 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Titanium nitride prepared by the urea-glass synthesis gives an active electrocatalyst for the oxygen reduction reaction
Wanrui Xie†
 ,
Ryan D. Van Daele†
,
Ryan D. Van Daele† ,
Allison Kaatz
,
Allison Kaatz ,
Leo Ellenson,
Fengrui Qu and
Bart M. Bartlett
,
Leo Ellenson,
Fengrui Qu and
Bart M. Bartlett *
*
Department of Chemistry, University of Michigan, USA. E-mail: bartmb@umich.edu
First published on 5th December 2025
Abstract
Rock-salt-structured titanium nitride (TiN) has emerged as a leading earth-abundant electrocatalyst for the oxygen reduction reaction (ORR). We compare TiN prepared in three ways starting from urea: the urea–glass method, by a direct reaction between urea and titanium(IV) chloride, and through a discrete monomeric complex. In the urea-glass route, a new-found [Ti4(µ-O)6(OC(NH2)2)12]4+ oxo-bridged titanium–urea precursor can be synthesized in a single-pot reaction at room temperature starting from titanium(IV) chloride, urea, and ethanol with a urea-to-titanium ratio of 6. Subsequent annealing of the polymeric gel that results at 750 °C in an N2 atmosphere yields phase-pure, TiN particles on the 100 µm size scale. TiN can be deposited as an ink with PiperlON®anion exchange dispersion onto a glassy carbon rotating disk electrode (RDE), and of the three synthesis methods, the urea-glass method gives the most active ORR catalyst. The onset potential for ORR activity is −131 mV vs. Hg/HgO, and Koutecký–Levich analysis of linear-sweep voltammetry recorded at varying rotation rates supports a two-electron reduction pathway to H2O2, with a rate constant of 0.0172 cm s−1. The higher activity is ascribed to a more oxygen-rich surface—boh defect sites and active oxygen species—afforeded by the oxo-bridged precursor, corrorborated by X-ray photoelectron spectroscopy (XPS) analysis.
Introduction
The concept of a green hydrogen economy has garnered significant attention due to concerns about the depletion of fossil fuel reserves and the environmental challenges stemming from their overconsumption.1 In the transportation sector, fossil fuels still dominate as the primary energy source.2 However, due to the accelerated depletion of fossil fuels, developing alternative sources for sustainable energy storage and conversion has gained much attention over the past quarter century. Alkaline anion-exchange membrane fuel cell (AEMFCs) and rechargeable metal-air batteries have emerged as promising technologies for clean energy storage and conversion. In these systems, the oxygen reduction reaction (ORR) at the cathode is a critical but challenging step, particularly due to the slow multi-electron transfer steps (especially *O–O bond breaking, *OOH, *OH intermediates).3,4 To address this limitation, selecting an appropriate catalyst that offers optimal adsorption of oxygen intermediates and facilitates O–O bond cleavage is crucial for enhancing the rate.5,6 Currently, platinum and iridium are effective catalysts, but their high cost and scarcity limit large-scale production. This situation underscores the urgent need to develop low-cost, high activity electrocatalysts.7 There has been a lot of recent attention on transition-metal sulfides, borides, carbides, and nitrides. Among these classes of materials, the transition-metal nitrides (TMNs) stand out as having high conductivity, scalable syntheses, and sufficient stability with respect to corrosion,8 due to their unique metallic electronic structure.9In particular, rock-salt structured titanium nitride (TiN) is highly stable, less expensive than platinum, and exhibits good electrical conductivity as an electrode.10–12 Accordingly, there are several examples of its use as an ORR electrocatalyst in AEMFCs.13–15 It is notably more active than W2N, NbN, and Ta3N5.16,17 To date, the primary synthesis routes for TMNs include direct ammonolysis of precursors, solvothermal methods, impregnation adsorption methods, chemical vapor deposition, and carbonization of metal–organic framework materials.18–20 These synthesis methods often require high temperatures (1000 °C) or high pressures (60 bar)21 and flowing toxic ammonia, which complicates the procedures in a way that limits scaling the reactions.
An alternative approach for synthesizing TMNs is the urea-glass pathway, which uses metal chlorides and urea as the precursors. Several papers have discussed this method to synthesize various metal nitrides.22–24 In general, the procedure is to dissolve the desired metal chloride salt in ethanol solvent and to add urea, which presumably forms a coordination complex that reacts further to form a glass (polymeric gel). The entire solution is then placed in a tube furnace under flowing nitrogen gas and heated to 750 °C, where urea decomposes to generate ammonia, water, and other products such as isocyanic acid (HNCO), biuret [HN(CONH2)2], cyanuric acid (C3H3N3O3), and ammelide (C3H4N4O2).25,26 This approach was initially reported for synthesizing TiN, VN, NbN, GaN, Mo2N, W2N, CrN, and others,27 and it has been expanded to include oxynitride materials as well.28–30
The glass decomposition proceeds through two potentially competing routes: one dominated by ammonia evolution, which ultimately favors metal nitride formation, and another that produces water, promoting the formation of metal hydroxides and, upon dehydration, metal oxides.28 The predominance of either pathway is closely linked to the urea-to-metal chloride molar ratio (R). When the quantity of urea in the reactant mixture is insufficient, ammonia generation is limited, which suppresses the formation of pure TMNs. Conversely, an excess of urea enhances ammonia production, thereby increasing the likelihood of nitride formation. Using titanium nitride synthesis as an example, it has been observed that a glassy intermediate containing anatase and rutile TiO2 first appears at approximately 400 °C, and upon heating to 600 °C, oxygen loss accompanied by metal reduction and nitridation is observed. Complete transformation from titania to titanium nitride typically occurs at around 800 °C.30 However, insufficient nitridation time leads to incomplete conversion and the persistence of oxide impurities.31 But TiN never forms directly,32 which leads to a question of the structure and composition of any reaction intermediates. In the urea-glass synthesis of the main group nitride AlN, an easily isolable homoleptic octahedral complex between Al3+ and six urea molecules bound through their O-atoms forms.33 Accordingly, the optimal urea-to-metal molar ratio is 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and lower R ratios lead to persistent aluminum oxide impurities.8,9 Although the structure of hexakis(urea) titanium(III) chloride complex is known,34 only the bis(urea) titanium(IV) chloride complex has been identified. The increased electropositivity of Ti(IV) over Ti(III) results in preference for X-type chloride ligands over L-type urea ligands.35
1, and lower R ratios lead to persistent aluminum oxide impurities.8,9 Although the structure of hexakis(urea) titanium(III) chloride complex is known,34 only the bis(urea) titanium(IV) chloride complex has been identified. The increased electropositivity of Ti(IV) over Ti(III) results in preference for X-type chloride ligands over L-type urea ligands.35
A critical question for our study is: how does the urea content in the reactant mixture influence not only the phase purity, but also the surface composition that maximizes ORR activity? It is known for ZrN that some 15 equivalents of urea are needed to give phase pure material with excellent electrical conductivity and a sufficiently hydroxylated surface that facilitates ORR reaction kinetics.36 We focus on TiN because there we have identified three ways to combine titanium(IV) chloride and urea to yield phase pure material: (1) urea-glass method in ethanol solvent under varying R ratios 2, 4, and 6; (2) a direct solventless reaction between liquid TiCl4 and urea; (3) a molecular precursor method from a known reaction between TiCl4 and two equivalents urea in an inert solvent (dichloromethane), which does not form a glass.37 All methods form phase-pure TiN, and all reduce O2 to H2O2, which is expected under basic conditions.38 We compare the electrocatalytic activity in light of the surface functionality to identify the link between the oxygen content in the reactant mixture and the ORR activity. By establishing the connection between the precursor structure and composition, and the required urea our findings pave the way for scalable, predictable synthesis of a variety of TMNs that are active ORR electrocatalysts.
Experimental
Synthesis of TiN
All products were stored under ambient conditions for further use. Details for the single crystal diffraction experiment and crystallographic data (Tables S1–S6) are presented in the SI.
Results and discussion
Synthesis insights, structural intermediates, and morphology of titanium nitride
Starting with an exploration of the traditional urea-glass route, we carried out the reaction using urea-to-metal chloride molar ratios R = 2, 4, and 6. The XRD patterns (Fig. 1a) show that when R < 6, both anatase and rutile phases of TiO2 are present, indicating incomplete nitridation. In contrast, when R = 6, phase-pure TiN results. In this R = 6 case, we isolate a molecular complex as an intermediate, [Ti4(µ-O)6(OC(NH2)2)12]4+, whose single-crystal structure is illustrated (Fig. 1b). Details for the crystal growth experiment, data collection, and the relevant tables are provided in the electronic supplementary information. In this complex, each Ti4+ adopts a distorted octahedral coordination environment comprising three urea ligands and three bridging oxygen atoms in a facial arrangement. The bridging oxos likely stem from residual water in the ethanol solvent. This mixed coordination stabilizes the structure by balancing charge distribution and steric effects. Unlike Al3+, which forms a homoleptic urea complex, Ti4+ is strongly oxophilic and readily undergoes hydrolysis when water is present; subsequent condensation leads to the oxo-bridged Ti–O–Ti network.40By integrating literature on urea thermal decomposition and the transformation of TiO2 to TiN under ammonlysis by flowing ammonia, we hypothesize a stepwise process in which the complex decomposes to yield both NH3 and H2O; Ti4+ undergoes hydrolysis with the generated H2O to afford titanium oxide; and nitridation by NH3 then replaces oxygen in the oxide to form TiN. This model suggests that ethanol, used to create the urea-glass, may not be required to generate TiN. Accordingly, we carried out a direct solventless reaction in which we physically mixed liquid TiCl4 with solid urea at R = 6 overnight in a dry nitrogen glovebox, subjected the mixture to tube-furnace treatment, and characterized the product by XRD (Fig. 1c). The data indicate that TiN with small quantities of anatase and rutile TiO2 can be produced without first forming a homogeneous ethanol solution.
We posit that oxide-rich precursors necessitate more extensive nitridation—i.e., greater consumption of urea—to obtain phase-pure titanium nitride. Accordingly, we turned to a known reaction to generate a molecular complex of titanium, TiCl4(OC(NH2)2)2, reported by Rivest in 1962,35 of urea in a non-reactive solvent, dichloromethane. This synthesis specifically minimizes O-atom incorporation in the precursor. We performed ATR-IR characterization of the complex. (Fig. S1), where we observe clear shifts in the N–H and C–H stretching bands of urea upon complex formation. Subjecting this isolated molecular complex to the same tube-furnace treatment yields phase-pure TiN in the absence of any oxide impurity (Fig. 1d). These results demonstrate that the extent of nitridation depends primarily on the precursor's structure: the greater the oxide content in the precursor, the more nitrogen from urea is needed to complete the TiO2 to TiN chemical transformation.
SEM images for TiN prepared by three routes are shown in Fig. 2. The TiCl4(OC(NH2)2)2 precursor route and the direct solventless route yield smaller particles than the urea-glass method. Urea-glass TiN consists of plate-like particles on the ∼100 µm scale, whereas the direct solventless route forms aggregates of similar lateral dimensions. Using TiCl4(OC(NH2)2)2 as the starting complex reduces particle aggregation. More images are provided in Fig. S2. EDX mapping (Fig. S3) indicates that for the sample derived from urea-glass synthesis, the average nitrogen atomic percentage is ∼29% on the plate surfaces and ∼47% on the smaller particles. For the direct solventless sample, the average nitrogen and oxygen atomic percentages are 27% and 73%, respectively. For TiN prepared from the complex, TiCl4(OC(NH2)2)2, the averages are 47% N and 53% O. Overall, the greater aggregation/larger particle size correlates with a higher oxygen content, whereas smaller particles show a higher nitrogen content.
As outlined in the introduction, previous research has shown that during urea–glass synthesis of transition-metal nitrides (TMNs), the initial step is formation of the corresponding metal oxide. Subsequently, nitrogen produced by urea decomposition gradually substitutes for oxygen in the oxide, leading to nitride formation.31,32 Taken together, the three TiN syntheses show that minimizing oxygen in the precursor or during processing reduces the amount of oxide observed in the product. These observations align with our hypothesis that greater oxide content in the precursor necessitates increased urea consumption (the nitriding source) to achieve complete conversion to TiN. Additionally, to confirm that urea is the only nitride source in our synthesis, we prepared TiN at R = 6 under both N2 and Ar atmospheres; the XRD patterns (Fig. S4) showed no differences, indicating that these gases are inert in this reaction. Furthermore, we isolated the powder obtained from the single-crystal growth experiment in the urea-glass synthesis, and subjected it to heat treatment in flowing nitrogen at 750 °C for 3 hours with a heating and cooling rate of 3 °C per minute. The XRD pattern (Fig. S5) confirms that this material yields phase-pure TiN.
Oxygen reduction reaction (ORR) activity of TiN prepared by the three methods
To further investigate the oxygen reduction reaction (ORR) activity and elucidate the reaction kinetics of TiN catalysts prepared by three different methods, LSV traces of three different TiN catalyst inks were recorded. Polarization curves were obtained by scanning the potential from 0 to −0.7 V vs. Hg/HgO at a scan rate of 100 mV s−1 in 0.1 M KOH, with rotation rates ranging from 1225 to 2500 rpm under O2-saturated conditions (Fig. 3a). For comparison, the experiment was also performed using a blank glassy carbon electrode (GCE). As expected, all three TiN catalysts exhibited higher current densities and more positive onset potentials compared to the control GCE. Furthermore, the limiting current density increased with higher rotation rates for all three TiN catalysts, consistent with typical ORR behavior. Interestingly, despite the smaller particle sizes and lower surface oxygen content of the TiN catalysts prepared by the direct solventless reation and the TiCl4(OC(NH2)2)2 complex, their ORR activity is inferior to that of TiN prepared by the urea-glass method. The onset potential of the urea-glass TiN was −0.131 V vs. Hg/HgO, accompanied by a higher current density compared to the other two. TiN prepared by the other synthesis methods exhibit onset potentials of approximately −0.193 V vs. Hg/HgO. These results suggest that a higher oxygen content on the TiN surface enhances ORR catalytic activity, which is consistent with the findings of Zhu et al., who demonstrated that introducing oxygen into TiN generates an oxygen-defect-rich TiOxNγ shell, that promotes ORR by creating abundant, defect-rich interfacial sites.41We also conducted RDE tests on TiN prepared by the urea-glass method with lower R values, 2 and 4 (Fig. S6). Interestingly, the R = 4 sample exhibits the highest ORR activity, even though it contains some remaining TiO2. This result suggests that the presence of an oxide may facilitate the ORR reaction. We do, however, observe a limit to this beneficial activity; the R = 2 sample shows a slightly more negative onset potential than the original R = 6 version. The enhanced ORR activity observed in the mixed-phase Ti samples agrees with the results of Zeng et al.,42 who found that introducing oxide species on nitride surfaces can facilitate charge transfer and stabilize oxygen intermediates, leading to improved electrocatalytic activity.
To evaluate the practical applicability of the TiN electrodes in fuel cells, stability is a critical factor. Fig. 3b presents the controlled potential chronoamperometry (CPC) data for the three types of TiN during an 8 hour ORR experiment. A consistent steady-state cathodic current density between 0.25 and 0.38 mA cm−2 is observed maintained throughout the duration without any signs of decline, demonstrating the catalyst's high stability. The corresponding Koutecký–Levich plot for the R = 6 urea-glass TiN RDE data is shown in Fig. 4a. The analyses for TiN prepared by the other two methods are provided in Fig. S7 and S8. TiN synthesized by all three different methods exhibits good linearity and parallel trends over the same potential range, confirming a consistent electron-transfer number per O2 molecule and a first-order dependence of ORR kinetics on O2 concentration.43,44 The electron-transfer number (n) for the ORR on TiN was determined from the slope, using the Koutecký–Levich equation:45 slope = (0.62nFAC0∗D02/3ν−1/6)−1, where n is the number of electrons transferred, A is the electrode area (0.2846 cm2), F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485C mol−1), C0 is the bulk concentration of O2 (1.2 × 10–3 M), D0 is the diffusion coefficient of O2 in 0.1 M KOH electrolyte (1.9 × 10–5 cm2 s−1, and ν is the kinematic viscosity of the electrolyte (1.09 × 10–2 cm2 s−1). Based on this analysis, the calculated number of electrons transferred during the ORR on traditional urea-glass synthesized TiN is 1.7 (Fig. 4b) which corresponds closely to the classical two-electron reduction pathway of O2 to H2O2 (O2 + 2H2O + 2e− + → H2O2 + 2OH−).32 Additionally, the y-intercept of the Koutecký–Levich plot corresponds to the kinetic current at different potentials. Using the equation ik = FAkf(E)C0, the forward rate constant (kf) (Fig. 4c) for the ORR on traditional urea glass synthesized TiN is determined to be approximately 1.72 × 10−2 cm s−1 within the studied potential window;25 this experiment was conducted in triplicate (Fig. S9 and Table S7). This rate constant is comparable to that observed on a bare GCE in a similar potential range (9.5 × 10−3 cm s−1 at −0.26 V vs. Hg/HgO in 0.1 M KOH), indicating competitive ORR kinetics for the urea-glass- synthesized TiN.46 In contrast, the TiN prepared from either the direct solventless or from the TiCl4(OC(NH2)2)2 complex displayed lower electron-transfer numbers during the ORR, indicating reduced activity. Taken together, the LSV and Koutecký–Levich analyses consistently demonstrate that a higher surface oxygen content correlates with enhanced ORR activity in TiN catalysts.
485C mol−1), C0 is the bulk concentration of O2 (1.2 × 10–3 M), D0 is the diffusion coefficient of O2 in 0.1 M KOH electrolyte (1.9 × 10–5 cm2 s−1, and ν is the kinematic viscosity of the electrolyte (1.09 × 10–2 cm2 s−1). Based on this analysis, the calculated number of electrons transferred during the ORR on traditional urea-glass synthesized TiN is 1.7 (Fig. 4b) which corresponds closely to the classical two-electron reduction pathway of O2 to H2O2 (O2 + 2H2O + 2e− + → H2O2 + 2OH−).32 Additionally, the y-intercept of the Koutecký–Levich plot corresponds to the kinetic current at different potentials. Using the equation ik = FAkf(E)C0, the forward rate constant (kf) (Fig. 4c) for the ORR on traditional urea glass synthesized TiN is determined to be approximately 1.72 × 10−2 cm s−1 within the studied potential window;25 this experiment was conducted in triplicate (Fig. S9 and Table S7). This rate constant is comparable to that observed on a bare GCE in a similar potential range (9.5 × 10−3 cm s−1 at −0.26 V vs. Hg/HgO in 0.1 M KOH), indicating competitive ORR kinetics for the urea-glass- synthesized TiN.46 In contrast, the TiN prepared from either the direct solventless or from the TiCl4(OC(NH2)2)2 complex displayed lower electron-transfer numbers during the ORR, indicating reduced activity. Taken together, the LSV and Koutecký–Levich analyses consistently demonstrate that a higher surface oxygen content correlates with enhanced ORR activity in TiN catalysts.
Although the electron-transfer numbers for the ORR carried on TiN derived from the direct solventless method and from the TiCl4(OC(NH2)2)2 complex is marginally less than 2, we assume a two-electron ORR mechanism leading to hydrogen peroxide. To confirm that H2O2 is indeed the product for the three TiN variants, we conducted a qualitative test using potassium permanganate as an indicator.47 As shown in Fig. S10, for the three types of TiN, following an 8-hour CPC experiment the intense violet ligand-to-metal charge-transfer (LMCT) band of permanganate vanishes immediately upon addition to the electrolyte solution, which is consistent with the expected reaction: 
Monitoring changes in electrode composition by XRD, SEM, and XPS analysis
To evaluate changes in the catalyst surface composition after the ORR, we employed a suite of characterization techniques. XRD shows that no new phases emerge after the ORR; only rock-salt structured TiN is present (Fig. S11). Moreover, SEM imaging shows no morphological changes to the TiN particles (Fig. S12); plate-like particles on the 10 µm scale are still observable.Despite seeing no changes in the bulk composition or morphology, X-ray photoelectron spectroscopy (XPS) does show that the electrocatalyst surface changes because of carrying out the ORR. Fig. 5 shows the O(1s) spectra collected pre- and post-ORR, with the binding energies and relative areas of each component provided in Table 1. All three preparation methods show a broad O(1s) feature that tails to higher binding energies and can be fit to two peaks. In particular, the high-binding-energy component (blue peak in Fig. 5) can be associated with surface hydroxide species; increases in this hydroxide environment correlate with enhanced ORR activity. The major component (brown peak in Fig. 5) is consistent with an amorphous surface TiO2 layer. Notably, there is an additional peak in the sample prepared by the urea-glass synthesis at notably lower binding energy (pink peak in Fig. 5). This additional O(1s) component observed prior to the ORR likely represents reduced active oxygen species (AOS) on the surface that arises during the synthesis from the oxygen-rich precursor complex. This pre-existing AOS could facilitate the rapid reaction of oxygenated intermediates during ORR, which has been observed in MnN,48 and helps explain why the urea-glass sample exhibits the highest ORR catalytic activity among the three preparation methods.
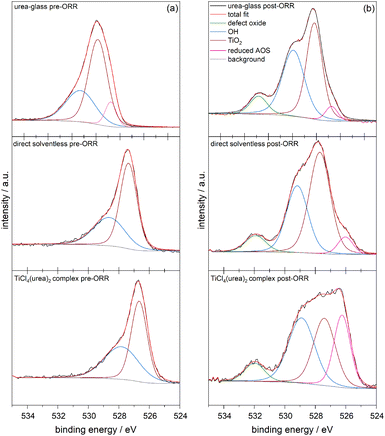 | ||
| Fig. 5 XPS O(1s) analysis of urea-glass synthesized TiN, direct solventless reaction TiN, and TiCl4(urea)2 TiN (a) before the ORR and (b) after the ORR. | ||
| Preparation method | B.E./eV pre-ORR | Area pre-ORR | B.E./eV post-ORR | Area post-ORR |
|---|---|---|---|---|
| Urea–glass | ||||
| Defect oxide | — | — | 532.8 | 0.090 |
| OH | 531.3 | 0.328 | 529.9 | 0.419 |
| TiO2 | 529.8 | 0.572 | 528.2 | 0.442 |
| Reduced AOS | 528.8 | 0.100 | 526.0 | 0.049 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Direct solventless | ||||
| Defect oxide | — | — | 531.9 | 0.081 |
| OH | 528.6 | 0.381 | 529.2 | 0.345 |
| TiO2 | 527.4 | 0.619 | 527.7 | 0.511 |
| Reduced AOS | — | — | 526.0 | 0.063 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| TiCl4(OC(NH2)2)2complex | ||||
| Defect oxide | — | — | 532.0 | 0.054 |
| OH | 527.8 | 0.467 | 528.2 | 0.253 |
| TiO2 | 526.7 | 0.533 | 526.6 | 0.406 |
| Reduced AOS | — | — | 526.0 | 0.287 |
After the ORR, comparable low binding-energy oxygen species have been observed across all samples, which could be attributed to oxygen substituting into nitrogen sites within the TiN lattice, resembling Ti3+–O or Ti2O3-like environments for the AOS. Notable is that as the peak area for this feature increases, the ORR activity decreases, hinting that having surface AOS inhibits the ORR. Both the solventless and TiCl4(urea)2 samples initially contain a substantial amount of this species. We also note that after the ORR, all three preparation methods show the emergence of a higher binding-energy feature at ∼532 eV, corresponding to a defect oxide species,49,50 which could include under-coordinated oxygen and other non-lattice oxygen environments that can arise from surface-adsorbed ORR intermediates.30 The urea-glass synthesis shows the highest ratio of defect oxide to AOS, which could explain the higher ORR activity if O2 binds to the defect sites and requires the AOS to be released as H2O2 product forms.
The Ti(2p) XPS data (Fig. S13) for the three TiN samples further clarify the changes in surface oxidation states before and after ORR. Although Ti(2p) spectra are difficult to fit due to the presence of shake-up features and plasmons—and over-fitting can therefore be misleading—we chose to emphasize the raw spectra and focus primarily on the most intense Ti(2p3/2) peak, the oxide-like feature.51 For the urea-glass TiN sample, prior to ORR the oxide-like peak in the envelope appears at 458.1 eV, and after ORR the high-binding-energy oxide portion decreases noticeably. This behavior is consistent with literature reports showing that when oxygen replaces nitrogen in titanium nitride, the effective charge on Ti decreases; consequently, the Ti(2p) binding energy shifts to lower values because Ti becomes more metallic (i.e., less positively charged).52 In Kroger Vink notation: 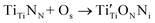 where Os is an O-atom introduced from the surface and Ni is an interstitial (or other non-lattice) nitrogen. A similar trend is observed for the other two samples. For the direct-solventless sample, the Ti(2p3/2) oxide-like feature decreases in binding energy after ORR, shifting from 456.2 eV to 454.5 eV. For the TiCl4(urea)2-derived sample, this feature decreases, from 455.2 eV to 454.8 eV, indicating that more oxygen is incorporated after ORR.
where Os is an O-atom introduced from the surface and Ni is an interstitial (or other non-lattice) nitrogen. A similar trend is observed for the other two samples. For the direct-solventless sample, the Ti(2p3/2) oxide-like feature decreases in binding energy after ORR, shifting from 456.2 eV to 454.5 eV. For the TiCl4(urea)2-derived sample, this feature decreases, from 455.2 eV to 454.8 eV, indicating that more oxygen is incorporated after ORR.
The N(1s) spectra (Fig. S14) also confirm surface oxidation during ORR. The Ti–N peak in the envelope weakens after ORR across the samples, consistent with the classic TiN oxidation behavior described by Saha and Tompkins, where nitrogen loss leads to a reduced nitride contribution in N(1s).53 A slight shift of the Ti–N peak toward lower binding energy is also observed, aligning with the expected perturbation of the Ti–N bonding environment. Among the three materials, the urea-glass TiN shows the smallest change in its N(1s) envelope, retaining a clear Ti–N peak with only modest attenuation. The TiCl4(urea)2-derived sample exhibits a more noticeably weakened and broadened envelope, whereas the direct-solventless TiN shows nearly complete loss of the Ti–N feature. Since nitrogen retention is closely associated with preserving conductive TiN character, the well-maintained N(1s) signature in the urea-glass TiN provides a strong chemical basis for its superior ORR performance.
Conclusions
This study demonstrates three different synthesis methods for preparing titanium nitride (TiN): a traditional urea-glass starting from TiCl4 and urea in ethanol, a direct solventless reaction between the TiCl4 and urea precursors and heating an isolable TiCl4(OC(NH2)2)2 complex. Combining the electrocatalytic activity with the surface composition by XPS, we note that in all cases, the electrode surface changes during the ORR to yield both defect oxides and residual active oxygen species. We find that the urea-glass synthesis generates an oxygen-rich intermediate, a polynuclear oxo-bridged Ti–urea complex, [Ti4(µ-O)6(OC(NH2)2)12]4+ as verified by single-crystal X-ray diffraction. TiN synthesized by this urea-glass method exhibits the lowest overpotential for the ORR through the right balance of defect oxide and reduced AOS on the surface. Future work will focus on synthesizing other transition metal nitrides using the urea glass method with control of oxygen stoichiometry, particularly in the initial glass-forming step.Author contributions
W. X. and R. D. V. D. contributed equally to this work. W. X. contributed through substantial contributions to the design of the work investigation, formal analysis, visualization of the data through graphics (XRD, LSV, SEM, XPS), and writing. R. D. V. D. contributed substantial contributions to the design of the work, investigation, formal analysis, visualization of the data through graphics and writing (TiN synthesis and SEM-EDS). A. K. contributed through investigation (TiN synthesis). L. E. contributed through investigation (LSV). F. Q. collected the single crystal structure. B. M. B. contributed through funding acquisition, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details for single crystal growth, data collection, and structure determination; crystallographic metrics and parameters, ATR-IR characterization, SEM images, EDX data, XRD patterns, additional LSV traces and electrochemical kinetics, photographs of the permanganate test, and additional XPS spectra. See DOI: https://doi.org/10.1039/d5ta03402g.CCDC 2447455 contains the supplementary crystallographic data for this paper.54
Acknowledgements
This research was supported by a grant from the U.S. Department of Energy, Basic Energy Sciences, Catalysis Science Program, under Award No. DE-SC0006587. The authors acknowledge the financial support of the University of Michigan College of Engineering and NSF grant #DMR-0420785 (Kratos Axis Ultra XPS), and technical support from the Michigan Center for Materials Characterization. A. K. thanks the Arnold and Mabel Beckman Foundation for scholarship support. R. D. V. D. acknowledges funding support from the Chemistry President's Challenge Fellowship at the University of Michigan.Notes and references
- D. Y. C. Leung, X. Fu, C. Wang, M. Ni, M. K. H. Leung, X. Wang and X. Fu, ChemSusChem, 2010, 3, 681–694 CrossRef CAS PubMed.
- F. Jaouen, E. Proietti, M. Lefevre, R. Chenniz, J. P. Dodelet, G. Wu, H. T. Chung, C. M. Johnston and P. Zelenay, Energy Environ. Sci., 2011, 4, 114–130 RSC.
- H. A. Firouzjaie and W. E. Mustain, ACS Catal., 2019, 10, 225–234 CrossRef.
- A. FUJISHIMA and K. HONDA, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- K. R. Yoon, C.-K. Hwang, S.-H. Kim, J.-W. Jung, J. E. Chae, J. Kim, K. A. Lee, A. Lim, S.-H. Cho, J. P. Singh, J. M. Kim, K. Shin, B. M. Moon, H. S. Park, H.-J. Kim, K. H. Chae, H. C. Ham, I.-D. Kim and J. Y. Kim, ACS Nano, 2021, 15, 11218–11230 CrossRef CAS PubMed.
- K. Song, Y. Feng, W. Zhang and W. Zheng, J. Energy Chem., 2022, 67, 391–422 CrossRef CAS.
- B. Guo, J. Sun, X. Hu, Y. Wang, Y. Sun, R. Hu, L. Yu, H. Zhao and J. Zhu, ACS Appl. Nano Mater., 2019, 2, 40–47 CrossRef CAS.
- K. Kawashima, R. A. Marquez, L. A. Simth, R. R. Vaidyula, O. A. C. Jaim, Z. Q. Wang, Y. J. Son, C. L. Cao and C. B. Mullins, Chem. Rev., 2023, 123, 12795–13208 CrossRef CAS PubMed.
- H. Yang, C. Weng, H. Wang and Z. Yuan, Coord. Chem. Rev., 2023, 496, 215410 CrossRef CAS.
- B. Avasarala and P. Haldar, Electrochim. Acta, 2010, 55, 9024–9034 CrossRef CAS.
- J. Theerthagiri, G. Durai, K. Karuppasamy, P. Arunachalam, V. Elakkiya, P. Kuppusami, T. Maiyalagan, H. S. Kim and J. of, Ind. Eng. Chem., 2018, 67, 12–27 CrossRef CAS.
- Z. Jin, P. Lin and D. Xiao, Sci. Rep., 2014, 4, 6712 CrossRef CAS PubMed.
- R. Onishi, M. Katayama, D. Cha and K. Takanabe, J. Electrochem. Soc., 2013, 160, 501–506 CrossRef.
- Y. Wang, R. Ohnishi, E. Yoo, P. He, J. Kubota, K. Domen and H. hou, J. Mater. Chem., 2012, 22, 15549–15555 RSC.
- H. Zhang, J. Liu, X. Li, X. Duan, M. Yuan, F. Cao, K. Sun, Y. Zhang, Y. Wang, Z. Gu, J. Li and J. Liu, RSC Adv., 2022, 12, 25035–25040 RSC.
- D. H. Youn, G. Bae, S. Han, J. Y. Kim, H. Park, S. H. Choi and J. S. Lee, J. Mater. Chem. A, 2013, 1, 8007–8015 RSC.
- D. Zhang, D. Zhang, Z. Xie, B. Xu, M. Hou, Y. Lei, T. Watanabe, B. Yang and F. Liang, Materials, 2023, 16, 7469 CrossRef CAS PubMed.
- Q. Luo, C. Lu, L. Liu and M. Zhu, Green Energy Environ., 2023, 8, 406–437 CrossRef CAS.
- R. S. Ningthoujam and N. S. Gajbhiye, Prog. Mater. Sci., 2015, 70, 50–154 CrossRef CAS.
- Z. Yang, H. Xu, T. Shuai, Q. Zhan, Z. Zhang, K. Huang, C. Dai and G. Li, Nanoscale, 2023, 15, 11777 RSC.
- S. Carole, N. Frety, S. Etienne-Calas, C. Merlet and R. M. Marin-Ayral, Mater. Sci. Eng., A, 2006, 419, 365–371 CrossRef.
- J. J. Brancho, A. D. Proctor, S. Panuganti and B. M. Bartlett, Dalton Trans., 2017, 46, 12081–12087 RSC.
- X. Hu, W. Tian, Z. Wu, X. Li, Y. Li and H. Wang, J. Colloid Interface Sci., 2024, 672, 610–617 CrossRef CAS PubMed.
- S. C. H. Bragulla, A. R. V. Seggern, J. Lorenz, C. Harms, M. Wark and K. A. Friedrich, ChemCatChem, 2024, 16, e202400613 CrossRef CAS.
- S. Tischer, M. Bornhorst, J. Amsler, G. Schoch and O. Deutschmann, Phys. Chem. Chem. Phys., 2019, 21, 16785–16797 RSC.
- P. M. Schaber, J. Colson, S. Higgins, D. Thielen, B. Anspach and J. Brauer, Thermochim. Acta, 2004, 424, 131–142 CrossRef CAS.
- C. Giordano, C. Erpen, W. Yao, B. Milke and M. Antonietti, Chem. Mater., 2009, 21, 5136–5144 CrossRef CAS.
- J. J. Brancho, A. D. Proctor, S. Panuganti and B. M. Bartlett, Dalton Trans., 2017, 46, 12081–12087 RSC.
- X. Hu, W. Tian, Z. Wu, X. Li, Y. Li and H. Wang, J. Colloid Interface Sci., 2024, 672, 610–617 CrossRef CAS PubMed.
- S. C. H. Bragulla, A. R. V. Seggern, J. Lorenz, C. Harms, M. Wark and K. A. Friedrich, ChemCatChem, 2024, 16, e202400613 CrossRef CAS.
- M. Afkari, P. Liu, V. Briois, J. Claverie and A. Wustrow, Inorg. Chem., 2025, 64, 14853–14863 CrossRef CAS PubMed.
- H. Zhang and J. F. Banfield, Chem. Rev., 2014, 114, 9613–9644 CrossRef CAS PubMed.
- H. Chaurasia, S. K. Tripathi, K. Bilgaiyan, A. Pandey, K. Mukhopadhyay, K. Agarwal and N. E. Prasad, New J. Chem., 2019, 43, 1900–1909 RSC.
- J. Li, X. Yang and T. Ishigaki, J. Phys. Chem. B, 2006, 110, 14611–14618 CrossRef CAS PubMed.
- R. Rivest, Can. J. Chem., 1962, 40, 2234–2242 CrossRef CAS.
- S. C. Bragulla, A. R. van Seggern, J. Lorenz, C. Harms, M. Wark and K. A. Friedrich, ChemCatChem, 2024, 16, e202400613 CrossRef CAS.
- R. Rivest, Can. J. Chem., 1962, 40, 2234–2242 CrossRef CAS.
- S. Li, L. Shi, Y. Guo, J. Wang, D. Liu and S. Zhao, Chem. Sci., 2024, 15, 11188–11228 RSC.
- A. Byeon, W. Yun, J. Kim and J. W. Lee, ChemElectroChem, 2023, 10, e202300234 CrossRef CAS.
- U. Schubert and B. Stoger, Chem. Eur. J., 2024, 30 Search PubMed.
- X. Zhu, Z. Zhao, J. Tang, X. Peng, H. Um, X. Li, Q. Xiao and W. Luo, Fundam Res., 2023, 5, 1134–1143 CrossRef PubMed.
- R. Zeng, H. Li, Z. Shi, L. Xu, J. Meng, W. Xu, H. Wang, Q. Li, C. J. Pollock, T. Lian, M. Mavrikakis, D. A. Muller and H. D. Abruna, Nat. Mater., 2024, 23, 1695–1703 CrossRef CAS PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods, Jogn Wiley & Son, 1944 Search PubMed.
- Y. Dong, Y. Wu, M. Liu and J. Li, ChemSusChem, 2013, 6, 2016–2021 CrossRef CAS PubMed.
- J. Xu, G. Dong, C. Jin, M. Huang and L. Guan, ChemSusChem, 2013, 6, 493–499 CrossRef CAS PubMed.
- M. Siddika, N. Hosen, R. H. Althomali, J. Y. Al-Humaidi, M. M. Rahman and M. A. Hasnat, Catalysis, 2024, 14, 164/1–11 Search PubMed.
- C. Kim, S. O. Park, S. K. Kwak, Z. Xia, G. Kim and L. Dai, Nat. Commun., 2023, 14, 5822 CrossRef CAS PubMed.
- R. Zeng, H. Li, Z. Shi, L. Xu, J. Meng, W. Xu, H. Wang, Q. Li, C. J. Pollock, T. Lian, M. Mavrikakis, D. A. Muller and H. D. Abruna, Nat. Mater., 2024, 23, 1695–1703 CrossRef CAS PubMed.
- G. Beamson and D. Briggs, High Resolution XPS of Organic Polymers - The Scienta ESCA300 Database, Wiley Interscience, 1992, Appendices 3.1 and 3.2 Search PubMed.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
- N. C. Saha and H. G. Tompkins, J. Appl. Phys., 1992, 72, 3072–3079 CrossRef CAS.
- M. V. Kuznetsov, J. F. Zhuravlev, V. A. Zhilyaev and V. A. Gubanov, J. Electron Spectrosc. Relat. Phenom., 1992, 58, 1–9 CrossRef CAS.
- N. C. Saha and H. G. Tompkins, J. Appl. Phys., 1992, 72, 3072–3079 CrossRef CAS.
- CCDC 2447455: Experimental Crystal Struture Determination, 2025, DOI:10.5517/ccdc.csd.cc2n4s5s..
Footnote |
| † W. X. and R. D. V. D. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |




