Nanostructural design of flexible mesoporous hydrogel electrodes via colloidal electrochemical deposition for highly efficient oxygen evolution reaction
Hiroki
Wago
a,
Shigenori
Mitsushima
 ab and
Yoshiyuki
Kuroda
ab and
Yoshiyuki
Kuroda
 *ab
*ab
aGraduate School of Engineering Science, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan. E-mail: kuroda-yoshiyuki-ph@ynu.ac.jp
bAdvanced Chemical Energy Research Center (ACERC), Institute of Advanced Sciences (IAS), Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan
First published on 14th October 2025
Abstract
Water electrolysis is a core technology for converting renewable energy into hydrogen and is useful for energy storage and transportation. Porous electrodes can efficiently promote the oxygen evolution reaction (OER) because of their high surface area; however, the generated bubbles block the electrode surface and reduce the number of reaction sites. Here, we demonstrate flexible mesoporous hydrogel electrodes with more than 90% porosity prepared by the colloidal electrochemical deposition of hybrid cobalt hydroxide nanosheets (Co-ns) as promising new materials for porous OER electrodes in alkaline media, combining a large surface area with high mass transport. The pore size of the flexible mesoporous hydrogel electrodes was controlled in the range of 25–45 nm by controlling the lateral size of the Co-ns. The pore surfaces of the flexible mesoporous hydrogel electrodes with larger pore sizes were utilized more efficiently in the depth along the thickness direction, demonstrating higher mass transport. Colloidal electrochemical deposition is also useful for constructing hierarchical structures, such as bilayer electrodes combining differently sized mesopores, placing larger pores with high mass transport in the top layer and small pores with a high surface area in the bottom layer, thereby achieving a higher OER current density than monolayer electrodes. Flexible mesoporous hydrogel electrodes, with facile control over mesopores and hierarchical structures, show great potential as new forms of mesoporous electrodes for water electrolysis.
1 Introduction
Renewable energy sources such as solar and wind power are useful for reducing carbon dioxide emissions and mitigating global warming; however, these energy sources have temporal and regional variability. Therefore, green hydrogen produced via water electrolysis using renewable energy is an important energy carrier that can be easily stored and transported.1,2 Commercial-scale water electrolysis technologies include alkaline water electrolysis (AWE)3 and proton exchange membrane water electrolysis (PEMWE).4 PEMWE enables operation at high current density and is suitable for miniaturization. However, its proton exchange membrane is strongly acidic, requiring a large amount of precious metals such as Pt for the cathode and Ir for the anode. In contrast, AWE, which uses a liquid alkaline electrolyte, can be constructed using base metals such as Fe, Ni, and Co, thereby reducing the cost of green hydrogen production.5,6 Therefore, improving the performance of AWE is crucial for the practical implementation of large-scale hydrogen production systems.The efficiency of water electrolysis is significantly limited by the sluggish oxygen evolution reaction (OER); therefore, the development of highly active anodes is important.7–9 Porous electrodes with large surface areas are effective in achieving high current density in water electrolysis.10,11 However, in porous electrodes, gas bubbles generated within the pores block mass transport at the reaction sites;12–14 for instance, when the pores of a porous anode are clogged by oxygen bubbles, its OER performance has been reported to be almost identical to that of a smooth electrode without pores.15
Several strategies have been proposed to address this issue, including optimization of the porous structure to facilitate the release of bubbles, design of patterned surfaces on electrodes to facilitate the detachment of bubbles, and removal of bubbles from the interior of porous electrodes by electrolyte flow.16–18 Hao and co-workers developed a porous electrode with columnar pores (approximately 10–30 μm in diameter), which facilitated the release of bubbles, by electrodeposition of Ni while using hydrogen bubbles as a template.16 Rocha and co-workers fabricated a 3D-printed Ni electrode with a mathematically designed periodic porous structure that maintains zero mean curvature, demonstrating enhanced bubble removal.17 Furthermore, Yang and co-workers reported that an electrolyzer employing a porous Ni microfiber electrode achieved both a high surface area and efficient bubble removal by introducing electrolyte flow inside the porous electrode.18 However, in all these cases, the bubble removal effect was primarily applicable to macroporous electrodes with pore sizes of tens of micrometers or larger. Effective approaches for fabricating microporous (pore size <2 nm) and mesoporous (pore size between 2–50 nm) electrodes, which can achieve significantly larger surface areas, remain limited.19
We recently proposed flexible mesoporous hydrogel electrodes composed of CoOOH nanosheets as porous electrodes with a large surface area and efficient mass transport properties.20 These were prepared via the electrochemical deposition of hybrid cobalt hydroxide nanosheets (Co-ns), specifically colloidal electrochemical deposition (Fig. 1(a)). Co-ns is cobalt hydroxide (Co(OH)2) modified with a tripodal ligand, tris(hydroxymethyl)aminomethane, on its surface. During electrolysis in an electrolyte containing dispersed Co-ns, cobalt hydroxide nanosheets (Co-ns) are deposited on the anode while the ligand undergoes oxidative decomposition, forming a mesoporous catalytic layer. Colloidal electrochemical deposition is a unique technique for depositing colloidal materials, similar to electroplating under milder conditions than electrophoretic deposition.21
The resulting catalyst layer features flexible mesopores enclosed by CoOOH nanosheets, which improves oxygen transport and enables high OER activity at high current densities (Fig. 1(b)). We hypothesized that this enhancement is due to the increased oxygen solubility owing to the high Laplace pressure of the electrolyte confined in the mesopores, which suppresses the nucleation of bubbles.19,22 Additionally, the unique flexibility of the mesoporous hydrogel electrode is expected to maintain an optimum uniform structure without impeding oxygen release. Because the diffusivity of molecules within mesopores depends on pore size, precise control of pore size is crucial for optimizing electrode performance; therefore, nanostructural control of flexible mesoporous hydrogel electrodes is important.
The pore size of the flexible mesoporous hydrogel electrodes was expected to be determined by the lateral size of the constituent CoOOH nanosheets. Thus, we aimed to control the pore size by controlling the lateral size of the Co-ns (Fig. 1(c)). Co-ns of various lateral sizes were used as building blocks for flexible mesoporous hydrogel electrodes. We have previously shown that the tripodal ligand acts as a surface stabilizing agent to suppress crystal growth, allowing the lateral size of the layered double hydroxide to be varied from 10 to 60 nm depending on the concentration of the tripodal ligand.23–25 Additionally, the lateral size of hybrid magnesium hydroxide nanosheets was controlled by reaction time because the hybrid nanosheets grow via the classical homogeneous nucleation and growth mechanism.24 These concepts may be applicable for the lateral size control of Co-ns.
