0.2 and ∼400 °C for x = 0.4–0.6, mark the onset of dehydration. The cell volume contracts and then increases again up to ∼800 °C, after which a new linear trend emerges, corresponding to the thermal expansion of the fully dehydrated phase. Similar behavior has been reported in other proton-conducting ceramics, including BaIn0.8Ti0.2O2.6,15 BaZr0.8Y0.2O3−δ,20 BaZr1−xYxO3−δ (x = 0.05, 0.1, 0.2) and BaCe0.8Y0.2O3−δ,21 BaZr1−xYxO3−δ (x = 0.1, 0.15, 0.2, 0.25 and 0.3),22 BaZr0.7Ce0.2O0.1O3−δ,23 BaZr0.9Y0.1O3−δ and BaZr0.7Ce0.2Y0.1O3−δ,24 BaZrxCe0.8−xY0.2O3−δ (0 ≤ x ≤ 0.8),25 BaZrxCe0.8−xY0.1Yb0.1O3−δ (x = 0.1, 0.4),26 and BaZr0.1Ce0.7Y0.1Yb0.1O3−δ.27
Upon cooling, rehydration occurs to varying degrees. For x = 0.6, the RT cell volume matches that of the extrapolated dehydrated phase, indicating minimal rehydration. For x = 0.2, the volume suggests a fully hydrated state, while intermediate compositions (x = 0.4, 0.5) exhibit partial rehydration. The degree of rehydration is related to the basicity of the samples, which depends on the Zr/Ce ratio (see Section 3.3.2). Between RT and 300 °C, the cell volume increases, approaching the expansion behavior of the hydrated phase. From 400 °C to 800 °C, a more rapid volume decrease is observed during the second heat treatment, again indicative of dehydration. Above 800 °C, all samples exhibit the thermal expansion behavior characteristic of the dehydrated phase, consistent with the first run.
Throughout these experiments, the X-ray chamber was continuously flushed with nitrogen, and no external water vapor was introduced. Hence, rehydration is attributed to residual moisture in the N2 gas or chamber environment, which, although limited, was sufficient to induce partial rehydration. This facile hydration motivated us to investigate the structure of the dehydrated phases under vacuum to ensure truly water-free conditions.
Since the x = 0.1 as-prepared sample is confirmed to be fully hydrated by TGA, and all samples were prepared under identical conditions, it is reasonable to assume that all as-prepared samples are initially fully hydrated. This assumption will be further confirmed by neutron diffraction data collected on the as-prepared samples.
3.2 Structural characterization by neutron diffraction
The structures of the as-prepared samples with compositions x = 0, 0.1, 0.2, 0.3, and 0.4 were investigated by neutron diffraction at RT. Neutron diffraction is particularly well-suited for resolving octahedral tilt systems in perovskite structures due to its sensitivity to oxygen positions, enabling accurate determination of tilt patterns that may be difficult to detect using X-ray diffraction alone. Fig. 2 shows the neutron diffraction patterns of these hydrated samples.The tolerance factor (t), defined as t = (rA + rO)/√2(rB + rO), where rA, rB, and rO are the ionic radii of the A-site cation, B-site cation, and oxygen anion, respectively, is a reliable predictor of octahedral tilting in perovskite structures. The ideal cubic perovskite (unit cell ap × ap × ap) exhibits no octahedral tilting (Glazer tilt system a0a0a0), typically observed when t ≈ 1. As the B-site ionic radius increases, t decreases, leading to less favorable B–O bonding and promoting cooperative BO6 octahedral tilting to accommodate the structural mismatch.
Fifteen space groups have been identified to describe all known tilt systems.28 A double perovskite unit cell (2ap × 2ap × 2ap) is commonly used to facilitate the interpretation of diffraction patterns. In addition to the main reflections, with all even Miller indices, superlattice reflections of mixed parity, arising from structural distortions such as tilting, can appear. These reflections are often weak in X-ray diffraction but are more readily observed with neutrons.
For the BaZrxCe0.8−xY0.1Yb0.1O2.9 system, the tolerance factor increases from 0.936 (x = 0) to 0.989 (x = 0.8). In a previous study, we reported that the x = 0.1 sample (t = 0.943) crystallizes in the tetragonal I4/mcm space group, corresponding to the a0a0c− tilt system.12 For lower t values (x = 0), the symmetry is expected to be the same or lower, while higher t values (x > 0.1) favor increased symmetry as t approaches 1.
| Composition | x = 0.0 | x = 0.1 | x = 0.2 | x = 0.3 | x = 0.4 | |
|---|---|---|---|---|---|---|
| Space group | I2/m | I4/mcm | I4/mcm | I4/mcm | I4/mcm | |
| Tilt system | a 0 b − c − | a 0 a 0 c − | a 0 a 0 c − | a 0 a 0 c − | a 0 a 0 c − | |
| a (Å) | 6.2371(6) | 6.1478(2) | 6.12442(18) | 6.1122(3) | 6.0999(4) | |
| b (Å) | 8.7250(8) | 6.1478(2) | 6.12442(18) | 6.1122(3) | 6.0999(4) | |
| c (Å) | 6.2536(5) | 8.8789(5) | 8.8273(4) | 8.7434(8) | 8.6423(12) | |
| β (°) | 91.267(6) | 90 | 90 | 90 | 90 | |
| V (Å3) | 340.23(5) | 335.58(2) | 331.10(2) | 326.64(4) | 321.57(5) | |
| a red (Å) | 4.4103(4) | 4.34715(14) | 4.33062(13) | 4.3219(2) | 4.3133(3) | |
| b red (Å) | 4.3625(4) | 4.34715(14) | 4.33062(13) | 4.3219(2) | 4.3133(3) | |
| c red (Å) | 4.4219(3) | 4.4395(2) | 4.4136(2) | 4.3417(4) | 4.3212(6) | |
| V red (Å3) | 85.057(12) | 83.895(5) | 82.775(5) | 81.66(2) | 80.393(13) | |
| c/a | 1.00265(12) | 1.02123(6) | 1.01917(5) | 1.01150(10) | 1.00182(15) | |
| c/b | 1.01363(12) | — | — | — | — | |
| a/b | 1.01096(13) | — | — | — | — | |
| φ (°) [001] | 12.09 | 11.6621(4) | 10.7358(4) | 9.2243(6) | 7.6772(8) | |
| φ′ (°) [010] | 4.44 | — | — | — | — | |
| Ba | Wyckoff position | 4i | 4b | 4b | 4b | 4b |
| x | 0.249(5) | 0 | 0 | 0 | 0 | |
| y | 0 | 0.5 | 0.5 | 0.5 | 0.5 | |
| z | 0.746(3) | 0.25 | 0.25 | 0.25 | 0.25 | |
| SOF | 1 | 1 | 1 | 1 | 1 | |
| U eq (Å2) | 0.021(4) | 0.0239(14) | 0.0257(12) | 0.0243(17) | 0.021(3) | |
| Ce/Zr/Y/Yb | Wyckoff position | 4e | 4c | 4c | 4c | 4c |
| x | 0.25 | 0 | 0 | 0 | 0 | |
| y | 0.25 | 0 | 0 | 0 | 0 | |
| z | 0.25 | 0 | 0 | 0 | 0 | |
| SOF Ce/Zr/Y/Yb | 0.8/0.0/0.1/0.1 | 0.7/0.1/0.1/0.1 | 0.6/0.2/0.1/0.1 | 0.5/0.3/0.1/0.1 | 0.4/0.4/0.1/0.1 | |
| U eq (Å2) | 0.0115(11) | 0.0121(11) | 0.0122(9) | 0.0141(12) | 0.014(2) | |
| O1 | Wyckoff position | 4i | 4a | 4a | 4a | 4a |
| x | 0.212(3) | 0 | 0 | 0 | 0 | |
| y | 0 | 0 | 0 | 0 | 0 | |
| z | 0.1737(18) | 0.25 | 0.25 | 0.25 | 0.25 | |
| SOF | 1 | 1 | 1 | 1 | 1 | |
| U eq (Å2) | 0.031(4) | 0.0329(15) | 0.0311(13) | 0.0305(19) | 0.026(3) | |
| O2 | Wyckoff position | 4g | 8h | 8h | 8h | 8h |
| x | 0 | 0.1984(4) | 0.2026(4) | 0.2094(6) | 0.2163(8) | |
| y | 0.3001(15) | 0.6984(4) | 0.7026(4) | 0.7094(6) | 0.7163(8) | |
| z | 0 | 0 | 0 | 0 | 0 | |
| SOF | 1 | 1 | 1 | 1 | 1 | |
| U eq (Å2) | 0.021(4) | 0.0311(8) | 0.0328(9) | 0.0362(14) | 0.038(2) | |
| O3 | Wyckoff position | 4h | — | — | — | — |
| x | 0.5 | — | — | — | — | |
| y | 0.270(2) | — | — | — | — | |
| z | 0 | — | — | — | — | |
| SOF | 1 | — | — | — | — | |
| U eq (Å2) | 0.029(4) | — | — | — | — | |
| R-Factors | R p (%) | 1.90 | 3.22 | 2.66 | 3.67 | 2.85 |
| R wp (%) | 2.42 | 4.03 | 3.51 | 4.81 | 3.75 | |
| R obs (%) | 2.32 | 3.22 | 2.67 | 4.18 | 3.23 | |
| χ 2 | 1.43 | 1.64 | 1.49 | 1.63 | 2.05 | |
• x = 0: 1.02(2), 1.08(4), 1.030(18)
• x = 0.1: 1.000(16), 1.012(12)
• x = 0.2: 0.992(16), 1.008(12)
• x = 0.3: 0.992(3), 0.996(16)
• x = 0.4: 1.02(5), 0.99(4)
These values approach full oxygen occupancy (1.000), deviating significantly from the nominal 0.967. Final refinements assumed full occupancy, indicating that oxygen vacancies have been filled by oxygen from incorporated water. This interpretation is consistent with TGA data showing full hydration of the as-prepared samples. The resulting empirical formula is BaZrxCe0.8−xY0.1Yb0.1O3 (x = 0–0.4), which corresponds to BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 when hydrogen is considered. Tables 1 and S1 summarize the results of the Rietveld refinements.
First, the intensities of the 311 and 313 superlattice reflections decrease progressively with increasing x, indicating a reduction in octahedral tilting. Second, the 400 and 004 diffraction peaks move closer together, tending to merge, which reflects a convergence of the lattice constants and a decreasing c/a ratio. The tetragonal distortion is quantified using the reduced perovskite cell parameters, with a = at/√2 and c = ct/2. Although the c/a ratio decreases as x increases, it remains greater than 1 at x = 0.4, confirming the persistence of tetragonal symmetry.
Third, the tilt angle φ, extracted from the refined structural parameters, decreases from 11.6621(4)° at x = 0.1 to 7.6772(8)° at x = 0.4, further corroborating the reduction in structural distortion. Finally, the spontaneous tetragonal strain et also decreases with increasing x.
At x = 0.5, the Goldschmidt tolerance factor t approaches unity, and the structure is best described by the cubic space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (see Section 3.3). The tetragonal-to-cubic phase transition as a function of composition was analyzed by plotting the composition dependence of et. The data were fitted to the equation 0 = a(xt − x) + bet + cet2 where xt is the critical composition at which the symmetry change occurs.29 The fit yields xt = 0.41, confirming that the x = 0.4 sample retains tetragonal symmetry, while the x = 0.5 sample adopts the cubic phase. The I4/mcm-to-Pm
m (see Section 3.3). The tetragonal-to-cubic phase transition as a function of composition was analyzed by plotting the composition dependence of et. The data were fitted to the equation 0 = a(xt − x) + bet + cet2 where xt is the critical composition at which the symmetry change occurs.29 The fit yields xt = 0.41, confirming that the x = 0.4 sample retains tetragonal symmetry, while the x = 0.5 sample adopts the cubic phase. The I4/mcm-to-Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase transition, driven by increasing tolerance factor t, is consistent with behavior observed in analogous systems such as SrTixZr1−xO3,30 Sr1−xBaxZrO3,31 and Sr1−xCaxTi0.5Mn0.5O3.32
m phase transition, driven by increasing tolerance factor t, is consistent with behavior observed in analogous systems such as SrTixZr1−xO3,30 Sr1−xBaxZrO3,31 and Sr1−xCaxTi0.5Mn0.5O3.32
In summary, increasing Zr content raises the tolerance factor t, while the tetragonal distortion, as reflected by superlattice reflection intensity, c/a ratio, tilt angle φ, and tetragonal strain et, decreases. These findings provide a comprehensive structural framework for interpreting the behavior of BaZrxCe0.8−xY0.1Yb0.1O2.9 (0 ≤ x ≤ 0.8) as a function of temperature, to be investigated further using X-ray diffraction under nitrogen and vacuum atmospheres.
3.3 Thermodiffraction of BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 (0 ≤ x ≤ 0.8) in N2 atmosphere
The nine as-prepared samples discussed herein are fully hydrated, as confirmed in the preceding section. As these samples were obtained from different synthesis batches, minor discrepancies between cell parameters derived from neutron and X-ray diffraction may occur. However, these discrepancies are negligible compared to the compositional differences between successive samples.Due to the weak intensity or absence of superlattice reflections in X-ray diffraction patterns, phase identification cannot rely solely on their presence. Instead, the splitting behavior of the h00, hh0, and hhh main reflections is used to determine the appropriate space group. Based on previous neutron diffraction results, three candidate space groups are considered: I2/m, I4/mcm, and Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, with reflection splitting behaviors as follows:
m, with reflection splitting behaviors as follows:
• h00 reflections: split into 3, 2, and 1 peaks for I2/m, I4/mcm, and Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, respectively.
m, respectively.
• hh0 reflections: split into 4, 2, and 1 peaks, respectively.
• hhh reflections: split into 2, 1, and 1 peaks, respectively.
This analysis enables reliable identification of the structural symmetry.
Fig. S1 presents the full X-ray diffraction patterns across a broad 2θ range for each composition as a function of temperature. Fig. 4a highlights the 400 reflection (representative of h00) for all compositions from RT to 1000 °C. At RT, increasing Zr content (x) shifts the 400 peak to higher 2θ values, consistent with substitution of larger Ce4+ by smaller Zr4+ cations. Upon heating, no consistent shift toward lower 2θ is observed due to competing thermal and chemical expansion effects, leading to an atypical cell volume evolution.
At RT, the 400 peak splitting diminishes with increasing x and eventually becomes a single peak at a composition to be discussed later. Neutron data confirm I2/m symmetry for the hydrated x = 0.0 sample, which is also used for X-ray refinement. Fig. 4b shows the 440 (hh0) and 444 (hhh) reflections for x = 0 at 25 °C, 500 °C, and 600 °C. At 500 °C, the 444 reflection remains split, consistent with I2/m symmetry, while at 600 °C all splitting disappears, indicating a transition to the cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase. Consequently, Rietveld refinements were performed using I2/m from RT to 500 °C and Pm
m phase. Consequently, Rietveld refinements were performed using I2/m from RT to 500 °C and Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m from 600 °C onward. This unusual I2/m → Pm
m from 600 °C onward. This unusual I2/m → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m transition has also been reported in BaZr0.3Ce0.5Y0.2O3−δ and BaZr0.2Ce0.6Y0.2O3−δ.25
m transition has also been reported in BaZr0.3Ce0.5Y0.2O3−δ and BaZr0.2Ce0.6Y0.2O3−δ.25
For x = 0.2, the 400 and 440 reflections are split at RT, whereas the 444 reflection is not (Fig. 4a and c), consistent with I4/mcm symmetry. By 500 °C, all splitting vanishes, indicating a transition to the cubic phase. Rietveld refinements used I4/mcm from RT to 400 °C and Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m from 500 to 1000 °C. A similar I4/mcm → Pm
m from 500 to 1000 °C. A similar I4/mcm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m transition was also observed for x = 0.1 and corroborated by literature.12
m transition was also observed for x = 0.1 and corroborated by literature.12
Fig. 5 shows the temperature dependence of the reduced cell volume, reduced cell parameters, and c/a ratio for each composition. For x = 0.1–0.4, the c/a ratio is used to determine the tetragonal-to-cubic (I4/mcm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) transition temperature. The tetragonal phase is characterized by c/a > 1; with increasing temperature, the distortion diminishes and c/a approaches unity. When the ratio reaches 1 within error, the phase is considered cubic.
m) transition temperature. The tetragonal phase is characterized by c/a > 1; with increasing temperature, the distortion diminishes and c/a approaches unity. When the ratio reaches 1 within error, the phase is considered cubic.
Only the x = 0 sample exhibits an I2/m → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m transition. Samples with x = 0.1–0.4 undergo an I4/mcm → Pm
m transition. Samples with x = 0.1–0.4 undergo an I4/mcm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m transition, with the transition temperature decreasing as x increases. For x = 0.5–0.8, no structural transition is observed; these samples retain cubic symmetry across the entire 25–1000 °C range, as supported by the c/a ratio of approximately 1.
m transition, with the transition temperature decreasing as x increases. For x = 0.5–0.8, no structural transition is observed; these samples retain cubic symmetry across the entire 25–1000 °C range, as supported by the c/a ratio of approximately 1.
As shown in Section 3.1, which details four representative compositions, the evolution of the pseudo-cubic subcell volume with temperature follows a similar trend for all samples (Fig. 5). At low temperatures, linear expansion corresponds to hydrated phases, from which the thermal expansion coefficient (TEC) is derived (see Section 3.3.3). Deviation from linearity above ∼300 °C indicates the onset of dehydration. The unit cell volume decreases until ∼600 °C, then increases up to ∼800 °C, after which a new linear regime is observed, corresponding to the thermal expansion of the fully dehydrated phase.
For compositions with RT cubic symmetry (x = 0.5–0.8), the reduced cell parameter tracks the unit cell volume. In contrast, for lower-symmetry compositions, the a and c parameters evolve nonlinearly with temperature and converge at the phase transition. Exceptions include x = 0.1 and x = 0.2, where a increases and c decreases nonlinearly before converging at the transition point.
Room-temperature cell volumes, parameters, and structural details for the hydrated BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 (0 ≤ x ≤ 0.8) compounds are presented in Table 2.
| Composition | Space group | Cell parameters (Å) | Cell volume (Å3) | Subcell parameters (Å) | c red/ared | Subcell volume (Å3) | t | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | β | a red | b red | c red | ||||||
| x = 0.0 | I2/m | 6.2303(6) | 8.7245(6) | 6.2492(5) | 91.179(6) | 339.61(5) | 4.4055(4) | 4.3622(3) | 4.4188(3) | — | 84.884(12) | 0.936 |
| x = 0.1 | I4/mcm | 6.1493(5) | 6.1493(5) | 8.8627(9) | 90 | 335.13(5) | 4.3482(3) | 4.3482(3) | 4.4313(4) | 1.01912(13) | 83.782(12) | 0.943 |
| x = 0.2 | I4/mcm | 6.12181(10) | 6.12181(10) | 8.8216(2) | 90 | 330.602(11) | 4.32877(7) | 4.32877(7) | 4.4108(1) | 1.01895(2) | 82.650(2) | 0.949 |
| x = 0.3 | I4/mcm | 6.1188(5) | 6.1188(5) | 8.6890(12) | 90 | 325.32(6) | 4.3266(3) | 4.3266(3) | 4.3445(6) | 1.00413(16) | 81.330(15) | 0.955 |
| x = 0.4 | I4/mcm | 6.1011(4) | 6.1011(4) | 8.6556(10) | 90 | 322.19(5) | 4.3141(2) | 4.3141(2) | 4.3278(5) | 1.00317(10) | 80.547(12) | 0.962 |
| x = 0.5 |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
4.29678(13) | 4.29678(13) | 4.29678(13) | 90 | 79.329(4) | — | — | — | 1 | 79.329(4) | 0.968 |
| x = 0.6 |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
4.27560(15) | 4.27560(15) | 4.27560(15) | 90 | 78.161(5) | — | — | — | 1 | 78.161(5) | 0.975 |
| x = 0.7 |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
4.2568(2) | 4.2568(2) | 4.2568(2) | 90 | 77.133(7) | — | — | — | 1 | 77.133(7) | 0.982 |
| x = 0.8 |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
4.2350(2) | 4.2350(2) | 4.2350(2) | 90 | 75.953(6) | — | — | — | 1 | 75.953(6) | 0.989 |
Fig. 6b compares the ΔV curve with the water content n(H2O), derived from TGA. The near-perfect overlap between ΔV and n(H2O) confirms that chemical expansion is directly attributable to proton incorporation. Similar analyses across all compositions are summarized in Fig. 6c, showing that water incorporation into BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 results in an average volume increase of 0.70(13) Å3. This corresponds to approximately 7 Å3 per water molecule. In comparison, proton-conducting BaTixIn1−xO2.5+x/2 (x < 0.25) exhibits a slightly lower volume increase upon hydration, less than 7 Å3 per water molecule, indicating good agreement with the values obtained in this study.33
| TEC (×10−6 K−1) in N2 | TEC (×10−6 K−1) in vacuum | ||
|---|---|---|---|
| x = 0 | 13.5 (25–400 °C) | 14.2 (25–200 °C) | 11.2 (500–1000 °C) |
| x = 0.1 | 12.9 (25–400 °C) | 11.7 (25–200 °C) | 9.1 (500–1000 °C) |
| x = 0.2 | 12.5 (25–400 °C) | 8.6 (25–1000 °C) | |
| x = 0.3 | 14.0 (25–300 °C) | 7.8 (25–1000 °C) | |
| x = 0.4 | 12.1 (25–300 °C) | 8.5 (25–1000 °C) | |
| x = 0.5 | 11.4 (25–400 °C) | — | |
| x = 0.6 | 11.5 (25–300 °C) | 8.2 (25–1000 °C) | |
| x = 0.7 | 10.3 (25–300 °C) | — | |
| x = 0.8 | 8.9 (25–300 °C) | 7.5 (25–1000 °C) | |
3.4 Thermodiffraction of BaZrxCe0.8−xY0.1Yb0.1O2.9 (0 ≤ x ≤ 0.8) in vacuum
Following the characterization of phase transitions and chemical expansion in hydrated samples, the dehydrated samples were analyzed to assess their thermal and structural behavior. Dehydration was achieved by heating the compounds to 1000 °C under vacuum conditions, ensuring complete removal of incorporated water. Thermodiffraction experiments were subsequently conducted during cooling from 1000 °C to RT, with XRD patterns collected at 100 °C intervals under vacuum (Fig. S2). The final diffraction pattern corresponds to the fully dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 compositions at RT.As with the hydrated series, phase identification and symmetry assignments were based on the careful analysis of peak splitting in the main reflections rather than relying exclusively on superlattice reflections. This approach provided a more reliable assessment of symmetry changes and transition points.
Fig. 7a presents the evolution of the 400 reflection for seven compositions as a function of temperature. At RT, the 400 peak shifts progressively to higher 2θ values with increasing zirconium content, consistent with the smaller ionic radius of Zr4+ relative to Ce4+, a trend also observed in the hydrated samples. During cooling from 1000 °C to RT, all compositions exhibit a continuous and linear shift of the 400 reflection toward higher 2θ values, which is characteristic of pure thermal contraction in the absence of hydration-induced structural distortions.
For the x = 0 composition, the 444 reflection is split at RT, indicating monoclinic symmetry (space group I2/m; see Fig. 7b). As temperature increases, the monoclinic unit cell parameters gradually converge, approaching a higher symmetry near the phase transition (Fig. 8). At 500 °C, the splitting of the 444 peak is no longer resolved, and successful Rietveld refinement was achieved in the tetragonal I4/mcm space group. At 800 °C, the error bar of the c/a ratio intersects unity, justifying a cubic structure assignment and refinement in the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. A similar structural evolution was observed for the x = 0.1 composition.
m space group. A similar structural evolution was observed for the x = 0.1 composition.
In all compositions where monoclinic symmetry was not detected at RT, the c/a ratio was calculated to differentiate between tetragonal and cubic phases. For example, the x = 0.4 sample exhibits c/a < 1 at RT, yet refinement across the entire temperature range, from RT to 1000 °C, was performed in the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m cubic space group. In cases where a tetragonal-to-cubic transition (I4/mcm → Pm
m cubic space group. In cases where a tetragonal-to-cubic transition (I4/mcm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) was suspected, the c/a ratio was plotted against temperature to determine the point at which it approached unity. Rietveld refinements were conducted in the appropriate space group based on these criteria.
m) was suspected, the c/a ratio was plotted against temperature to determine the point at which it approached unity. Rietveld refinements were conducted in the appropriate space group based on these criteria.
For compositions with x = 0.4 to 0.8, no symmetry change was detected throughout the entire temperature range; these samples retained cubic symmetry (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) from 1000 °C down to RT.
m) from 1000 °C down to RT.
The temperature dependence of the unit cell volume for the dehydrated samples exhibits linear behavior for compositions that remain cubic (x = 0.4, 0.6, and 0.8; similar behavior is inferred for x = 0.5 and 0.7), as illustrated in Fig. 8. For samples with lower Zr content (x = 0 to 0.3), slight deviations from linearity are observed near the transition points. These deviations, however, are considerably smaller than those seen in the hydrated samples. This nearly linear dependence of the cell volume on temperature indicates the absence of chemical expansion, further confirming that rehydration did not occur, resulting in fully dehydrated phases. As temperature increases, the lattice parameters expand and converge smoothly, indicating continuous thermal expansion toward the transition temperatures.
The unit cell volumes, lattice parameters, and refined structural parameters for the dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 compounds at RT are summarized in Table 4.
| Composition | Space group | Cell parameters (Å) | Cell volume (Å3) | Subcell parameters (Å) | c red/ared | Subcell volume (Å3) | t | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | β | a red | b red | c red | ||||||
| x = 0.0 | I2/m | 6.20664(17) | 8.76090(15) | 6.22860(13) | 90.0725(19) | 338.685(13) | 4.38876(12) | 4.38045(7) | 4.40429(9) | — | 84.671(3) | 0.936 |
| x = 0.1 | I2/m | 6.1797(13) | 8.7182(9) | 6.2029(7) | 90.122(15) | 334.19(9) | 4.3697(9) | 4.3591(4) | 4.3861(4) | — | 83.54(2) | 0.943 |
| x = 0.2 | I4/mcm | 6.12181(10) | 6.12181(10) | 8.8216(2) | 90 | 330.01(14) | 4.3511(4) | 4.3511(4) | 4.3580(15) | 1.0016(3) | 82.50(3) | 0.949 |
| x = 0.3 | I4/mcm | 6.1278(4) | 6.1278(4) | 8.6775(18) | 90 | 325.84(7) | 4.3330(2) | 4.3330(2) | 4.3387(9) | 1.0013(2) | 81.460(17) | 0.955 |
| x = 0.4 |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
4.31297(11) | 4.31297(11) | 4.31297(11) | 90 | 80.228(4) | — | — | — | 1 | 80.228(4) | 0.962 |
| x = 0.6 |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
4.27104(9) | 4.27104(9) | 4.27104(9) | 90 | 77.911(3) | — | — | — | 1 | 77.911(3) | 0.975 |
| x = 0.8 |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
4.22638(13) | 4.22638(13) | 4.22638(13) | 90 | 75.493(4) | — | — | — | 1 | 75.493(4) | 0.989 |
As observed for the hydrated samples, the thermal expansion coefficient systematically decreases with increasing zirconium content. Specifically, TEC values drop from 14.2 × 10−6 K−1 for x = 0 to 7.5 × 10−6 K−1 for x = 0.8. This trend aligns with literature reports for related systems, such as BaZrxCe0.8−xY0.2O3−δ (0.2 ≤ x ≤ 0.7), in which TECs were also found to decrease with increasing Zr content, from 11.3 × 10−6 K−1 (x = 0.2) to 8.4 × 10−6 K−1 (x = 0.7).35
Finally, with the exception of x = 0, the TECs of hydrated phases are systematically higher than those of their dehydrated counterparts. A similar trend has been reported for fully hydrated BaIn0.8Ti0.2O2.6(H2O)0.4 (TEC = 14.1 × 10−6 K−1) compared to its dehydrated form, BaIn0.8Ti0.2O2.6 (TEC = 12.5 × 10−6 K−1).15
3.5 Structural variations in hydrated and dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9: composition dependence and hydration effects at room temperature
Fig. 9, compiled from data reported in Tables 2 and 4, presents the composition dependence at RT of the unit cell volume, lattice parameters, and c/a ratio for fully hydrated BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 and dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 compounds, as determined by X-ray diffraction.A linear contraction of the unit cell volume with increasing Zr content is observed, reflecting the substitution of larger Ce4+ ions (0.87 Å, sixfold coordination) with smaller Zr4+ ions (0.72 Å). As previously discussed in the Section 3.2.5, for compositions exhibiting tetragonal symmetry, increasing Zr content results in a reduction of tetragonal distortion, characterized by a decreasing c/a ratio. Since the c/a ratio directly correlates with the octahedral tilt angle, this trend enables a meaningful comparison of tilt angles between hydrated and dehydrated phases.
For compositions with monoclinic symmetry, three ratios, c/a, a/b, and c/b, were derived from the reduced cell parameters. For x = 0, the average of these three ratios is 1.0086 for the hydrated phase, significantly higher than that of the dehydrated counterpart (1.0036). For x = 0.1, the c/a ratio of the hydrated phase (1.0191) also exceeds the average of the three monoclinic ratios for the dehydrated phase (1.0041). For x > 0.1, hydrated tetragonal phases consistently exhibit higher c/a ratios than their dehydrated analogues.
These results clearly demonstrate that hydration significantly affects the c/a ratio and, by extension, the octahedral tilt angle. Specifically, hydration increases the tilt angle. To our knowledge, this is the first experimental evidence showing that the incorporation of water molecules into a proton-conducting perovskite structure leads to an increase in the octahedral tilt angle.
3.6 Phase diagrams of fully hydrated and dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 (0 ≤ x ≤ 0.8)
Fig. 10 presents the phase diagrams, from RT to 1000 °C, of fully hydrated BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 (0 ≤ x ≤ 0.8) in N2 atmosphere and dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 (0 ≤ x ≤ 0.8) in vacuum, as determined by temperature-dependent X-ray diffraction. The observed phase transition sequences are as follows: | ||
| Fig. 10 Phase diagrams of (a) hydrated BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 (0 ≤ x ≤ 0.8) under nitrogen and (b) dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 (0 ≤ x ≤ 0.8) under vacuum. | ||
BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 in N2:
(Note: phase transformations are reported here instead of phase transitions, as the water content decreases with increasing temperature).
• x = 0: I2/m → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m
m
• x = 0.1–0.4: I4/mcm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m
m
• x = 0.5–0.8: no phase transition; remains Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m
m
BaZrxCe0.8−xY0.1Yb0.1O2.9 in vacuum:
• x = 0 and 0.1: I2/m → I4/mcm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m
m
• x = 0.2 and 0.3: I4/mcm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m
m
• x = 0.4–0.8: no phase transition; remains Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m
m
Previous studies reported different phase sequences, such as I2/m → R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c → Pm
c → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m for x = 0.1 and R
m for x = 0.1 and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c → Pm
c → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m for x = 0.4, under dry and wet N2 conditions using X-ray diffraction.26
m for x = 0.4, under dry and wet N2 conditions using X-ray diffraction.26
In those studies, the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group (tilt system a−a−a−) was used for refinement of both hydrated and dehydrated samples, in particular for x = 0.4 at RT. In contrast, I4/mcm (tilt system a0a0c−) is preferred for the hydrated phase in the present study, based on several lines of evidence. I4/mcm was unambiguously identified for x = 0.1, 0.2 and 0.3 by the splitting of h00 reflections together with superlattice peaks. For x = 0.4, the h00 splitting is less well resolved, but the persistence of superlattice reflections strongly suggests that the structure remains I4/mcm (Fig. 2b). In addition, for x = 0.4, neutron-diffraction Rietveld refinements show that the I4/mcm model yields superior agreement factors (Rp = 2.85%, Rwp = 3.75%, Robs = 3.23%, GOF = 2.05) compared with the R
c space group (tilt system a−a−a−) was used for refinement of both hydrated and dehydrated samples, in particular for x = 0.4 at RT. In contrast, I4/mcm (tilt system a0a0c−) is preferred for the hydrated phase in the present study, based on several lines of evidence. I4/mcm was unambiguously identified for x = 0.1, 0.2 and 0.3 by the splitting of h00 reflections together with superlattice peaks. For x = 0.4, the h00 splitting is less well resolved, but the persistence of superlattice reflections strongly suggests that the structure remains I4/mcm (Fig. 2b). In addition, for x = 0.4, neutron-diffraction Rietveld refinements show that the I4/mcm model yields superior agreement factors (Rp = 2.85%, Rwp = 3.75%, Robs = 3.23%, GOF = 2.05) compared with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c model (Rp = 3.19%, Rwp = 4.13%, Robs = 4.15%, GOF = 2.26), supporting the choice of I4/mcm. Finally, Pm
c model (Rp = 3.19%, Rwp = 4.13%, Robs = 4.15%, GOF = 2.26), supporting the choice of I4/mcm. Finally, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m was preferred over R
m was preferred over R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c for the dehydrated x = 0.4 phase at RT. The X-ray diffraction pattern shows no splitting of h00, hh0, or hhh reflections, nor the presence of superlattice peaks, confirming cubic Pm
c for the dehydrated x = 0.4 phase at RT. The X-ray diffraction pattern shows no splitting of h00, hh0, or hhh reflections, nor the presence of superlattice peaks, confirming cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry (tilt system a0a0a0) at RT (Fig. 7c).
m symmetry (tilt system a0a0a0) at RT (Fig. 7c).
Notably, for compositions with x > 0.1, the phase transition temperatures are consistently lower in the dehydrated series compared to their hydrated counterparts (Fig. 10). In both series, the transition temperatures for I2/m → I4/mcm and I4/mcm → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m decrease with increasing Zr content. This compositional trend is consistent with literature reports for BaZrxCe0.8−xY0.2O3−δ (0 ≤ x ≤ 0.8) in O2 with 3.1% H2O,25 where the following phase sequences were observed:
m decrease with increasing Zr content. This compositional trend is consistent with literature reports for BaZrxCe0.8−xY0.2O3−δ (0 ≤ x ≤ 0.8) in O2 with 3.1% H2O,25 where the following phase sequences were observed:
• x = 0.0, 0.1: I2/m → Imma → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m
m
• x = 0.2, 0.3: I2/m → Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m
m
• x = 0.4–0.8: Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m over the entire temperature range
m over the entire temperature range
These behaviors can be rationalized using bond valence sum (BVS) analysis applied to both actual and hypothetical cubic structures, highlighting the role of lattice distortions and ionic radii in driving structural transitions.
3.7 BVS as a predictor for octahedral tilting
Bond valence sum (BVS) analysis is a powerful method for evaluating cation stability in irregular coordination environments. It is calculated using the equation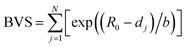 where dj is the distance to the j-th anion site (dj is provided in the CIF files available in the supplementary information), R0 and b are empirically tabulated constants (b = 0.37, R0 = 2.09 (Ce), 1.937 (Zr), 2.014 (Y), and 1.985 (Yb)),13,36 and N is the coordination number of the central cation.
where dj is the distance to the j-th anion site (dj is provided in the CIF files available in the supplementary information), R0 and b are empirically tabulated constants (b = 0.37, R0 = 2.09 (Ce), 1.937 (Zr), 2.014 (Y), and 1.985 (Yb)),13,36 and N is the coordination number of the central cation.
Beyond its traditional use in assessing bonding environments, BVS can serve as a predictor of octahedral tilting, similar in concept to the Goldschmidt tolerance factor. However, BVS offers a key advantage: it explicitly accounts for oxygen vacancies, enabling more accurate structural predictions in nonstoichiometric systems.
Importantly, reduced unit cell volumes remain consistent across different space groups (e.g., I2/m, I4/mcm, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), allowing direct comparisons of actual BVS values to those of hypothetical cubic analogues. For this analysis, RT structural data were used:
m), allowing direct comparisons of actual BVS values to those of hypothetical cubic analogues. For this analysis, RT structural data were used:
• Fully hydrated phases: neutron diffraction for x = 0 to 0.4
• Fully hydrated phases in N2: X-ray diffraction for x = 0.5 to 0.8
• Dehydrated phases in vacuum: X-ray diffraction for x = 0.4, 0.6, and 0.8
BVS values were computed at the B-site (Zr/Ce/Y/Yb), weighted by cation proportions. A coordination of six oxygen atoms was assumed for totally hydrated samples and 5.8 for dehydrated ones, reflecting the presence of oxygen vacancies. For example, for the fully hydrated x = 0 sample obtained from neutron diffraction refinement, the oxidation states for Ce, Y, and Yb are 4.04, 3.29, and 3.04, respectively, giving BVS = 3.86. In the hypothetical case that the structure adopts cubic symmetry while maintaining the same volume as the monoclinic structure, the corresponding oxidation states become 4.47, 3.64, and 3.37, yielding BVS = 4.27.
As x increases, the BVS of real hydrated structures gradually rises, while that of the hypothetical cubic counterparts decreases, both converging near x ≈ 0.5. For example, at x = 0.4, the hypothetical cubic structure yields a BVS of 4.10, while the actual tetragonal structure gives 3.96. At x = 0.5, the BVS reaches 4.03, and the phase adopts cubic symmetry, as confirmed by the disappearance of spontaneous tetragonal strain (see Section 3.2.5). For x > 0.5, BVS values decline steadily, reaching 3.81 at x = 0.8, very close to the ideal 3.8, confirming stability of the cubic phase.
For dehydrated phases, although unit cell volumes are lower than those of the corresponding hydrated samples (Tables 2 and 4), oxygen vacancies exert a stronger influence on BVS than volume contraction. As a result, BVS values for dehydrated phases (red crossed squares) are significantly lower than those for the corresponding hydrated samples, ranging from 4.17 (x = 0.0) to 4.02 (x = 0.3). As x increases, BVS approaches the ideal value more rapidly than in the hydrated series. Cubic symmetry is already observed at x = 0.4 in the dehydrated samples, compared with x = 0.5 in the hydrated case. (Note: for x = 0.0–0.3 in the dehydrated phases, the oxygen positions have not been refined; consequently, BVS values are not shown for these compositions). From x = 0.4 to 0.8, BVS continues to decrease, reaching 3.74 at x = 0.8, which is sufficient to stabilize the cubic structure.
Since BVS values generally decrease with increasing temperature, and dehydrated phases exhibit lower BVS values at room temperature compared to their hydrated counterparts, they are more prone to adopting cubic symmetry at lower temperatures. This trend is confirmed for compositions with x = 0.2 and 0.3 (Fig. 10).
This analysis underscores the value of BVS as a predictive tool for understanding symmetry transitions in perovskite-type oxides. By quantifying local bonding environments, including the effects of oxygen vacancies, BVS complements the Goldschmidt tolerance factor, capturing subtle compositional and thermal influences on structural distortions that are otherwise difficult to assess.
4. Conclusions
In this work, we successfully obtained both fully hydrated and fully dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 phases. The fully hydrated state was unambiguously confirmed by TGA and neutron diffraction, while the fully dehydrated state was validated by the near-linear cell-volume behavior during cooling. Using these well-defined phases, we systematically investigated the structural evolution by neutron and X-ray diffraction during heating in N2 atmosphere and cooling under vacuum. Water uptake was found to strongly affect the crystal structure by increasing octahedral tilting and inducing chemical expansion, which correlated with unit-cell volume changes and TGA-measured water loss. Phase diagrams further revealed that increasing zirconium content reduces symmetry and lowers phase-transition temperatures. While the Goldschmidt tolerance factor alone could not fully capture structural stability, bond-valence-sum (BVS) analysis, which accounts for oxygen vacancies, provided improved predictive capability for octahedral tilting and phase transitions.Overall, this study establishes the reliable preparation of fully hydrated and dehydrated perovskite phases, clarifies their structure–hydration relationships, and demonstrates the value of BVS analysis as a complementary tool for designing next-generation ceramic proton conductors with enhanced performance.
Author contributions
Lozane Hamze: writing – review & editing, visualization, investigation, formal analysis, data curation. Olivier Joubert: writing – review & editing, funding acquisition. Eric Quarez: writing – original draft, writing – review & editing, validation, supervision, project administration, methodology, conceptualization.Conflicts of interest
There are no conflicts to declare.Data availability
Further data relevant to this article are available on request from the corresponding author.All data supporting the results of this work are available in the main article and in the supplementary information (SI). Supplementary information: crystallographic data at RT for fully hydrated BaZrxCe0.8−xY0.1Yb0.1O2.9(H2O)0.1 (x = 0, 0.1, 0.2, 0.3, and 0.4), without hydrogen localization and derived from neutron diffraction data, as well as for x = 0.5, 0.6, 0.7, and 0.8 obtained from XRD data, and for dehydrated BaZrxCe0.8−xY0.1Yb0.1O2.9 (x = 0.4, 0.6, and 0.8) obtained from XRD data, have been deposited via the joint CCDC/FIZ Karlsruhe service under deposition numbers 2471699–2471710.37a–l The corresponding CIF files, anisotropic thermal displacement parameters, and XRD patterns are available in the SI. See DOI: https://doi.org/10.1039/d5ta07218b.
Acknowledgements
The authors acknowledge support from the French National Research Agency (ANR) under France 2030 program and reference ANR-22-PEHY-0006 (project PEPRH2-PROTEC). The author would like to thank ILL for providing beam time and Dr Emmanuelle Suard for her help with the measurements on the D2B powder diffractometer, Dr Pierre Emmanuel Petit responsible for the diffractometers in the IMN's characterization platform PLASSMAT, Nantes, France.References
- L. Yang, S. Wang, K. Blinn, M. Liu, Z. Liu, Z. Cheng and M. Liu, Science, 2009, 326, 126–129 CrossRef CAS
.
- N. A. Danilov, I. A. Starostina, G. N. Starostin, A. V. Kasyanova, D. A. Medvedev and Z. Shao, Adv. Energy Mater., 2023, 13, 2302175 CrossRef CAS
.
- M. B. Hanif, S. Rauf, Z. ul Abadeen, K. Khan, Z. Tayyab, S. Qayyum, M. Mosiałek, Z. Shao, C.-X. Li and M. Motola, Matter, 2023, 6, 1782–1830 CrossRef CAS
.
- S. Guo, L. Jiang, Y. Li, P. Zhong, S. A. Ismail, T. Norby and D. Han, Adv. Funct. Mater., 2024, 34, 2304729 CrossRef CAS
.
- C. Tang, Y. Yao, N. Wang, X. Zhang, F. Zheng, L. Du, D. Luo, Y. Aoki and S. Ye, InfoMat, 2024, 6, e12515 CrossRef CAS
.
- U. Tariq, M. Z. Khan, O. Gohar, Z. U. Din Babar, F. Ali, R. A. Malik, I. A. Starostina, I. A. Samia, J. Rehman, I. Hussain, M. Saleem, A. Ghaffar, M. A. Marwat, K. Zheng, M. Motola and M. B. Hanif, J. Power Sources, 2024, 613, 234910 CrossRef CAS
.
- J. Rehman, M. B. Hanif, M. Z. Khan, M. Ullah, I. A. Starostina, M. T. Muhammad and Z. Li, Energy Fuels, 2024, 38, 22637–22665 CrossRef CAS
.
- H. Nie, Z. Liu, M. Xiao, G. Yang, T. Li, I. A. Starostina, D. A. Medvedev, W. Wang, W. Zhou and R. Ran, Adv. Funct. Mater., 2025, 35, 2416651 CrossRef CAS
.
- J. Wang, W. Chen, Y. Wang, J. Wei, W. Zhang, C. Sun and S. Peng, Mater. Sci. Eng., B, 2025, 317, 118188 CrossRef CAS
.
- L. Hamze, A. Le Gal La Salle, O. Joubert and E. Quarez, Int. J. Hydrogen Energy, 2025, 139, 316–324 CrossRef CAS
.
- S. Choi, C. J. Kucharczyk, Y. Liang, X. Zhang, I. Takeuchi, H.-I. Ji and S. M. Haile, Nat. Energy, 2018, 3, 202–210 CrossRef CAS
.
- L. Hamze, E. Suard, O. Joubert and E. Quarez, Solid State Ionics, 2024, 417, 116682 CrossRef CAS
.
- V. Petříček, M. Dušek and L. Palatinus, Z. Kristallogr. Cryst. Mater., 2014, 229, 345–352 CrossRef
.
- J. F. Berar and P. Lelann, J. Appl. Crystallogr., 1991, 24, 1–5 CrossRef CAS
.
- E. Quarez, S. Noirault, M. T. Caldes and O. Joubert, J. Power Sources, 2010, 195, 1136–1141 CrossRef CAS
.
- S. Noirault, E. Quarez, Y. Piffard and O. Joubert, Solid State Ionics, 2009, 180, 1157–1163 CrossRef CAS
.
- A. Jarry, E. Quarez, K. Kravchyk and O. Joubert, Solid State Ionics, 2012, 216, 11–14 CrossRef CAS
.
- A. Jarry, E. Quarez and O. Joubert, Solid State Ionics, 2014, 256, 76–82 CrossRef CAS
.
- A. Jarry, O. Joubert, E. Suard, J. M. Zanotti and E. Quarez, Phys. Chem. Chem. Phys., 2016, 18, 15751–15759 RSC
.
- C. Hiraiwa, D. Han, A. Kuramitsu, A. Kuwabara, H. Takeuchi, M. Majima and T. Uda, J. Am. Ceram. Soc., 2013, 96, 879–884 CrossRef CAS
.
- A. K. E. Andersson, S. M. Selbach, C. S. Knee and T. Grande, J. Am. Ceram. Soc., 2014, 97, 2654–2661 CrossRef CAS
.
- D. Han, N. Hatada and T. Uda, J. Am. Ceram. Soc., 2016, 99, 3745–3753 CrossRef CAS
.
- G. C. Mather, G. Heras-Juaristi, C. Ritter, R. O. Fuentes, A. L. Chinelatto, D. Pérez-Coll and U. Amador, Chem. Mater., 2016, 28, 4292–4299 CrossRef CAS
.
- G. Hudish, A. Manerbino, W. G. Coors and S. Ricote, J. Am. Ceram. Soc., 2018, 101, 1298–1309 CrossRef CAS
.
- D. Han, X. Liu, T. S. Bjørheim and T. Uda, Adv. Energy Mater., 2021, 11, 2003149 CrossRef CAS
.
- K. Nomura, H. Shimada, Y. Yamaguchi, W. Shin, Y. Okuyama and Y. Mizutani, J. Electrochem. Soc., 2022, 169, 024516 CrossRef CAS
.
- I. Ivanov, D. Tsvetkov, V. Sereda, D. Malyshkin, A. Sereda, P. Zakiryanov, R. Yagovitin and A. Zuev, Chim. Techno Acta, 2024, 11, 202411422 CrossRef CAS
.
- C. J. Howard and H. T. Stokes, Acta Crystallogr., Sect. B, 1998, 54, 782–789 CrossRef
.
- M. A. Carpenter, A. I. Becerro and F. Seifert, Am. Mineral., 2001, 86, 348–363 CrossRef
.
- T. K.-Y. Wong, B. J. Kennedy, C. J. Howard, B. A. Hunter and T. Vogt, J. Solid State Chem., 2001, 156, 255–263 CrossRef CAS
.
- B. J. Kennedy, C. J. Howard, G. J. Thorogood and J. R. Hester, J. Solid State Chem., 2001, 161, 106–112 CrossRef CAS
.
- I. Qasim, P. E. R. Blanchard, B. J. Kennedy, T. Kamiyama, P. Miao and S. Torii, J. Solid State Chem., 2014, 213, 293–300 CrossRef CAS
.
- V. Jayaraman, A. Magrez, M. Caldes, O. Joubert, F. Taulelle, J. Rodriguez-Carvajal, Y. Piffard and L. Brohan, Solid State Ionics, 2004, 170, 25–32 CrossRef CAS
.
- Y. Zhang, Inorg. Chem., 1982, 21, 3886–3889 CrossRef CAS
.
- Yu. G. Lyagaeva, D. A. Medvedev, A. K. Demin, P. Tsiakaras and O. G. Reznitskikh, Phys. Solid State, 2015, 57, 285–289 CrossRef CAS
.
- N. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B, 1991, 47, 192–197 CrossRef
.
-
(a)
CCDC 2471699: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz07x
; (b) CCDC 2471700: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz08y
; (c) CCDC 2471701: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz09z
; (d) CCDC 2471702: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0b0
; (e) CCDC 2471703: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0c1
; (f) CCDC 2471704: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0d2
; (g) CCDC 2471705: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0f3
; (h) CCDC 2471706: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0g4
; (i) CCDC 2471707: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0h5
; (j) CCDC 2471708: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0j6
; (k) CCDC 2471709: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0k7
; (l) CCDC 2471710: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nz0l8
.
| This journal is © The Royal Society of Chemistry 2026 |