Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Upcycle to recycle: triglyceride-derived magnesium soaps as stable, sustainable and efficient catalysts for poly(ethylene terephthalate) glycolysis
Lorenzo
Pedrini
 a,
Anshul
Jain
a,
Anshul
Jain
 a,
Lauren
Kenny
a,
David T.
Mannion
b,
Kieran N.
Kilcawley
b and
Stephen J.
Connon
a,
Lauren
Kenny
a,
David T.
Mannion
b,
Kieran N.
Kilcawley
b and
Stephen J.
Connon
 *a
*a
aSchool of Chemistry, Trinity Biomedical Sciences Institute, Trinity College Dublin, 152-160 Pearse St., Dublin 2, Ireland. E-mail: connons@tcd.ie
bFood Quality and Sensory Science Department, Teagasc, Food Research Centre, Moorepark, Fermoy, Co Cork, Ireland
First published on 20th January 2026
Abstract
The glycolysis of poly(ethylene terephthalate) is an intensively researched process, yet simple, highly active catalysts from bulk sustainable sources without metal ions of known toxicity remain elusive. We report the development of magnesium carboxylate catalysts capable of performance comparable to the most active literature systems in the glycolysis of waste PET. A systematic study demonstrated the inexpensive/environmentally/toxicologically safe magnesium stearate to be superior to a range of group I/II metal variants, while glycolysis catalysed by an analogue derived from a dietary medium-chain fatty acid proved extraordinarily efficient (368 gBHET gcat−1 h−1). The use of commercial cooking oils as a source of magnesium soap catalysts was then demonstrated. The soap derived from medium-chain fatty acid triglyceride-rich coconut oil exhibited particularly impressive activity (267 gBHET gcat−1 h−1), while performance superior to magnesium stearate was even possible using a catalyst synthesised from fast-food restaurant waste cooking oil.
Sustainability spotlightThe chemical recycling of polyethylene terephthalate (PET) by glycolysis is dependant on efficient catalysis. The most common highly-active catalysts rely on Zn2+ ions of concern from an aquatic toxicity perspective. The staggering volumes of PET synthesised annually mean that highly active, sustainable catalysts will need to be available on large scales if chemical PET recycling is to play a key role in the emerging circular economy. This study details the development of magnesium soap catalysts prepared from vegetable oils which possess superior activity to the majority of literature systems, including zinc acetate. These materials can even be synthesised from waste cooking oil – thereby providing a sustainable methodology whereby upcycled waste oil can be utilised catalytically to recycle plastic waste. |
Poly(ethylene terephthalate) (PET, 1) is a petroleum-derived thermoplastic commonly used to manufacture, inter alia, beverage bottles, food packaging and textiles. In 2023, 25.7 billion kg of PET was produced: equating to a global production rate of >800 kg s−1.1 Given the staggering speed/scale of PET manufacturing, the steady growth of worldwide plastics production/low overall recycling rates,2 the >2500 years estimated half-life of waste PET in landfill,3 together with the urgent need to reduce humanity's dependence on fossil fuel resources; it is obvious that future methods for recycling the deluge of plastic waste will need to be rapid, circular and – importantly – highly sustainable.
The majority of non-destructive PET recycling is currently mechanical.1 The PET is sorted, cleaned and reduced in size, then melted and reformed into flakes/pellets for reuse.4 Without resource-intensive interventions/additives, polymer chain scission and retained/generated contaminants are problematic,4,5 which can lead to inferior quality recyclate,6–8 increased downcycling (and eventual landfill). By contrast, chemical recycling9 – involving the depolymerisation of the PET waste to monomers, which can be purified and used to synthesise pristine polymer without requiring blending with virgin (petroleum-derived) PET – is a complementary, circular methodology more forgiving in terms of waste stream heterogeneity5 which can also be used as a platform for upcycling.10–12 Both mechanical and chemical recycling will be needed to ensure future PET sustainability,13–15 yet currently only 0.1% of plastics production stems from chemical recycling.1
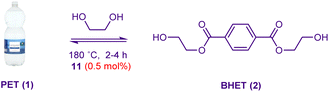
The most common form of PET chemical recycling is catalysed solvolysis; with hydrolytic, glycolytic, alcoholytic, and aminolytic variants possible.16–21 PET glycolysis is arguably the most industrially-developed circular chemical recycling pathway. Glycolysis (Fig. 1A) involves the addition of ethylene glycol to PET (1) to afford bis-hydroxyethyl terephthalate (BHET, 2). BHET is crystalline and can be either repolymerised directly to pristine PET or upcycled to value added materials.22,23 Such is the potential value, volume and utility of the compound that a future, more circular BHET-enabled polymer materials economy has been envisioned.24 The high boiling point of ethylene glycol is advantageous as it allows high-temperature depolymerisation to occur at atmospheric pressures, however the transesterification process is an equilibrium between 1, 2 and incompletely depolymerised oligomers, which can complicate purification and necessitate excess ethylene glycol.22 BHET yields in the 65–≥80% range are common after several hours reaction time:16 a comparison of efficacy across studies is often confounded by inconsistent methods of measuring yield (i.e. NMR spectroscopic analysis or HPLC of the crude material vs. mass of crystallised product).16–22
Catalysis is a key part of any glycolytic depolymerisation regimen, as in the absence of a catalyst only traces of product are formed at temperatures up to 196 °C. An extensive variety of both homo- and heterogeneous catalysts for the process have been developed22 – efforts to rank these in order of reactivity are complicated by the breadth of reaction conditions employed (temperature, concentration, catalyst loading) and the physical nature of the polymer substrate (pristine, post-consumer waste-derived, flakes vs. powders or pellets, particle size etc.).16 Recently Cao and coworkers25 used a variant of space time yield to compare catalyst performance in terms of mass of BHET produced per gram of catalyst utilised per unit time (gBHET gcat−1 h−1). While this data should not be extrapolated to potential yields of continuous processes because it often derives from batch operations, it is nonetheless an interesting and serviceable method of comparing catalyst efficacy.