A key issue in the evaluation of the pore size of flexible mesoporous hydrogel electrodes is the observation of realistic mesoporous structures swollen with electrolyte using electron microscopy under vacuum; thus, it cannot be guaranteed that the pore size will be maintained after drying. Therefore, we focused on the scanning electron microscopy (SEM) technique using a room-temperature ionic liquid as both the conducting coating and the swelling agent of the hydrogel, instead of an aqueous electrolyte (Fig. 1(c)). Ionic liquids are salts composed of organic cations, such as alkylimidazolium, that remain liquid at room temperature and have almost no vapor pressure. Despite being ion conductors, ionic liquids can be used as conductive coatings because they do not cause charge-up due to electron beam irradiation during SEM observation.26 Furthermore, by replacing water in hydrated materials such as biological samples, SEM observation can be performed while maintaining almost the same structure as the swollen state with water.27 Therefore, it is expected that the swollen structures of flexible mesoporous hydrogel electrodes can be estimated by SEM with ionic liquid substitution.
Flexible mesoporous hydrogel electrodes were prepared by colloidal electrochemical deposition of Co-ns, offering high flexibility in material design. For instance, colloidal electrochemical deposition allows the self-repair of degraded electrodes.28–30 Furthermore, self-repairing composite catalysts have been prepared by electrostatic assembly of Co-ns and β-FeOOH nanorods serving as a self-repairing support and a highly active catalyst, respectively.31 By using the methods mentioned above to create flexible mesoporous hydrogel electrodes with varied pore sizes, it is possible to fabricate bilayer electrodes consisting of layers with different pore sizes by repeated deposition under different conditions. Therefore, bilayer mesoporous hydrogel electrodes consisting of layers with different pore sizes were prepared, and the relationship between their nanostructures and mass transport properties was investigated for the rational design of porous electrodes (Fig. 1(d)). The bilayer structure is promising for optimizing electrolyte flow in the pores.32
In this study, we demonstrate the usefulness of colloidal electrochemical deposition of Co-ns of variable sizes as a powerful tool for the nanostructural design of flexible mesoporous hydrogel electrodes with controlled pore sizes. The pore size in the swollen state was evaluated by substituting the aqueous electrolyte within the mesoporous hydrogel electrodes with an ionic liquid. The OER performance with varied pore sizes and catalyst layer thicknesses was precisely evaluated to clarify the effect of pore size on mass transport properties during the OER in flexible mesoporous hydrogel electrodes. These findings were applied to fabricate bilayer mesoporous hydrogel electrodes with different pore sizes in the internal and surface layers, and the pore configuration that facilitates the removal of gas molecules was investigated to achieve both an increased surface area and improved mass transport properties in mesoporous electrodes with small pore sizes.
2 Experimental section
2.1 Materials
Cobalt chloride hexahydrate (CoCl2·6H2O) was purchased from Kanto Chemical Co., Inc. Tris(hydroxymethyl)aminomethane (Tris–NH2), hydrochloric acid (HCl), ethanol, and ammonia (NH3) solution were purchased from FUJIFILM Wako Pure Chemical Corporation. Potassium hydroxide (KOH) was purchased from Toagosei Co., Ltd. 1-Butyl-3-methylimidazolium tetrafluoroborate was purchased from Tokyo Chemical Industry Co., Ltd Ni wire and foam were purchased from Nilaco Co. A Ni plate was purchased from Koei Co., Ltd Pt wire (φ0.3 mm) was purchased from Tanaka Kikinzoku K. K. The Ni wire and plate were etched by boiling them in 6 M HCl for 6 min prior to use. All other reagents and materials were used as received.2.2 Synthesis of Co-ns with different sizes
Co-ns is a hybrid layered metal hydroxide with a Tris–NH2 ligand attached to the surface of the Brucite-type Co(OH)2 sheet.23 Co-ns was synthesized based on a previous report28 by mixing a Tris–NH2 aqueous solution (1.0 M, 100 mL) with a CoCl2·6H2O aqueous solution (0.1 M, 100 mL) and heating the mixture at 90 °C for various durations. To increase the size of Co-ns, the heating duration was varied to 1, 5, and 10 d. Additionally, to synthesize larger Co-ns, the concentration of the Tris–NH2 aqueous solution was reduced to 0.10 M, and the mixture was heated for 10 d. The resulting precipitate was filtered through a Millipore membrane (pore size: 0.2 μm) and washed twice with deionized water. The wet precipitate was dispersed in deionized water (approximately 100 mL) without drying and sonicated for 30 min to obtain the Co-ns dispersion. The Co content of the Co-ns dispersion was measured using inductively coupled plasma atomic emission spectroscopy (ICP-AES). The amount of dispersion used for the electrochemical tests was calculated using the determined Co concentrations.The particle size of each sample was determined using transmission electron microscopy (TEM). The Co-ns exhibited circular shapes with slightly torn edges, corresponding to distorted disk-shaped nanosheets. Disk aggregates with irregular intensities (aggregates of nanosheets) were excluded from the particle size evaluation. Because dispersed nanosheets are much more suitable for electrochemical deposition than aggregates, the exclusion of aggregates from the particle size evaluation is acceptable for analyzing the relationship between particle size and the colloidal electrochemical deposition ability. The aspect ratio, calculated from the longitudinal and minor axes of each particle, ranged from 1.0 to 1.2, indicating that the particles were not perfectly isotropic in the plane, whereas no particles with extremely high aspect ratios were observed. Because the size of the particles could only be directly determined by the longitudinal axis, particle size histograms were constructed using the longitudinal axis as the representative value for the size of the Co-ns. Based on the average longitudinal sizes of 21, 34, 42, and 54 nm obtained from the histograms of each sample, the samples were designated as ns21, ns34, ns42, and ns54, respectively.
2.3 Preparation of flexible mesoporous hydrogel electrodes
Electrochemical measurements were conducted using a three-electrode cell composed of perfluoroalkoxy alkanes (PFAs). A Ni plate (approximately 0.7 × 0.7 cm2) was used as the working electrode, a reversible hydrogen electrode (RHE) as the reference electrode, and a Ni coil as the counter electrode. A KOH aqueous solution (1.0 M, 250 mL) was used as the electrolyte. The Co-ns dispersion was added to the electrolyte at a concentration of 80 ppm (catalyst mass basis) and stirred at approximately 600 rpm. For some samples, the concentration was increased to 60 and 80 ppm to increase the film thickness. Constant-current electrolysis was performed at 800 mA cm−2 for 20 min to 100 h, forming a hydrogel-like catalytic layer composed of CoOOH nanosheets on the working electrode.20 The thickness of the catalytic layer was controlled by the electrolysis duration. Afterward, electrochemical evaluation was carried out using cyclic voltammetry (CV) (0.5–2.5 V vs. RHE, 5.0 mV s−1, 1 cycle), CV (0.5–1.6 V vs. RHE, 50 mV s−1, 1 cycle), and electrochemical impedance spectroscopy (EIS) (1.6 V vs. RHE, 10–105 Hz, amplitude 10 mV). The electrochemical measurements were performed using a BioLogic VSP-3e potentiogalvanostat. The sample names were designated based on the substrate and Co-ns used, such as Ni/ns21, Ni/ns34, Ni/ns42, and Ni/ns54. The reproducibility of the measurements is shown in Fig. S1.2.4 Preparation of bilayer mesoporous hydrogel electrodes
Bilayer mesoporous hydrogel electrodes were prepared by alternately depositing ns21 and ns42. First, a 1 M KOH electrolyte with one type of Co-ns dispersion was used, and a Ni plate that had not yet been coated with the catalyst was employed as the working electrode. Constant current electrolysis was performed at 800 mA cm−2 for 4 h (1st layer). The OER performance was evaluated using the method described in Section 2.3. The working electrode was then removed from the cell and stored in pure water. The electrolyte in the cell was completely replaced with a new electrolyte containing Co-ns of a different size, and the electrode was reinstalled in the cell. The same electrochemical process (800 mA cm−2, 4 h) was repeated to deposit the 2nd layer on top of the 1st layer. The sample names were formatted as Ni/1st layer/2nd layer, and Ni/ns21/ns42 and Ni/ns42/ns21 samples were prepared.2.5 Characterization
For the analysis of Co-ns with different particle sizes, a portion of the sample was dried in an oven at 90 °C, then ground in a mortar, and the resulting powder was analyzed by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and ICP-AES. XRD patterns were recorded on a Rigaku Ultima IV diffractometer with Cu Kα radiation at a scan rate of 10° min−1 over a 5–90° range. The sample was finely ground and mounted on a nonreflective Si sample holder for measurement. The baseline of Co-based samples was affected by the X-ray fluorescence of Co; therefore, the baseline was corrected, using the baseline curve obtained from an XRD pattern of crystalline CoCl2·6H2O as a reference. Fourier-transform infrared (FTIR) spectra were obtained using a JASCO FT/IR-6600 spectrometer equipped with KBr disks. Elemental analysis was performed by ICP-AES using an ICPE-9000 system. The sample powder was dissolved in a small amount of 6 M HCl and diluted to 100 mL in a volumetric flask for measurement. The amount of Co deposited on the flexible mesoporous hydrogel electrode was measured by dissolving the catalyst deposited on the electrode in a small amount of 6 M HCl and diluting it to 50 mL in a volumetric flask for analysis. The film thickness of the flexible mesoporous hydrogel electrode was measured by confocal laser scanning microscopy (CLSM) using a KEYENCE Laser Microscope VK-X200 with a laser (λ = 408 nm). After the electrochemical measurements, the mesoporous hydrogel electrode was removed from the cell and the excess KOH solution was rinsed off by gently applying a small amount of pure water to the gel. After scratching the electrode surface, as previously reported,20 the film thickness of the mesoporous hydrogel electrode was calculated from the height difference. Field emission scanning electron microscopy (FE-SEM) images were obtained using a Hitachi SU8010 microscope. The method for observing the swollen state using an ionic liquid is described in Section 2.6. The Co-ns particle size was measured using a TEM (JEOL JEM-2100F) at an acceleration voltage of 200 kV. Holey carbon-coated Cu grids (Okenshoji Co., Ltd) were used for the measurement. The Co-ns dispersion was diluted with ethanol and dried on a copper grid.2.6 SEM observation of flexible mesoporous hydrogel electrodes swollen with ionic liquid
To observe the hydrogel electrode in the swollen state in liquid, the electrolyte solution inside the hydrogel was replaced with an ionic liquid and observed using FE-SEM. The ionic liquid used was 1-butyl-3-methylimidazolium tetrafluoroborate ([BMI+][BF4−]), a hydrophilic ionic liquid that was used in a previous report27 to maintain the swollen state of biological samples. First, ethanol solutions were prepared with [BMI+][BF4−] concentrations of 10, 15, and 20 mM. A 50 mg portion of the prepared solution was then dropped onto the hydrogel electrode and left under reduced pressure (ca. −0.099 MPaG) in a vacuum chamber for 2 h. Subsequently, the electrode was fixed directly onto the FE-SEM sample holder using carbon tape and introduced into the FE-SEM. When the ionic liquid was present in large amounts, all the mesopores at the outermost surfaces were filled with the ionic liquid, making it impossible to distinguish between the framework and voids in the SEM images. Therefore, the concentration of the ionic liquid in ethanol was varied, and the area where the ionic liquid was absorbed only by the framework of the nanosheets, leaving the pore regions as voids, was explored. For comparison, electrodes that underwent the same procedure using ethanol without the ionic liquid were also observed to verify the effect of the ionic liquid. Because the CoOOH nanosheets are conductive, metal deposition was not performed under any conditions.3 Results and discussion
3.1 Characterization of Co-ns with different lateral sizes
The XRD patterns of the prepared Co-ns powders show broad peaks assignable to a pseudo-hexagonal lattice of disordered layered materials, similar to those reported in previous studies (Fig. S2(a)).23,30 The 110 and 100 diffractions, originating from the in-plane periodicity, show a decrease in peak width as the size of Co-ns increases. The crystallite size along the [110] axis of ns21, ns34, ns42, and ns54 estimated according to Scherrer's equation were 10.2, 10.8, 11.5, and 13.1 nm, respectively. The crystallite size obtained using Scherrer's equation may not perfectly match the actual size owing to the influence of defects and the bending of very thin nanosheets. Therefore, the discussion of crystallite size by XRD was kept qualitative, and the size of the Co-ns was further analyzed in detail using TEM. Additionally, the basal diffraction of ns54 was very broad, which was probably due to the reduced amount of organic ligands modified on the cobalt hydroxide sheet owing to the decreased concentration of Tris–NH2 in the synthetic solution, resulting in uneven interlayer interactions and disruption of stacking.The FTIR spectra of the prepared Co-ns powders show absorption bands due to lattice vibrations of cobalt hydroxide sheets at 600–700 cm−1, C–H stretching vibrations at 2700–3000 cm−1, and C–O stretching vibrations at 1000–1100 cm−1, indicating modification by Tris–NH2 (Fig. S2(b)).23 These absorption bands are reduced in ns54, confirming that the reduction in the concentration of the organic ligand led to a decrease in the amount of Tris–NH2 modified on a cobalt hydroxide sheet.
In addition, powdered Co-ns of each size were added to pure water at a concentration of 2000 ppm and subjected to ultrasonic treatment for 10 min. As a result, the dispersions of ns21, ns34, and ns42 became transparent brown, whereas ns54 turned light brown and opaque with brown precipitates (Fig. S3). When these liquids were irradiated with laser light, the Tyndall effect was observed, indicating that the Co-ns particles were dispersed in pure water rather than dissolved. The dispersion ability was similar for ns21, ns34, and ns42, but was significantly reduced for ns54. Because tripodal ligands act as surface stabilizers to prevent aggregation, the significantly low modification amount of ns54 was probably the reason for this difference.
The TEM images of each Co-ns sample obtained from the dispersions synthesized under different conditions are shown in Fig. 2(a)–(d). The shapes of the particles were irregular but could be approximated as elliptical. The uniform contrast suggests that these images show the nanosheets viewed vertically. The maximum width of the particles (i.e., the size along the longitudinal axis) was used as a representative measure of particle size (Fig. S4). The aspect ratios of the primary particles observed by TEM were 1.0–1.2, and no particles with extremely high aspect ratios were observed. Thus, the longitudinal axis is appropriate as a representative value for the Co-ns particle size.
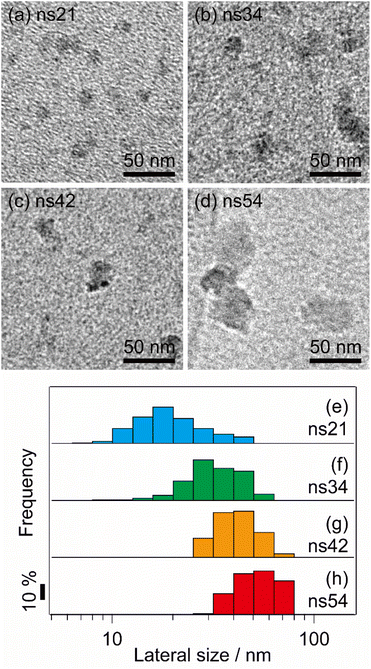 | ||
| Fig. 2 TEM images of (a) ns21, (b) ns34, (c) ns42 and (d) ns54, and the histogram of lateral sizes of (e) ns21, (f) ns34, (g) ns42 and (h) ns54. | ||
Histograms of the maximum width of the primary particles show that the average sizes of the Co-ns were 21 (coefficient of variation is 40%), 34 (coefficient of variation is 28%), and 42 nm (coefficient of variation is 23%) when the heating time was changed to 1, 5, and 10 d, respectively, indicating that the Co-ns grew laterally with increasing heating time (Fig. 2(e)–(h)). This trend is consistent with our previous study on hybrid magnesium hydroxide.24 When the concentration of the Tris–NH2 solution was decreased to 0.1 M and heated for 10 d, the average size of Co-ns was 54 nm (coefficient of variation is 22%), showing that the particle size was further increased by decreasing the ligand concentration (Fig. 2(h)). This is consistent with the trend observed in our previous study on hybrid MgAl layered double hydroxides.25
3.2 Characterization of flexible mesoporous hydrogel electrodes
The appearance of the Ni/ns21 mesoporous hydrogel electrode formed after 4 h of electrochemical deposition is shown in Fig. 3(a). Compared to the appearance of the electrode after drying (Fig. 3(b)), the Ni/ns21 electrode was swollen by the electrolyte. The catalyst film remained stable against weak mechanical forces, such as vigorous stirring of the electrolyte and rinsing with water, although it could be peeled off by strong mechanical forces, such as scratching with a metal rod. Mesoporous hydrogel electrodes have also been reported to be stable under the electrochemical stress caused by potential cycling,28,29,32,33 although a degraded NiCoOx layer is detached from a Ni substrate under the same condition. | ||
| Fig. 3 Photographs of flexible mesoporous hydrogel electrodes (Ni/ns21) after (a) electrolysis for 4 h, (b) drying, and (c) substitution with ionic liquid. | ||
According to our previous report,29 surface functional groups of Co-ns are decomposed by anodic reactions to form CoOOH nanosheets. XRD patterns showed the disappearance of the basal diffraction (001) and shifts of 100 and 110 diffractions. FTIR and SEM-EDX showed a large decrease in organic functional groups (Fig. S5).
The thickness of the mesoporous hydrogel electrode was measured using CLSM, as reported previously.20 A scratch was made on the electrode surface with a needle, and the height difference between the film surface and the substrate was determined from the height profile obtained using CLSM, which was used to calculate the film thickness of the mesoporous hydrogel electrode in the swollen state (Fig. S6). The height profiles at the film and substrate surfaces were fitted linearly using the least-squares method, and the distance between the lines was used as the thickness. It was difficult to form a film thickness of over 60 μm for ns54 possibly because of poor dispersibility as shown in Fig. S3(d). The aggregated Co-ns should have a lower frequency of collisions with the electrode surface. The film thickness increases rapidly in the first 10 h up to 30–40 μm, and then gradually increased along with the duration of electrolysis (Fig. S7).
In the electrochemical evaluation of the mesoporous hydrogel electrode, the cyclic voltammograms obtained at a scan rate of 50 mV s−1 exhibited peaks corresponding to Co2+/3+ and Co3+/4+, which were attributed to the redox behavior of Co3+ in CoOOH (Fig. 4).34,35 These results indicate that the deposited catalyst is electrochemically active. The charge associated with the reduction peak (Qc) between 0.8–1.5 V vs. RHE in the cyclic voltammogram was proportional to the amount of Co in the deposited CoOOH (mCo) (Fig. S8).20 Additionally, regardless of the average size of the Co-ns used, all the plots follow the same correlation, confirming that the electrochemical reaction efficiency of Co atoms is constant across all hydrogel electrodes.
Furthermore, when the amount of deposited Co, calculated from Qc was plotted as a function of the thickness of the catalyst layer, S-shaped curves were observed (Fig. S9). The relative density (dr) of each mesoporous hydrogel electrode was calculated using the following equation (eqn (1)):
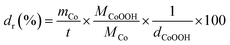 | (1) |
 | ||
| Fig. 5 Relationship between the apparent density of Co (dr) and the thickness of catalyst layers: (a) Ni/ns21, (b) Ni/ns34, (c) Ni/ns42, (d) Ni/ns54, and (e) Ni only. | ||
The porosity of the electrodes was calculated using the density in the plateau region for each mesoporous hydrogel electrode, resulting in 91.5% (Ni/ns21), 93.2% (Ni/ns34), 94.1% (Ni/ns42), and 97.0% (Ni/ns54). This trend shows that larger Co-ns formed a mesoporous structure with higher porosity. Assuming that the wall thickness of the mesoporous hydrogel electrodes is constant regardless of the size of the Co-ns, the increase in porosity corresponds to an increase in pore size. The porosity of these flexible mesoporous hydrogel electrodes composed of CoOOH nanosheets was found to be very high compared to rigid CoOOH platelets made by electrolysis of Co2+ on a Ni substrate (57.0%),20,36 demonstrating the exceptionally high porosity of the flexible mesoporous hydrogel electrode. The thin pore walls, consisting of a few nanosheets stacked in the direction of the wall thickness, likely contributed to high porosity.
FE-SEM images of the flexible mesoporous hydrogel electrodes were obtained using a 10 mM ionic liquid solution (Fig. 6(a)–(d)). In the FE-SEM images, the bright areas represent the CoOOH nanosheets deposited on the surface of the electrode. Each nanosheet area had an elongated shape, and both the long and short axes were larger than the average long diameter of ns21. This suggests that they correspond to parallelly stacked nanosheets, as shown in Fig. 6(e). These assemblies form the pore walls of the mesoporous structures; in other words, the dark areas represent pores surrounded by nanosheets. From the above discussion, the mesopores were classified as slit-shaped pores between the aggregates of the nanosheets. The ionic liquid-assisted FE-SEM observations demonstrated that the thickness of pore walls of flexible mesoporous hydrogel electrodes is independent of the lateral size of Co-ns. Therefore, the difference in the porosity also consistently explains the difference in pore size.
The FE-SEM image of the hydrogel electrode substituted only with ethanol showed that the size of the bright areas was similar to that observed in the FE-SEM images of the ionic liquid-substituted samples, indicating that the CoOOH nanosheets were stacked in assemblies (Fig. S10). During ethanol substitution, it was assumed that structural changes, such as pore destruction and contraction, occurred owing to the evaporation of ethanol.
These changes correspond to the concentrated bright areas and extremely large dark areas in the FE-SEM images. Therefore, the FE-SEM images of the electrode after ethanol substitution show pores that have become uneven due to contraction and destruction, which differs from the conditions during the electrochemical measurements. Even the sample substituted with the ionic liquid does not necessarily demonstrate the exact swollen state in the electrolyte solution because of the unavoidable risk of structural deformation during substitution and drying; however, the fact that the structural uniformity is not destroyed has a significant effect owing to the use of ionic liquid. For the sample swollen with the ionic liquid, the relative relationship between the pore sizes of the samples can be sufficiently discussed.
From these FE-SEM images, the pore size distribution was obtained to compare the structure and uniformity of the hydrogel electrodes swollen with the ionic liquid and those dried in air. The average interwall distance of each slit-shaped pore observed in the FE-SEM images was used as the representative pore size in the histograms (Fig. 6(a)–(d)). It was found from the FE-SEM images of the samples substituted with ionic liquids that the average pore size (Dp) increased with an increase in the size of the Co-ns (Fig. 6(f)–(i)). Moreover, the coefficient of variation of pore size was 49–54%. The relatively large coefficient of variation is probably due to polydispersity of the lateral size of Co-ns; therefore, the controlled nucleation and growth24 will be useful to improve monodispersity.
The average pore size of the hydrogel electrodes substituted only with ethanol was 20–30 nm for all Co-ns used, and no significant dependence on the size of the Co-ns was observed (Fig. S10(f)–(i)). Additionally, the coefficient of variation was higher (65–72%) compared to those obtained for the ionic liquid-substituted samples. It is suggested that pores of 20–30 nm were predominantly formed due to the contraction of the gaps between assemblies of nanosheets during the evaporation of ethanol, and larger gaps were simultaneously formed. This also supports the idea that the observation of the ionic liquid-substituted samples is much more suitable for the evaluation of pore sizes. Such large gaps were hardly observed in the ionic liquid-substituted samples.
Consequently, the substitution of an aqueous electrolyte with an ionic liquid to maintain the swollen state of the hydrogel electrode is useful for preventing the destruction of the mesoporous structure, even in a vacuum, during FE-SEM observation, allowing the evaluation of mesoporous structures that are closer to the state in the electrolyte solution. Furthermore, it was found that an increase in the size of the Co-ns contributed to an increase in the size of the slit-shaped mesopores.
3.3 OER current and mass transportation of flexible mesoporous hydrogel electrodes
The OER polarization curves of the flexible mesoporous hydrogel electrodes with different pore sizes, rigid mesoporous Co3O4 electrodes,20 rigid CoOOH platelets,20 and Ni foam (Fig. S11) are shown in Fig. 7. Among the flexible mesoporous hydrogel electrodes, those with similar mCo values were selected, as shown in the figure. The current density on a logarithmic scale has a linear relationship with potential, following Tafel's law at a relatively low overpotential. With an increase in current density, the polarization curve deviated from the ideal Tafel relationship, with the increase in current density becoming gradual and finally approaching the diffusion-limited current density. The generated gas bubbles may have hindered mass transport within the pores, delaying the transport of OH− ions from outside the electrode to the reaction sites.In the polarization curves, the flexible mesoporous hydrogel electrodes exhibit similar i–E curves in the low-potential region in which the polarization curves satisfy Tafel's law. In this region, it can be assumed that the nucleation of oxygen bubbles is suppressed within the mesopores, and the transport of dissolved oxygen hardly affects the OER overpotential. The upper limit of the current density at which the polarization curve follows Tafel's law depends on the pore size of the mesoporous hydrogel electrodes. The upper limit increases with increasing pore size.
To clarify the effect of mass transport, the thicknesses of the flexible mesoporous hydrogel electrodes were controlled by changing the duration of electrochemical deposition for Ni/ns21, Ni/ns34, Ni/ns42, and Ni/ns54. The thickness-dependent change in the polarization curves is shown in Fig. 9 and S12. Ni/ns21 and Ni/ns42 showed an increase in the current density along with an increase in thickness in both the low and high overpotential regions, whereas the increase in the current density of Ni/ns42 in the high overpotential region was more pronounced than that of Ni/ns21 because of more efficient mass transport in larger mesopores (Fig. 8(a) and (b)). The current density of the rigid mesoporous Co3O4 electrodes in the high overpotential region (E > approx. 1.65 V vs. RHE) hardly depends on the thickness, owing to mass transport limitations.
 | ||
| Fig. 8 Polarization curves of (a) Ni/ns21, (b) Ni/ns42, and (c) rigid mesoporous Co3O4 electrodes with different thicknesses. | ||
The geometric current density at 1.6 V vs. RHE (igeo-1.6 V), where the effect of thickness and pore size on mass transport was relatively obvious, was plotted as a function of the thickness of the catalyst layer (Fig. 9). Ni/ns21 exhibited a linear relationship between igeo-1.6 V and the thickness up to about 50 μm. Ni/ns34 and Ni/ns42 exhibited the same relationship up to about 70 and 90 μm, respectively, and the maximum current density also increased along with the increase in the mesopore size. This indicates that an increase in the pore size of the flexible mesoporous hydrogel electrodes improves their mass transport ability. The maximum depth of the catalyst layer used to efficiently utilize the catalytically active sites increases as the pore size increases.
 | ||
| Fig. 9 Relationship between the OER current density at 1.6 V vs. RHE (igeo-1.6 V) and the thickness of catalyst layers for (a) Ni/ns21, (b) Ni/ns34, (c) Ni/ns42, (d) Ni/ns54, and (e) Ni only. | ||
Furthermore, to evaluate the effect of the suppression of mass transport in the porous electrodes separately from the OER performance, the overpotential due to mass transport ΔEmass was calculated as the difference in the electrode potential from the ideal potential extrapolated from Tafel's relationship as a function of current density (Fig. S13). A larger ΔEmass indicated that the reaction was more restricted by mass transport under OER conditions. The ΔEmass curves roughly consisted of two parts, low and high current density regions. The slope of the curve has units of Ω cm2 and is denoted herewith as the mass transportation resistance (Rmass).
ΔEmass increased along with the increase in the current density with a small slope in the low current density region. In the high current density region, the slope of the curves increased suddenly to a large and common Rmass value (0.32 ± 0.1 Ω cm2) (filled symbols in Fig. 10). Because these values are nearly identical, this suggests that the increase in ΔEmass is primarily governed by a common phenomenon, meaning that the rate determining process is the detachment of gas bubbles from the outer surface of electrodes rather than from interior of mesopores. The limiting current densities of the flexible mesoporous hydrogel and macroporous electrodes were in a similar range, although the overpotentials differed (Fig. 7). At the limiting current density, the outermost surface of the electrodes was covered with a layer of oxygen bubbles, and mass transport was restricted by the detachment of bubbles from the outermost surface. Thus, the mass transport inside the pores is no longer important. The inflection points between the low and high current density regions were approximately 550 mA cm−2 (Ni/ns21), 640 mA cm−2 (Ni/ns34), 830 mA cm−2 (Ni/ns42), and 950 mA cm−2 (Ni/ns54). Thus, the interior of the mesopores was more efficiently utilized when the pore size increased.
ΔEmass in the low current density region represents the overpotential associated with mass transport within the mesopores. The mass transport resistance of flexible mesoporous hydrogel electrodes in the low current density region (open symbols in Fig. 10(a)–(d)) decreased along with the increase in the pore size (Dp) from 0.15 Ω cm2 (Ni/ns21) to 0.03 Ω cm2 (Ni/ns54). Contrarily, the Rmass values of rigid porous electrodes, such as rigid CoOOH platelets, rigid mesoporous Co3O4 electrodes and Ni foam, were almost constant (0.17 Ω cm2) or even increased along with the increase in pore size (open symbols in Fig. 10(e)–(g)). Mass transport was enhanced in larger mesopores because the diffusion coefficients of oxygen molecules and OH− in the mesopores increased with increasing pore size.
The diffusion coefficient of substances such as OH− decreases significantly in mesopores. For example, Nakasaka et al. summarized the diffusion coefficients of organic substances in various porous materials.37 According to the relationship between diffusion coefficients of substances in mesopores and pore size in log–log graphs in the literature, diffusion coefficients are expected to be proportional to Dp to a power of 5–6, making them extremely sensitive to the pore size. Accordingly, it is reasonable that a small increase in the mesopore size significantly changed the diffusion coefficient of the substances.
Macroporous electrodes such as rigid CoOOH platelets and Ni foam showed inflection points at lower current densities than the flexible mesoporous hydrogel electrodes (Fig. 7). The mass-transport resistance was also higher than that of flexible mesoporous hydrogel electrodes (Fig. 10). The effect of bubbles was reduced only in small mesopores like flexible mesoporous hydrogel electrodes, possibly due to high Laplace pressure.† Once oxygen bubbles clogged the pores, diffusion of substances was blocked. Thus, an increase in the diffusion coefficient was not effective for the macropores.
The mass transport overpotential (ΔEmass) for the rigid mesoporous Co3O4 electrodes was similar to those of Ni/ns21 and Ni/ns34 (Fig. S13). Despite a larger deposition amount of Co compared to that of the flexible mesoporous hydrogel electrodes, the rigid mesoporous Co3O4 electrodes exhibited a lower OER current density, suggesting that the pore surfaces were not effectively utilized. As reported previously, an increase in the thickness of the rigid mesoporous Co3O4 electrodes exhibited an increase in the electric double-layer capacitance, which was proportional to the electrochemically active surface area; however, no increase in the OER current density was observed in the high current density region (>100 mA cm−2).20 Thus, the effective surface of the mesopores was limited only near the surface of the catalyst layer. Consequently, flexible mesoporous hydrogel electrodes demonstrate high mass transport ability owing to their nanoscale, flexible, and uniform mesoporous structure. Detailed analysis of mesoporous structures will support the quantitative analysis of the pore size effects on mass transport behavior by EIS diffusion modeling, porosity–tortuosity correlation, and simulations.
It should be noted that the flexible hydrogel electrodes are stable at constant operation, which can be confirmed by the stable potential profile during the long-term constant current electrolysis for the deposition of Co-ns (Fig. S14). The catalyst layers are also stable under intermittent operation (4000 cycles by accelerated durability test simulating start and stop operation38) because cobalt hydroxide nanosheets (Co-ns) function as self-repairing catalysts.28–31
3.4 Preparation and evaluation of bilayer mesoporous hydrogel electrodes
The electrochemical deposition of Co-ns enables the creation of complex nanostructures such as those fabricated by electroplating. Bilayer electrodes consisting of two mesoporous hydrogel layers with different pore sizes were prepared as a proof-of-concept. A bilayer electrode consisting of ns21 and ns42 layers on the substrate and electrolyte sides, respectively, was designated as Ni/ns21/ns42, whereas the opposite structure, in which ns42 and ns21 were on the substrate and electrolyte sides, respectively, was designated as Ni/ns42/ns21. The porosities of Ni/ns21/ns42 and Ni/ns42/21 were 98.2% (total thickness 30 μm) and 97.6% (total thickness 24 μm), which are between the porosities of Ni/ns21 (94.6%, thickness 28 μm) and Ni/ns42 (99.2%, thickness 30 μm). Thus, it is reasonable to assume that the bilayer structures consist of both ns21 and ns42.The thickness of the first layer in the bilayer electrodes was estimated electrochemically using the relationship between the amount of Co deposited and the thickness, as shown in Fig. S9. The thickness of the second layer was estimated from the difference between the total thickness measured using CLSM and that of the first layer. The thicknesses of the first and second layers of Ni/ns21/ns42 were 17 and 13 μm, respectively, and those of Ni/ns42/ns21 were 20 and 4 μm, respectively. However, the deposited amounts of Co (mCo) based on the charge in cyclic voltammograms (Qc) were 0.104 and 0.025 μg cm−2 for the first and second layers of Ni/ns21/ns42, respectively, and 0.092 and 0.041 μg cm−2 for the first and second layers of Ni/ns42/ns21, respectively. The exceptionally low thickness of the second layer of Ni/ns42/21 was probably due to the penetration of the ns21 nanosheets into the mesopores of the first ns42 layer, as illustrated in Fig. 11(a) and (b). Therefore, a catalyst layer denser than that of Ni/ns21/ns42 was formed. The mesoporous surface structures were observed by FE-SEM using the ionic liquid Fig. 11(c) and (d). The top surfaces of Ni/ns21/ns42 and Ni/ns42/ns21 comprised large and small CoOOH nanosheets, corresponding to ns42 and ns21, respectively.
The polarization curves of the bilayer electrodes (Fig. 11(e) and (f)) show a higher OER current density for Ni/ns21/ns42 and a lower OER current density for Ni/ns42/ns21 compared to the single-layer electrodes Ni/ns21 and Ni/ns42. The relationship between the OER current density at 1.6 V vs. RHE (igeo-1.6 V) and the amount of deposited Co (mCo) is shown in Fig. 12. If the active sites of the CoOOH nanosheets are efficiently used for the OER, igeo-1.6 V is expected to correlate linearly with mCo (dashed line in Fig. 12), whereas the plots curve downward, exhibiting decreased efficiency of active sites at larger mCo. The plot of Ni/ns21/ns42 is close to the dashed line whereas that for Ni/ns42/ns21 is the lowest among the single-layer electrodes. These features originate from mass transport effects. Accordingly, Ni/ns21/ns42 exhibited a 60% higher current density than Ni/ns21 and Ni/ns42 at the same mCo.
The mass transport resistance (Rmass) of the bilayer electrodes is plotted in Fig. 10 according to Fig. S13. Although the Rmass value of Ni/ns21/ns42 in the high-current-density region was similar to those of the other samples, that of Ni/ns42/ns21 was much larger than those of the other samples, which was possibly due to poor mass transportation in the mesopores of Ni/ns42/ns21. The Rmass of Ni/ns42/ns21 in the low-current-density region was lower than the common value observed in the high-current-density region, Rmass of Ni/ns42/ns21 showed an exceptionally large value.
When a layer with smaller mesopores is placed on the electrolyte side (second layer), mass transport is restricted to the outer small mesopores, and the retardation of the reaction also affects the reaction in the layer on the substrate side. When a layer with smaller mesopores is placed on the substrate side (first layer), the electrochemically active surface area increases without limiting mass transport. The polarization curves of Ni/ns21/ns42 and Ni/ns42 exhibit similar shapes, whereas those of Ni/ns21/ns42 appear at a potential 14 mV lower than that of Ni/ns42. This difference can be attributed to the increased electrochemical surface area and electronic conductivity of Ni/ns42. Because the outermost surfaces of these electrodes are almost the same, their mass-transport-limited behavior at high current densities should be similar. As the constituents of the flexible mesoporous hydrogel electrodes are all CoOOH, the Tafel slopes at low current densities should also be the same. Thus, the shift in the polarization curve owing to the increase in the electrochemically active surface area caused by the introduction of the ns21 layer into Ni/ns42 is reasonable. The denser CoOOH in the ns21 layer compared to that in the ns42 layer improves electronic conductivity of the electrode, which may decrease ohmic resistance affecting the overpotential at high current density.
According to the above assumption, Ni/ns42/ns21 should exhibit performance similar to that of Ni/ns21, whereas the mass transport overpotential of Ni/ns42/ns21 was larger than that of Ni/ns21 (Fig. 10(a) and (i)). The pore size of the ns21 layer in Ni/ns42/ns21 (Dp = 24.3 nm) was similar to or slightly smaller than that of Ni/ns42 because ns21 penetrated the mesopores of ns42. Therefore, the oxygen molecules generated at the surface of the ns42 layer (first layer) were difficult to remove from the electrode and passed through the ns21 layer and/or the ns42 layer penetrated by ns21. The supply of OH− from the electrolyte was also retarded by this structure because the pore entrance was too narrow to gather substances in the interior. Therefore, the polarization curve of Ni/ns42/ns21 was similar to that of Ni/ns21 in the low current density region but exhibited a significantly higher overpotential than that of Ni/ns21 in the high current density region because of its poorer mass transport ability.
Studies on the thickness-dependent OER activity under mass transportation-limited conditions are still limited; thus, further studies on the mass transportation in mesoporous electrodes are needed. Recently, Ni foam, possessing macropores in micrometer scale, coated with highly active OER catalysts such as NiFe layered double hydroxides, has been reported to exhibit both high OER activity and good mass transport at large current densities (e.g. 1 A cm−2 at 1.55 V vs. RHE).39–41 These activities are higher than those of the flexible mesoporous hydrogel electrodes (e.g. 1 A cm−2 at 1.69 V vs. RHE), although the pore size ranges are significantly different. Flexible mesoporous hydrogel electrodes would be applicable for macroporous substrates such as Ni foam. Such a hierarchical structure will be useful to improve the overall performance of OER electrodes. Moreover, the use of highly active catalysts, such as NiFeCo oxides, is also promising.42 For industrial applications, the development of highly active and durable OER catalysts forming flexible mesoporous hydrogel electrodes is required. The cost of the preparation process would be reduced by achieving colloidal electrochemical deposition without organic modification.30
Utilizing the colloidal electrochemical deposition of Co-ns, complex structures such as multilayer and hierarchical mesoporous structures can be controlled, enabling the design of optimum mesoporous structures that achieve both large electrochemically active surface areas and low mass transportation overpotentials.
4 Conclusions
In this study, Co-ns, a highly durable self-repairing catalyst, was found to be a useful building block for constructing flexible mesoporous hydrogel electrodes with efficient mass transport via electrochemical deposition. The enhanced mass transport in flexible mesoporous hydrogel electrodes enabled efficient use of mesopore surfaces as active sites for OER deep inside thick catalyst layers (up to 90 μm), whereas conventional rigid mesoporous electrodes activated only near the surface (<3 μm). Using an ionic liquid as a swelling agent, the unique mesoporous structures of the flexible mesoporous hydrogel electrodes were observed using FE-SEM, which enabled a detailed analysis of their mass transport properties.As Co-ns are deposited on a substrate by colloidal electrochemical deposition, the morphology of flexible mesoporous hydrogel electrodes, including multilayer structures with enhanced mass transport compared to unimodal electrodes, can be controlled by electroplating. This technique is applicable for the construction of complex hierarchical structures for practical electrodes. Therefore, flexible mesoporous hydrogel electrodes will open a new avenue for designing gas-evolving electrodes with efficient mass-transport abilities.
Author contributions
H. W. performed all experiments and data analysis and wrote the draft of the manuscript under the supervision of Y. K. and S. M. Y. K. conceptualized the study and designed the experiments. Y. K. participated in funding acquisition. All authors approved the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
All relevant data are available from the corresponding author on reasonable request.The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: additional electrochemical data, XRD patterns, FTIR spectra, photographs, TEM image, EDX spectrum, CLSM image, SEM images, and additional graphs and illustrations. See DOI: https://doi.org/10.1039/d5ta05023e.
Acknowledgements
This work was supported by JSPS KAKENHI (grant number 24K01580).Notes and references
- W. Sun, G. P. Harrison and P. E. Dodds, Int. J. Hydrogen Energy, 2022, 47, 9103–9114 CrossRef CAS
.
- A. M. Oliveira, R. R. Beswick and Y. Yan, Curr. Opin. Chem. Eng., 2021, 33, 100701 CrossRef
.
- K. Zeng and D. Zhang, Prog. Energy Combust. Sci., 2010, 36, 307–326 CrossRef CAS
.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS
.
- F. M. Sapountzi, J. M. Gracia, C. J. Weststrate, H. O. A. Fredriksson and J. W. Niemantsverdriet, Prog. Energy Combust. Sci., 2017, 58, 1–35 CrossRef
.
- M.-I. Jamesh and X. Sun, J. Power Sources, 2018, 400, 31–68 CrossRef CAS
.
- M. Yu, E. Budiyanto and H. Tuysuz, Angew. Chem., Int. Ed., 2022, 61, e202103824 CrossRef CAS PubMed
.
- M. S. Burke, L. J. Enman, A. S. Batchellor, S. Zou and S. W. Boettcher, Chem. Mater., 2015, 27, 7549–7558 CrossRef CAS
.
- S. Wang, A. Lu and C. J. Zhong, Nano Convergence, 2021, 8, 4 CrossRef CAS PubMed
.
- L. Trotochaud and S. W. Boettcher, Scr. Mater., 2014, 74, 25–32 CrossRef CAS
.
- J. Qi, W. Zhang and R. Cao, ChemCatChem, 2018, 10, 1206–1220 CrossRef CAS
.
- L. Wang, X. Huang, S. Jiang, M. Li, K. Zhang, Y. Yan, H. Zhang and J. M. Xue, ACS Appl. Mater. Interfaces, 2017, 9, 40281–40289 CrossRef CAS PubMed
.
- A. Angulo, P. van der Linde, H. Gardeniers and M. Modestino, Joule, 2020, 4, 555–579 CrossRef CAS
.
- J. Eigeldinger and H. Vogt, Electrochim. Acta, 2000, 45, 4449–4456 CrossRef CAS
.
- A. J. Appleby and J. J. G. Crepy, Int. J. Hydrogen Energy, 1978, 3, 21–37 CrossRef CAS
.
- M. Hao, V. Charbonneau, N. N. Fomena, J. Gaudet, D. R. Bruce, S. Garbarino, D. A. Harrington and D. Guay, ACS Appl. Energy Mater., 2019, 2, 5734–5743 CrossRef CAS
.
- F. Rocha, R. Delmelle, C. Georgiadis and J. Proost, Adv. Energy Mater., 2023, 13, 2203087 CrossRef CAS
.
- F. Yang, M. J. Kim, M. Brown and B. J. Wiley, Adv. Energy Mater., 2020, 10, 2001174 CrossRef CAS
.
- P. Tiwari, G. Tsekouras, K. Wagner, G. F. Swiegers and G. G. Wallace, Int. J. Hydrogen Energy, 2019, 44, 23568–23579 CrossRef CAS
.
- R. Nakajima, H. Wago, T. Taniguchi, Y. Sasaki, Y. Nishiki, Z. Awaludin, T. Nakai, A. Kato, S. Mitsushima and Y. Kuroda, Chem. Commun., 2024, 60, 2536–2539 RSC
.
- B. Chi, J. Li, X. Yang, H. Lin and N. Wang, Electrochim. Acta, 2005, 50, 2059–2064 CrossRef CAS
.
- C. Zhang, Y. Xu, P. Lu, X. Zhang, F. Xu and J. Shi, J. Am. Chem. Soc., 2017, 139, 16620–16629 CrossRef CAS PubMed
.
- Y. Kuroda, T. Koichi, K. Muramatsu, K. Yamaguchi, N. Mizuno, A. Shimojima, H. Wada and K. Kuroda, Chem.–Eur. J., 2017, 23, 5023–5032 CrossRef CAS
.
- K. Muramatsu, Y. Kamiusuki, Y. Kuroda, H. Wada, A. Shimojima and K. Kuroda, Dalton Trans., 2021, 50, 3071–3380 RSC
.
- Y. Oka, Y. Kuroda, T. Matsuno, K. Kamata, H. Wada, A. Shimojima and K. Kuroda, Chem.–Eur. J., 2017, 23, 9362–9368 CrossRef CAS
.
- S. Kuwabata, A. Kongkanand, D. Oyamatsu and T. Torimoto, Chem. Lett., 2006, 35, 600–601 CrossRef CAS
.
- S. Arimoto, M. Sugimura, H. Kageyama, T. Torimoto and S. Kuwabata, Electrochim. Acta, 2008, 53, 6228–6234 CrossRef CAS
.
- Y. Kuroda, T. Nishimoto and S. Mitsushima, Electrochim. Acta, 2019, 323, 134812 CrossRef CAS
.
- Y. Kuroda, S. Takatsu, T. Taniguchi, Y. Sasaki, I. Nagashima, A. Inomata, Y. Nishiki, A. Zaenal, T. Nakai, A. Kato and S. Mitsushima, J. Sol-Gel Sci. Technol., 2022, 104, 647–658 CrossRef CAS
.
- R. Nakajima, T. Taniguchi, Y. Sasaki, Y. Nishiki, Z. Awaludin, T. Nakai, A. Kato, S. Mitsushima and Y. Kuroda, ChemSusChem, 2023, 16, e202300384 CrossRef CAS
.
- Y. Kuroda, D. Mizukoshi, V. Yadav, T. Taniguchi, Y. Sasaki, Y. Nishiki, Z. Awaludin, A. Kato and S. Mitsushima, Adv. Energy Sustainability Res., 2024, 5, 2400196 CrossRef CAS
.
- F. Rocha, C. Georgiadis, K. van Droogenbroek, R. Delmelle, X. Pinon, G. Pyka, G. Kerckhofs, F. Egert, F. Razmjooei, S.-A. Ansar, S. Mitsushima and J. Proost, Nat. Commun., 2024, 15, 7444 CrossRef CAS PubMed
.
- A. Abdel Haleem, K. Nagasawa, Y. Kuroda, Y. NIshiki, A. Zaenal and S. Mitsushima, Electrochemistry, 2021, 89, 186–191 CrossRef CAS
.
- M. E. G. Lyons and M. P. Brandon, Int. J. Electrochem. Sci., 2008, 3, 1425–1462 CrossRef CAS
.
- L. Han, S. Dong and E. Wang, Adv. Mater., 2016, 28, 9266–9291 CrossRef CAS PubMed
.
- A. D. Jagadale, D. P. Dubal and C. D. Lokhande, Mater. Res. Bull., 2012, 47, 672–676 CrossRef CAS
.
- Y. Nakasaka, T. Tago, K. Yano and T. Masuda, Chem. Eng. Sci., 2010, 65, 226–231 CrossRef CAS
.
- A. A. Haleem, K. Nagasawa, Y. Kuroda, Y. Nishiki, A. Zaenal and S. Mitsushima, Electrochemistry, 2021, 89, 186–191 CrossRef
.
- G. Deng, J. Zhao, H.-S. Hu, X.-Y. Wang, M. Zhu and Y.-Y. Feng, Int. J. Hydrogen Energy, 2021, 46, 37333–37339 CrossRef CAS
.
- Y. Liu, Q. Song, T. Xu, Q. Kong, G. He, H. Sun, H. Li, Z. Yuan, X. Ma, X. Su, X. P. Dai, Q. Zhang, Z. X. Li, Y. C. Wei and X. Zhang, Appl. Catal., B, 2025, 363, 124811 CrossRef CAS
.
- X. Xu, T. Wang, L. Su, Y. Zhang, L. Dong and X. Miao, ACS Sustainable Chem. Eng., 2021, 9, 5693–5704 CrossRef CAS
.
- P. K. Bhoj, R. A. Chavan, S. A. Mane, D. M. Ulisso, J. Heo, I. W. P. Chen, J. B. Yadav, T. D. Dongale, B. R. Sathe and A. V. Ghule, Int. J. Hydrogen Energy, 2024, 88, 604–616 CrossRef CAS
.
- R. Evans, U. M. B. Marconi and P. Tarazona, J. Chem. Soc., Faraday Trans., 1986, 82, 1763–1787 RSC
.
- J. W. Bullard and E. J. Garboczi, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 79, 011604 CrossRef PubMed
.
Footnote |
† The nucleation of bubbles might be suppressed in the confined space of mesopores due to high Laplace pressure (i.e., high solubility of oxygen molecules). The Laplace pressure for a slit-shaped pore is described by ΔP = 2σ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/Dp (see ref. 43 and 44) where ΔP is the Laplace pressure, σ is the surface tension, θ is the contact angle and Dp is the width of the slit-shaped pore. θ/Dp (see ref. 43 and 44) where ΔP is the Laplace pressure, σ is the surface tension, θ is the contact angle and Dp is the width of the slit-shaped pore. |
| This journal is © The Royal Society of Chemistry 2026 |