A diverse array of organocatalysts have been shown to promote PET glycolysis.26 Acids, bases and hydrogen bond-donating systems have been utilised. This catalyst class represents a broad canvas in terms of structural diversity, and recently systems from sustainable sources/biodegradable analogues27 have been designed. For instance, Liu et al. demonstrated the ability of a cholinium acetate organocatalyst 3 (Fig. 1B) to promote the glycolysis of small PET particles,28 while in a seminal study, Yue and coworkers29a disclosed the superiority of a basic ionic liquid 4 over counterparts incorporating less basic anions for the depolymerisation of PET flakes – with 72% yield possible. It is important to note that the decomposition of such imidazolium hydroxide salts to carbenes has been documented.29b Sardon and Dove et al.30 solved the problem associated with the inherent instability which can bedevil organocatalytic systems at elevated temperatures through the design of the protic salt 5 which exhibited excellent stability and recyclability under reaction conditions; at the expense of catalyst activity.
A plethora of metal ion-based catalysts are also known. Of these, Zn2+ systems are common and exhibit impressive activity. The zinc acetate-based deep eutectic solvent 6 proved highly efficacious in the glycolysis of PET pellets at 190 °C,31 while Cao et al. recently developed a heterogenous oxygen vacancy (Vo)-rich Fe/ZnO nanosheet system 7 reported to possess the highest reported activity of any glycolysis catalyst.25 Interestingly, according to analysis by Cao et al.,25 organocatalytic processes were less efficient (<10 gBHET gcat−1 h−1) while the 6 glycolysis processes with associated space time yields >100 gBHET gcat−1 h−1 were promoted by either nanoscale catalysts31–33 and/or materials incorporating Zn2+ ions requiring energy-intensive synthesis.34,35 For example, the preparation of the highly active catalyst 7 requires a pyrolytic step involving heating at 350 °C for 3 h. In addition, while Zn2+ ions are undoubtedly advantageous components of glycolysis catalysts, they are also aquatic eco-toxins of considerable concern.36–38 The EU European Chemicals Agency (ECHA) has labelled both Zn(OAc)2 and ZnO as “very toxic to aquatic life with long lasting effects”.39
Given the aforementioned rapid (and burgeoning) global rates of PET generation, it follows that a circular and sustainable future would require PET recycling methodologies which are commensurately rapid, sustainable and applicable across the world, on enormous scale. The sheer volumes of plastic to be depolymerised will necessitate the production of even the most efficient catalysts in quantities sufficiently large enough to warrant consideration regarding their sustainability, efficacy, ecotoxicity and biodegradability. As an example, if just 10% of the PET produced in 2023 had been chemically recycled using a catalyst at 0.5 wt% loading; then 12.85 million kg of catalyst would have been required.
We are therefore interested in the design of PET glycolysis catalysts which are highly active yet are ecologically safe and potentially sustainably sourced. Herein we report the development of simple magnesium-soap catalysts (Fig. 1C) known to be of little/no toxicological/ecological concern, the activities of which surpasses organocatalytic/metal-ion based systems and is superior to all but the most efficacious nanoscale metal-based catalysts. A study of the influence of fatty acid chain length on activity led to the development of catalysts synthesisable in one pot from either pure or waste cooking oil (i.e.8, Fig. 1C) which are active in the glycolysis of post-consumer PET bottle waste (86.8–267.2 gBHET gcat−1 h−1) at loadings of 0.3–1.5 wt%.
Results
Zinc acetate is a commonly-used glycolysis catalyst18 which has been shown to be superior to other transition metal-based (e.g. Pb, Co, Mn) analogues.40 A systematic examination of the catalytic activities of acetates derived from more earth-abundant41 alkali- and alkaline earth elements is absent from the literature. Accordingly, the glycolysis of PET flakes from waste bottles (5 × 5 mm) in the presence of a variety of metal acetates was carried out at 180 °C for 2 h (Table 1). A challenging catalyst loading of 0.5 mol% was chosen to better allow catalyst performance to be compared and to maximise catalyst solubility in the reaction medium.| Entry | Catalyst | Cation Lewis acidity (v.u.)a | Conv.b,c (%) | Yieldb,d (%) |
|---|---|---|---|---|
| a Valence units. From ref. 45. b Average of at least two experiments agreeing within 6%. c Based on unreacted 1. d Isolated yield after recrystallisation. e Reaction using commercial pristine PET pellets (3–5 mm) for 4 h. | ||||
| 1 | Zn(OAc)2 | 0.405 | 88 | 61 |
| 2 | LiOAc | 0.215 | 37 | 29 |
| 3 | NaOAc | 0.159 | 30 | 25 |
| 4 | Mg(OAc)2 | 0.337 | 89 | 70 |
| 5 | Ca(OAc)2 | 0.264 | 91 | 67 |
| 6 | Sr(OAc)2 | 0.222 | 76 | 58 |
| 7 | Ba(OAc)2 | 0.194 | 76 | 60 |
| 8 | MgCl2 | 0.337 | 0 | 0 |
| 9 | Mg(hydrocinnamate)29 | 0.337 | 90 | 75 |
| 10 | [N8888][hydrocinnamate] 10 | 59 | 43 | |
| 11 | Mg(stearate)211 | 0.337 | 90 | 74 |
| 12e | [N8888][hydrocinnamate] 10 | 50 | 32 | |
| 13e | Mg(stearate)211 | 0.337 | 81 | 67 |
Zinc acetate was a competent catalyst (entry 1); which outperformed group I acetates (entries 2 and 3). Dove et al.42 recently found that Zn(OAc)2 promoted glycolysis of PET at 15 mol% loading to higher conversion than Mg(OAc)2. Interestingly, under these lower catalyst concentration conditions magnesium- and calcium acetates promoted the reaction to similar conversion levels as Zn(OAc)2 (entries 4–5), however 2 was isolated in higher yield. Ca(OAc)2 had not found application in PET glycolysis in the literature, however it is mentioned one recent patent that it catalyses the PET glycolytic equilibrium more efficiently than Zn(OAc)2 at 3.5 mol% loading.43 Similarly, the larger alkaline earth metal acetates had not previously been reported as glycolysis catalysts. Given the small difference in activity between Ca(OAc)2 and Mg(OAc)2, their Strontium- and Barium analogues these were subsequently evaluated (entries 6–7). While catalyst activity was appreciable, neither approached that associated with Mg(OAc)2.
It is clear that the M2+ ion-based catalysts evaluated were more active than their M+ analogues and that inside a group that there was a weak correlation between cation Lewis-acidity when bound to oxyanions44 and activity (e.g. entries 2 vs. 3 and entries 4–5 vs. 6–7). Comparisons across groups are not instructive – e.g. Zn2+ is more Lewis-acidic towards oxyanions than Mg2+, yet the latter seems the marginally more beneficial, while Li+ and Sr2+ have similar Lewis-acidities but very different activity profiles. Here it is perhaps useful to note that Zn2+ is a ‘borderline’ Lewis-acid in Pearson's Hard and Soft Acids and Bases (HSAB) classification while other cations utilised in Table 1 are ‘hard’.45 Therefore the relative affinity of the Zn2+ cation for ethylene glycol, PET and acetate could be substantially different to those associated with ‘harder’ cations. In addition, comparisons between LiOAc and Sr(OAc)2 are complicated by (inter alia) their ligand stoichiometry. The acetate ligand is certainly a significant contributor to catalysis – its exchange for chloride leads to a complete loss of activity (entry 8).
We have previously shown that PET glycolysis catalysed by ammonium and phosphonium-based ionic liquids depends on the properties (lipophilicity in particular) of both the catalyst anion and cation.27,46 Hydrocinnamate emerged from these studies27 as a particularly effective anion (rationalised in part due to increased lipophilicity and π-stacking capability which could facilitate interaction with the PET surface). Accordingly, magnesium hydrocinnamate (9) was synthesised and found to promote the formation of BHET in improved yield relative to magnesium acetate (entries 4 and 9). A lipophilic hydrocinnamate-based ionic liquid devoid of a metal ion proved markedly inferior (i.e.10, entry 10). Our goal was to identify active yet ecologically safe and sustainable catalysts. The finding that neither the zinc cation nor acetate anion was a sine qua non for catalytic activity, coupled with the promising performance of a magnesium-based catalyst equipped with a more lipophilic ligand (i.e.9) led to the hypothesis that the fatty-acid derived magnesium stearate (11) could serve as a powerful and sustainable catalyst system.
Magnesium stearate is an inexpensive soap derived from edible fats and oils which is used as a lubricant and an additive (no. E470b) in dietary supplements, pharmaceuticals and food products. In 2024, >280 million kg of this material was synthesised and production is expected to exceed 425 million kg by 2033.47 The compound is readily biodegradable,48 non-toxic49 at high doses (even to 2500 mg per kg per day),49 non-genotoxic50 and has been affirmed by the US FDA as generally recognised as safe (GRAS) for use in food products.51
Additionally, it has been given a ‘green half circle’ label by the US Environmental Protection Agency which signifies that “the chemical is expected to be of low concern based on experimental and modeled data”.52 Magnesium stearate is not classified by the EU European Chemicals Agency (ECHA) as either toxic to humans or harmful to aquatic life.40 Commercial 11 comprises a mixture of predominantly (>90%) stearate (C18) and palmitic (C16) ligands.53 To ensure homogeneity of the sample, we prepared 11 from pure stearic acid. Gratifyingly, this lipophilic material was as efficacious as 9 and superior to Mg(OAc)2 as a glycolysis catalyst (entry 11). To ensure that the relative catalyst activities were not dependant on the PET source, the glycolysis of more challenging virgin commercial PET pellets was also carried out in the presence of either the metal-free catalyst 10 or the metal-based 11; with results consistent with the trends observed using waste PET flakes (entries 12–13). The catalyst is also stable – TGA analysis of the catalyst indicated no decomposition before 300 °C.
The effect of the ethylene glycol loading on the BHET-forming equilibrium was next examined. We investigated PET waste glycolysis using either 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 or 1
4 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w) ratios of PET
10 (w/w) ratios of PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol at 180 °C catalysed by 11 (0.5 mol%, Table 2). In parallel sets of duplicate experiments, the isolated yield of 2 after recrystallisation was compared with that determined by 1H NMR spectroscopic analysis of the reaction in the presence of an internal standard.
ethylene glycol at 180 °C catalysed by 11 (0.5 mol%, Table 2). In parallel sets of duplicate experiments, the isolated yield of 2 after recrystallisation was compared with that determined by 1H NMR spectroscopic analysis of the reaction in the presence of an internal standard.
| Entry | PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol (w/w) ethylene glycol (w/w) |
Time (h) | Conv.a,b (%) | Isolated yielda,c (%) | NMR yielda,d (%) |
|---|---|---|---|---|---|
| a Average of two experiments agreeing within 5%. b Based on unreacted 1. c Isolated yield of 2 after recrystallisation. d Yield of 2 determined by quantitative 1H NMR spectroscopic analysis of the reaction mixture using (E)-stilbene as an internal standard. e Data from Table 1. f Quantitative 1H NMR spectroscopic analysis of the residual mother liquor using (E)-stilbene as an internal standard revealed the presence of an additional 11% yield of BHET product which did not crystallise. | |||||
| 1e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2 | 90 | 74 | — |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2 | — | — | 88 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 4 4 |
4 | 91 | 74 | — |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
4 | — | — | 87 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
2 | 86 | 71 | — |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
2 | — | — | 82 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
4 | 97 | 80f | — |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
4 | — | — | 95 |
Under conditions identical to those previously utilised (i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 PET
4 PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol) the amount of BHET product formed (determined by 1H NMR spectroscopy) was greater than that obtained after recrystallisation (entries 1–2). Small amounts of oligomeric material were also detected. Extension of the reaction time to 4 h led to no change, indicating that equilibrium under these conditions had been reached after 2 h (entries 3–4). In the presence of 10 mass equivalents of glycol, both isolated- and NMR product yields are lower after 2 h (entries 5–6) – most likely due to catalyst dilution – however a 4 h reaction time afforded 80% isolated yield of pure BHET (entry 7). Quantitative analysis of the mother liquor indicated that 11% of BHET remained unrecrystallised – meaning that higher overall yields could be obtainable at industrial scale after mother liquor processing. This was confirmed by quantitative NMR spectroscopic analysis of replicate reaction mixtures; which provided a BHET yield of 95% with only trace amounts of oligomeric products formed (entry 8).
ethylene glycol) the amount of BHET product formed (determined by 1H NMR spectroscopy) was greater than that obtained after recrystallisation (entries 1–2). Small amounts of oligomeric material were also detected. Extension of the reaction time to 4 h led to no change, indicating that equilibrium under these conditions had been reached after 2 h (entries 3–4). In the presence of 10 mass equivalents of glycol, both isolated- and NMR product yields are lower after 2 h (entries 5–6) – most likely due to catalyst dilution – however a 4 h reaction time afforded 80% isolated yield of pure BHET (entry 7). Quantitative analysis of the mother liquor indicated that 11% of BHET remained unrecrystallised – meaning that higher overall yields could be obtainable at industrial scale after mother liquor processing. This was confirmed by quantitative NMR spectroscopic analysis of replicate reaction mixtures; which provided a BHET yield of 95% with only trace amounts of oligomeric products formed (entry 8).
While magnesium stearate is an inexpensive, safe and environmentally benign species, we were nevertheless interested in catalyst recovery and reuse. It was found that filtration of small amounts of the metallic soap was challenging, however a 62% mass recovery of the stearate post-glycolysis was possible. The recovered catalyst was successfully used in a subsequent glycolysis reaction without loss of activity (SI). With better filtration methodologies and larger scales, improved catalyst recovery rates may well be possible at scale.
Nature provides a cornucopia of medium- and long chain fatty acids. Given the efficacy of the stearate-derived system, an examination of the influence of the fatty acid chain length on catalyst activity seemed prudent. Reactions were carried out at 0.5% loading for 1 h (Table 3). Under these conditions equilibrium was unlikely to be reached, so more potent catalysts could be more easily distinguished from less active analogues. Beginning with the short-chain magnesium caproate 12 (entry 1), product yield increased as the chain length increased to 10- and 14-carbon atoms (i.e.13 and 14, entries 2–3) and then declined: the C18 acid-derived stearate catalyst (11, entry 4) and its monounsaturated analogue 15 (entry 5) possessed similar activity to 12. Further augmentation of the chain (i.e.16, entry 6) led to a precipitous decline in product yield. By comparison, Zn(OAc)2 and the active hydrocinnamate-based ionic liquid 17 (ref. 27) promoted considerably slower glycolysis than 13–14 (entries 7–8) while the literature organocatalysts urea and TBD·MSA (5) afforded only traces of product (entries 9–10). The performance of catalysts prepared via anhydrous deprotonation of fatty acids using Bu2Mg with those prepared via NaOH-mediated carboxylate formation followed by cation exchange with MgCl2 were virtually identical – indicating that NaOH is not a contributor to catalysis.
| Entry | Catalyst | Carbon chain length | Yield (%)a,b |
|---|---|---|---|
| a Average of at least two experiments agreeing within 5%. b Yield of 2 determined by quantitative 1H NMR spectroscopic analysis of the reaction mixture using (E)-stilbene as an internal standard. | |||
| 1 | Mg(caproate)212 | 6 | 49 |
| 2 | Mg(caprate)213 | 10 | 59 |
| 3 | Mg(myristate)214 | 14 | 57 |
| 4 | Mg(stearate)211 | 18 | 44 |
| 5 | Mg(oleate)215 | 18 | 49 |
| 6 | Mg(lignocerate)216 | 24 | 23 |
| 7 | Zn(OAc)2 | 2 | 39 |
| 8 | [P8888][hydrocinnamate] 17 | — | 33 |
| 9 | Urea | — | 1 |
| 10 | TBD·MSA 5 | — | 2 |
The activity of the pure fatty acid-derived promoters 11–15 raised the possibility of preparing potentially more sustainable catalysts directly from cooking oils. Intriguingly, the changes in catalyst activity as the ligand chain length increased suggested that cooking oils with different fatty acid compositions could possess different activity profiles. Magnesium soap catalysts derived from supermarket-purchased rapeseed oil (RS-cat), sunflower oil (SO-cat) and coconut oil (CO-cat) were synthesised (Fig. 2) via saponification and quenching with MgCl2 (obtainable from seawater54).
 | ||
| Fig. 2 Synthesis and fatty acid composition of magnesium soap catalysts derived from (waste) cooking oil. | ||
In addition, two catalysts were prepared from ‘vegetable oil’ of unknown composition sourced from a local fast-food restaurant; one from fresh, unused oil (i.e.RES-cat) and another from a waste sample of the same oil after 5 days use in that restaurant, immediately prior to disposal (i.e.W-RES-cat). High mass yields of the solid catalysts from the oils were obtained in each case. These largely insoluble materials were characterised IR spectroscopy, solid-state 1H NMR spectroscopy and fatty acid composition analysis. The latter was broadly in line with values expected from the literature data associated with precursor oils (Fig. 2 and see SI).55
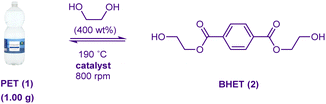
The cooking-oil derived catalysts were evaluated in the glycolysis of PET bottle waste at 190 °C (Table 4). Under these conditions the very insoluble magnesium stearate (11) at 1.5 wt% loading could promote the formation of 2 in 35.8% yield in 30 min (entry 1). Gratifyingly, catalysts prepared from supermarket-bought cooking oil (i.e.RS-cat, SO-cat and CO-cat) exhibited improved activity (entries 2–4). The CO-cat catalyst (the only soap in the study enriched in the catalytically more competent medium-chain fatty acid ligands) proved particularly efficacious. The restaurant-sourced oil-based RES-cat was also found to be useful (entry 5), and while diminished performance of the corresponding waste oil-based W-RES-cat was observed (entry 6), it is noteworthy that this catalyst is still significantly superior to 11 under these conditions. An experiment using W-RES-cat involving adding the ethylene glycol in two equal charges, one at t = 0 min and the other at t = 15 min (based on the hypothesis that initial conditions would feature higher catalyst concentrations which would facilitate bulk polymer destruction followed later by increased ethylene glycol loadings to drive the equilibria towards BHET formation) led to a marginal improvement (entry 7).
| Entry | Catalyst | Loading (wt%) | Time (min) | NMR yielda (%) | Isolated yieldb (%) | STYc (gBHET gcat−1 h−1) |
|---|---|---|---|---|---|---|
| a Yield of 2 determined by quantitative 1H NMR spectroscopic analysis of the reaction mixture using (E)-stilbene as an internal standard. b Isolated yield of 2 after recrystallisation in replicate experiments. c Space time yield (gBHET gcat−1 h−1) = massBHET/(masscat × time). d Ethylene glycol (total loading 400 wt%) added in 2 equal charges, one at t = 0 min and a second at t = 15 min. e Volume reduced to 40 mL instead of 50 mL prior to recrystallisation. f Volume reduced to 60 mL prior to recrystallisation. g Recycling of waste textile (T-shirt)-PET. | ||||||
| 1 | 11 | 1.5 | 30 | 35.8 | — | — |
| 2 | RS-cat | 1.5 | 30 | 72.4 | — | — |
| 3 | SF-cat | 1.5 | 30 | 67.7 | — | — |
| 4 | CO-cat | 1.5 | 30 | 79.6 | — | — |
| 5 | RES-cat | 1.5 | 30 | 66.4 | — | — |
| 6 | W-RES-cat | 1.5 | 30 | 54.8 | — | — |
| 7 | W-RES-cat | 1.5 | 15 + 15d | 58.5 | — | — |
| 8 | 13 | 1.5 | 30 | 94.2 | — | — |
| 9 | 13 | 0.5 | 60 | 92.4 | 84.1 | 222.5 |
| 10 | 13 | 0.3 | 60 | 91.0 | 83.6 | 368.7 |
| 11 | CO-cat | 0.3 | 60 | 78.5 | — | — |
| 12 | CO-cat | 0.3 | 70 | 91.2 | — | — |
| 13 | CO-cat | 0.4 | 60 | 89.4 | 80.8 | 267.2 |
| 14 | W-RES-cat | 1.5 | 50 | — | 83.0 | 87.9 |
| 15 | W-RES-cat | 1.5 | 50 | — | 83.4e | 88.3 |
| 16 | W-RES-cat | 1.5 | 50 | — | 80.7f | 85.4 |
| 17 | W-RES-cat | 1.5 | 50 | — | 80.0g | 84.7 |
Next the loading of the two most active catalysts were optimised. The decanoate system 13 could be utilised at loadings as low as 0.3 wt% (entries 8–10); resulting in an 83.6% yield of recrystallised BHET, which corresponds to a space time yield of 368.7 gBHET gcat−1 h−1. In a similar fashion, it was found that CO-cat could be utilised at 0.4 wt% to afford a product output of 267.2 gBHET gcat−1 h−1 (entries 11–13). At a loading of 1.5 wt% W-RES-cat could promote the formation of 2 in high isolated yield in just 50 min (entry 14). This reaction was repeatable on 5 g scale (SI). Modification of the crystallisation volume to maximise yield from the first crop was not impactful (entries 15–16), however, depolymerisation of textile PET T-shirt waste with similar efficiency to that observed using bottle waste (entry 17) was possible. TGA analysis of both the cooking-oil derived catalysts and 13 indicated high stability – no decomposition before 300 °C.
Discussion and conclusion
At low catalyst loadings, magnesium carboxylates can serve as highly active catalysts for PET glycolysis. The safe and environmentally benign lubricant/food additive magnesium stearate (11) proved superior both the more environmentally-concerning zinc acetate and other group I and II metal acetates. It appears clear that the observed activity trends depend on both the metal and the ligand. With regards to the metal, in general more Lewis-acidic cations outperformed less Lewis-acidic members of the same group, however, comparisons between groups proved more complex. It is also noteworthy that a magnesium cation also proved superior to a quaternary ammonium variant – again indicating (although analysis is complicated by ligand stoichiometry) involvement of the metal ion in catalysis. The ligand is also key: exchange of acetate for the considerably less basic chloride led to a complete ablation of activity, implying that general base catalysis by the ligand plays a role. An investigation into the influence of ligand chain length on catalyst efficacy revealed that medium chain fatty acid-derived systems are optimal; resulting in the identification of magnesium decanoate (13) as a simple glycolysis catalyst par excellence which considerably outperformed zinc acetate and selected organocatalysts at low loadings. The advantages associated with medium chain fatty acid-derived ligands is ascribable to a combination of the lipophilicity required for interaction with the hydrophobic PET surface and solubility: higher homologues (i.e.11, 15 and 16) proved increasingly insoluble in the reaction medium. The product output associated with catalysis by 13 (368.7 gBHET gcat−1 h−1) is higher than all but 2 nanoscale catalysts detailed in Cao et al.'s25 recent comprehensive comparison of literature systems (see the SI associated with ref. 25). Two points are noteworthy here (i) we calculated ‘space time yield’ based only on isolated yields of BHET and (ii) this figure is based on a 60 min reaction time to provide more potentially meaningful data regarding the actual potential of this process in terms of product production per hour reaction time.Soap-based catalysts prepared from cooking oils of varying triglyceride chain lengths and unsaturation – rapeseed oil, sunflower oil and coconut oil (global 2022 production: 26.7, 20.3 and 3.2 billion kg respectively56) – together with analogues synthesised from one ‘vegetable oil’ of unlabelled composition from a local restaurant and a sample of same after 5 days of restaurant use, all proved to be highly active glycolysis catalysts, which is advantageous as future industrial availability of this catalyst class would not be dependent on any one crop. The superiority of the C18-fatty acid-enriched RS-cat, SF-cat and RES-cat to 11 is interesting and may be related to unsaturation (see SI); as 15 is also superior to 11. The coconut oil-derived CO-cat possessed particularly outstanding efficacy (267.2 gBHET gcat−1 h−1): if this level of activity could be replicated at scale (assuming identical catalyst synthesis- and BHET-product yields), then the hypothetical diversion of just 1% of 2022 global coconut oil production to the synthesis of CO-cat would have been sufficient to depolymerise 7.4 billion kg of PET (or 29% of 2023 global production) with potential BHET yields of 7.9 billion kg.
CO-cat comprises higher levels of medium chain fatty acid-derived ligands, which is consistent with both the literature lipid composition of cooking oils55 and the superiority of 13 and 14 over both shorter- and longer-chain catalyst homologues. It is noteworthy that medium chain fatty acids are also obtained on large scale as a byproduct of palm refining and can also be produced from the fermentation of a variety of organic waste streams.57 They are a normal constituent of the human diet which can improve metabolic function and cognition.58
It is estimated that global production of used cooking oil is 41–67 billion kg, the incorrect disposal of which is an environmental hazard.59 In this context, it is notable that W-RES-cat is capable of impressive activity far superior to that associated with magnesium stearate at loadings as low as 1.5 wt%; which highlights the considerable promise of waste cooking oil as an abundant future feedstock for more sustainable PET-glycolysis catalysts.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental procedures and characterisation data. See DOI: https://doi.org/10.1039/d5su00862j.Acknowledgements
This publication has emanated from research conducted with the financial support of Taighde Éireann – Research Ireland under grant number 19/FFP/6527. We thank BiteBox Ltd for donations of cooking oil and waste cooking oil. For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.References
- Plastics – the fast facts, Plastics Europe, https://plasticseurope.org/wp-content/uploads/2024/11/PE_TheFacts_24_digital-1pager.pdf, accessed 02/09/25.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, ACS Sustain. Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS PubMed.
- E. Bezeraj, S. Debrie, F. J. Arraez, P. Reyes, P. H. M. Van Steenberge, D. R. D'hooge and M. Edeleva, RSC Sustain., 2025, 3, 1996–2047 RSC.
- M. D. M. C. López, A. I. Ares Pernas, M. J. Abad López, A. L. Latorre, J. M. López Vilariño and M. V. González Rodríguez, Mater. Chem. Phys., 2014, 147, 884–894 CrossRef.
- F. P. La Mantia and M. Vinci, Polym. Degrad. Stab., 1994, 45, 121–125 CrossRef CAS.
- F. Welle, Resour. Conserv. Recycl., 2011, 55, 865–875 CrossRef.
- Z. Guo, J. Wu and J. Wang, RSC Sustain., 2025, 3, 2111–2133 RSC.
- A. Carniel, N. F. dos Santos, F. Smith Buarque, J. V. Mendes Resende, B. D. Ribeiro, I. M. Marrucho, M. A. Zarur Coelho and A. M. Castro, Green Chem., 2024, 26, 5708–5743 RSC.
- H. Zhao, Y. Ye, Y. Zhang, L. Yang, W. Du, S. Wang and Z. Hou, Chem. Commun., 2024, 60, 13832–13857 RSC.
- K. Wang, C. Guo, J. Li, K. Wang, X. Cao, S. Liang and J. Wang, J. Environ. Chem. Eng., 2024, 12, 113539 CrossRef CAS.
- U. R. Gracida-Alvarez, H. Xu, P. T. Benavides, M. Wang and T. R. Hawkins, ACS Sustain. Chem. Eng., 2023, 11, 514–524 CrossRef CAS.
- U. S. Chaudhari, Y. Lin, V. S. Thompson, R. M. Handler, J. M. Pearce, G. Caneba, P. Muhuri, D. Watkins and D. R. Shonnard, ACS Sustain. Chem. Eng., 2021, 9, 7403–7421 CrossRef CAS.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, ACS Sustain. Chem. Eng., 2023, 11, 965–978 CrossRef CAS.
- M. Wang, Y. Li, L. Zheng, T. Hu, M. Yan and C. Wu, Polym. Chem., 2024, 15, 585–608 RSC.
- T. El Darai, A. Ter-Halle, M. Blanzat, G. Despras, V. Sartor, G. Bordeau, A. Lattes, S. Franceschi, S. Cassel, N. Chouini-Lalanne, E. Perez, C. Déjugnat and J. C. Garrigues, Green Chem., 2024, 26, 6857–6885 RSC.
- M. Muszyński, J. Nowicki, M. Zygadło and G. Dudek, Molecules, 2023, 28, 6385–6411 CrossRef PubMed.
- S. C. Kosloski-Oh, Z. A. Wood, Y. Manjarrez, J. P. de los Rios and M. E. Fieser, Mater. Horiz., 2021, 8, 1084–1129 RSC.
- E. Barnard, J. J. Rubio Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3789 RSC.
- I. Vollmer, M. J. G. P. van der Laan, F. Meirer, J. T. F. Keurentjes and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15402–15423 CrossRef CAS PubMed.
- A. McNeeley and Y. A. Liu, Ind. Eng. Chem. Res., 2024, 63, 3355–3399 CrossRef CAS.
- A. McNeeley and Y. A. Liu, Ind. Eng. Chem. Res., 2024, 63, 3400–3424 CrossRef CAS.
- C. C. Westover and T. E. Long, Sustain. Chem., 2023, 4, 363–393 CrossRef CAS.
- J. Cao, H. Liang, J. Yang, Z. Zhu, J. Deng, X. Li, M. Elimelech and X. Lu, Nat. Commun., 2024, 15, 6266 CrossRef CAS PubMed.
- C. Jehanno, M. M. Pérez-Madrigal, J. Demarteau, H. Sardon and A. P. Dove, Polym. Chem., 2019, 10, 172–186 RSC.
- L. Pedrini, C. Zappelli and S. J. Connon, ACS Sustain. Chem. Eng., 2025, 13, 1424–1430 CrossRef CAS PubMed.
- Y. Liu, X. Yao, X. Yao, H. Yao, Q. Zhou, J. Xin, X. Lu and S. Zhang, Green Chem., 2020, 22, 3122–3131 RSC.
- (a) Q. F. Yue, C. X. Wang, L. N. Zhang, Y. Ni and Y. X. Jin, Polym. Degrad. Stab., 2011, 96, 399–403 CrossRef CAS; (b) A. K. L. Yuen, A. F. Masters and T. Maschmeyer, Catal. Today, 2013, 200, 9 CrossRef CAS.
- C. Jehanno, I. Flores, A. P. Dove, A. J. Müller, F. Ruipérez and H. Sardon, Green Chem., 2018, 20, 1205–1212 RSC.
- B. Liu, W. Fu, X. Lu, Q. Zhou and S. Zhang, ACS Sustain. Chem. Eng., 2019, 7, 3292–3300 CrossRef CAS.
- Y. Liu, X. Wang, Q. Li, T. Yan, X. Lou, C. Zhang, M. Cao, L. Zhang, T.-K. Sham, Q. Zhang, L. He and J. Chen, Adv. Funct. Mater., 2022, 33, 2210283 CrossRef.
- L.-X. Yun, H. Wu, Z.-G. Shen, J.-W. Fu and J.-X. Wang, ACS Sustain. Chem. Eng., 2022, 10, 5278–5287 CrossRef CAS.
- M. Zhu, Z. Li, Q. Wang, X. Zhou and X. Lu, Ind. Eng. Chem. Res., 2012, 51, 11659–11666 CrossRef CAS.
- M. Zhu, S. Li, Z. Li, X. Lu and S. Zhang, Chem. Eng. J., 2012, 185–186, 168–177 CrossRef CAS.
- N. R. Brun, M. Lenz, B. Wehrli and K. Fent, Sci. Total Environ., 2014, 476–477, 657–666 CrossRef CAS PubMed.
- A. H. Mandal, S. Ghosh, D. Adhurjya, P. Chatterjee, I. Samajdar, D. Mukherjee, K. Dhara, N. C. Saha, G. Piccione, C. R. Multisanti, S. Saha and C. Faggio, Aquacult. Rep., 2024, 36, 102038 CrossRef.
- US Environmental Protection Agency, Aquatic Life Fact Sheet for Zinc, 1993, https://www.epa.gov/sites/default/files/2015-06/documents/ny_al_433_03121998.pdf, accessed 03/09/25 Search PubMed.
- European Chemicals Agency, ECHA database, https://echa.europa.eu/information-on-chemicals, accessed 03/09/25.
- S. Baliga and W. T. Wong, J. Polym. Sci., Part A, 1989, 27, 2071–2082 CrossRef CAS.
- W. M. Haynes, D. R. Lide and T. J. Bruno, CRC Handbook of Chemistry and Physics, CRC Press, 97th edn, 2016–2017, pp. 14–17 Search PubMed.
- A. J. Spicer, A. Brandolese and A. P. Dove, ACS Macro Lett., 2024, 13, 189–194 CrossRef CAS PubMed.
- M. Cecchetto, M. Bertola, A. Dal Moro, M. Modesti and S. Guerra, Process for depolymerizing polyethylene terephthalate by glycolysis, WO2022243832A1, 2022.
- O. C. Gagné and F. C. Hawthorne, Acta Crystallogr. B, 2017, 73, 956–961 CrossRef PubMed.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- D. Bura, L. Pedrini, C. Trujillo and S. J. Connon, RSC Sustain., 2023, 1, 2197–2201 RSC.
- ChemAnalyst, Magnesium Stearate market analysis 2015-2035, https://www.chemanalyst.com/industry-report/magnesium-stearate-market-3076, accessed 03/09/25.
- Irish Environmental Protection Agency, Magnesium Stearate Safety Sheet, https://leap.epa.ie/docs/74a61e4f-0963-4ac8-8533-f786e2e4710c.pdf, accessed 03/09/25.
- D. Søndergaard, O. Meyer and G. Würtzen, Toxicology, 1980, 17, 51–55 CrossRef PubMed.
- C. A. Hobbs, K. Saigo, M. Koyanagi and S.-M. Hayashi, Toxicol. Rep., 2017, 4, 554–559 CrossRef CAS PubMed.
- US Food and Drug Administration, https://hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances%26id=MAGNESIUMSTEARATE, accessed 03/09/25.
- US Environmental Protection Agency, Safer ingredients list, https://www.epa.gov/saferchoice/safer-ingredients, accessed 03/09/25.
- S. P. Delaney, M. J. Nethercott, C. J. Mays, N. T. Winquist, D. Arthur, J. L. Calahan, M. Sethi, D. S. Pardue, J. Kim, G. Amidon and E. J. Munson, J. Pharm. Sci., 2017, 106, 338–347 CrossRef CAS PubMed.
- S. Randazzo, F. Vicari, J. López, M. Salem, R. Lo Brutto, S. Azzouz, S. Chamam, S. Cataldo, N. Muratore, M. Fernández de Labastida, V. Vallès, A. Pettignano, G. D’Alì Staiti, S. Pawlowski, A. Hannachi, J. L. Cortina and A. Cipollina, J. Clean. Prod., 2024, 436, 140412 CrossRef CAS.
- J. Orsavova, L. Misurcova, J. V. Ambrozova, R. Vicha and J. Mlcek, Int. J. Mol. Sci., 2015, 16, 12871–12890 CrossRef CAS PubMed.
- UN Food and Agriculture Organization, Coconut Oil Production, 2022; Crops/Regions/World list/Production Quantity, Corporate Statistical Database (FAOSTAT), 2025, https://www.fao.org/faostat/en/#data/QCL, accessed 03/09/25 Search PubMed.
- P. Stamatopoulou, J. Malkowski, L. Conrado, K. Brown and M. Scarborough, Processes, 2020, 8, 1571 CrossRef CAS.
- P. G. Roopashree, S. S. Shetty and N. S. Kumari, J. Funct.Foods, 2021, 87, 104724 CrossRef CAS.
- A. Kumar, S. Bhayana, P. K. Singh, A. D. Tripathi, V. Paul, V. Balodi and A. Agarwal, Discov. Sustain., 2025, 6, 119 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |