 Open Access Article
Open Access ArticleA case study in the emerging bioeconomy: biobased solvents dihydrolevoglucosenone and 2-methyltetrahydrofuran
Giuseppe
Angellotti
a,
Giovanna
Li Petri
a,
Chiara
Valenza
ab,
Rafael
Luque
*c,
Rosaria
Ciriminna
*a and
Mario
Pagliaro
 *a
*a
aIstituto per lo Studio dei Materiali Nanostrutturati, CNR, via U. La Malfa 153, 90146 Palermo, Italy. E-mail: mario.pagliaro@cnr.it; rosaria.ciriminna@cnr.it
bDipartimento di Ingegneria Civile, dell'Energia, dell'Ambiente e dei Materiali, Università degli Studi Mediterranea di Reggio, Calabria, 89122 Reggio Calabria, Italy
cUniversidad Espíritu Santo (UEES), Samborondón EC091650, Ecuador. E-mail: rluquealvarez@gmail.com
First published on 16th December 2025
Abstract
Obtained via catalytic conversion of substrates sourced from sugars respectively derived from cellulose or from hemicellulose, dihydrolevoglucosenone (tradenamed “Cyrene”) and 2-methyltetrahydrofuran (2-MeTHF, tradenamed “Ecomeo”) are biobased solvents that can be used to replace lipophilic petroleum-derived solvents in numerous applications, ranging from use as cleaning agents in the electronics industry to extraction of natural products. Whereas most of the petroleum-based solvents replaced have significant detrimental effects on human health or the environment, this is not the case for both Cyrene and 2-MeTHF. For example, in early 2023, 2-MeTHF was added to the list of permitted solvents for foodstuffs and food ingredients approved for use in food and feed production in EU countries. The analysis of their commercial uptake presents two remarkable case studies in the emerging bioeconomy whose outcomes confirm the validity of bioeconomy management guidelines concerning successful biobased productions.
Sustainability spotlightIn the bioeconomy, energy is efficiently obtained from renewable energy sources whereas chemicals and polymers, currently chiefly derived from oil feedstocks, are efficiently sourced from biological resources. This study presents the analysis of the commercial uptake of two biobased solvents (dihydrolevoglucosenone and 2-methyltetrahydrofuran) whose outcomes confirm the validity of recent bioeconomy management guidelines concerning successful biobased production. Work is particularly relevant in the context of the United Nations Sustainable Development Goals (SDGs), particularly SDG9 9 (Industry, Innovation and Infrastructure) and SDG 12 (Responsible Consumption and Production). |
1 Introduction
In the bioeconomy, energy in the form of electricity and heat is obtained from renewable energy sources whereas chemicals and polymers, currently chiefly derived from oil feedstocks, are sourced from biological resources.1 Widely employed in the manufacturing of paints and coatings, pharmaceutical, chemical and cosmetic products, as extraction solvents in the natural product and food industries and as cleansing agents in the electronics and laundry industries, solvents are amid the main products of the chemical industry. Most solvents are sourced from oil.Unfortunately, many petroleum-derived solvents are toxic and highly regulated chemicals causing health, safety and environmental concerns. Examples include widely employed xylenes, hexane, NMP (N-methyl-2-pyrrolidone) and DMF (dimethylformamide). For example, both NMP and DMF are classified in EU countries as “hazardous” (H360) by the Registration, Evaluation, Authorization and Restriction of CHemicals (REACH) regulation. In the last three decades, plentiful research and entrepreneurial efforts have been devoted to identify and commercialize biobased solvents free of detrimental effects on human health or the environment.2
Among biobased solvents, dihydrolevoglucosenone (or 6,8-dioxabicyclo[3.2.1] octanone) is a cellulose-derived solvent whose commercialization with the trade name “Cyrene” started around 2014.3 Cyrene is the tradename given by the team of Clark at the University of York's Green Chemistry Centre of Excellence to dihydrolevoglucosenone. A Norway-based company (Circa Group) subsequently commercialized production starting from cellulose sourced from sawdust to make the precursor platform molecule levoglucosenone (LGO).
Similarly, 2-methyltetrahydrofuran (2-MeTHF, also called 2-methyloxolane, 2-MeOx) is a solvent produced from hemicellulose by hydrogenation of furfural, whose chemical and physical properties are comparable to those of hexane.4
Both Cyrene and 2-MeTHF can be used to replace oil-derived solvents in numerous applications that range from use as cleaning solvents to extraction of natural products, but with little or no detrimental effects on human health or the environment. For example, in early 2023, 2-MeTHF was added to the list of permitted solvents for foodstuffs and food ingredients approved for use in food and feed production in EU countries, with specified conditions and maximum residue limits.5 Similarly, Cyrene is non-mutagenic and biodegradable, has low acute oral toxicity and is barely toxic to the environment.6
Providing guidelines for the education of bioeconomy company managers, in 2022 Pagliaro and co-workers suggested that customers will buy bioproducts “driven only by higher product performance (quality) and lower prices and reliable (stable and smooth) supply and not by ‘green’ or ‘bio’ allures of their company's production”.7 To lower product price, the team suggested focusing on high-margin bioproducts made in (digitally controlled) small, modular plants relying on green synthetic processes of high energy and material efficiency rather than in large plants requiring high capital expense and operational expenditures.
The study of the efforts to manufacture and commercialize these two biobased solvents shows evidence that bioeconomy management guidelines concerning successful biobased production in these cases were correct.
2 The case of dihydrolevoglucosenone
As mentioned in the Introduction, Circa Group established the “Furacell” production process starting from cellulose sourced from sawdust to make the precursor platform molecule LGO via catalytic pyrolysis of cellulose using phosphoric acid in sulfolane (Scheme 1) obtaining LGO in 40% yield.8 | ||
| Scheme 1 Two-step synthesis of dihydrolevoglucosenone from cellulose (reproduced from ref. 9, CC BY-NC 3.0 Creative Commons License). | ||
A dipolar aprotic solvent, Cyrene is a colorless viscous liquid with 1.25 g mL−1 density (at room temperature) and a high boiling point (227 °C), which offers the possibility to conduct for instance organic reactions taking place in a wide range of temperatures.9 Furthermore, it is also miscible with water, which facilitates solvent removal from reaction mixtures using liquid–liquid extraction, or in industrial scale reactions, separating water via distillation, with nearly complete solvent recovery for reuse.9 “Building on easy removal and recycling”, wrote Kokotos and co-workers in 2022 in an elegant review describing its applications as solvent in organic reactions, “Cyrene can become the future number one choice as a green solvent”.9
Regardless of the most promising results concerning applications of the solvent in numerous segments of the organic solvent market and numerous awards received for innovation in sustainability, by late 2024 the world's first dihydrolevoglucosenone manufacturer filed for bankruptcy.10 In early 2022 the company had increased prices for LGO by 25% and by 15% for Cyrene ascribing the price increases to “rising costs for most inputs and growing customer demand for supply”.11
One year before, the company (which in the early 2020s manufactured Cyrene on a relatively small scale in Tasmania)12 had successfully raised over €50 million in private capital to fund the construction of a 1000 t per year plant “for the manufacture of Cyrene to be located in France”,13 with a large chemical distribution company based in Germany having “provided a letter of intent covering the entire Cyrene production capacity of the French plant”.13
Aiming to meet the “growing demand for alternatives given government regulations and concerns about human health and the environment”,14 the company was targeting the market of traditional toxic petroleum-based solvents, such as dimethylformamide (DMF). The DMF market is expected to reach $545.4 million by 2030, growing at an average CAGR of 4.8% between 2024 and 2030”.14
To understand the scope of the challenge, it is helpful to learn that whereas the bulk price of Cyrene on the bulk scale is about $59 per kg (namely $59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per t),15 the current (2025 first quarter) bulk price of DMF varies between $575 per t in China and $1570 per t in the USA.16 It is also instructive to learn that researchers based in the Netherlands wrote in a research article published in 202017 that according to a previous article published in 2018 “the bulk price of Cyrene may be approximately 2 € per kg, which is comparable with traditional solvents”.17 However, analysis of the mentioned 2018 study shows that Dumesic and co-workers reported therein that, based on discussion with the Norway-based company, economic modeling suggests selling prices below $10
000 per t),15 the current (2025 first quarter) bulk price of DMF varies between $575 per t in China and $1570 per t in the USA.16 It is also instructive to learn that researchers based in the Netherlands wrote in a research article published in 202017 that according to a previous article published in 2018 “the bulk price of Cyrene may be approximately 2 € per kg, which is comparable with traditional solvents”.17 However, analysis of the mentioned 2018 study shows that Dumesic and co-workers reported therein that, based on discussion with the Norway-based company, economic modeling suggests selling prices below $10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton when conducted in plants having a 5–10 kton per year capacity.18 The authors further noted that LGO currently had a high market price (>$10
000 per ton when conducted in plants having a 5–10 kton per year capacity.18 The authors further noted that LGO currently had a high market price (>$10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton) due to its small scale of production.
000 per ton) due to its small scale of production.
The selling price of LGO in 2018 was >$10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per ton, which means that upon partial hydrogenation of LGO under solvent-free conditions followed by catalyst separation and isolation of dihydrolevoglucosenone, its price would be substantially higher than that of LGO, translating into the aforementioned average bulk price of $59
000 per ton, which means that upon partial hydrogenation of LGO under solvent-free conditions followed by catalyst separation and isolation of dihydrolevoglucosenone, its price would be substantially higher than that of LGO, translating into the aforementioned average bulk price of $59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per t.15 Indeed, reviewing methods to produce LGO, Kudo and co-workers aptly noted in 2021 that “the current sales price of the reagent-grade Cyrene is greater than $100 per L”.19
000 per t.15 Indeed, reviewing methods to produce LGO, Kudo and co-workers aptly noted in 2021 that “the current sales price of the reagent-grade Cyrene is greater than $100 per L”.19
Under these circumstances, no competition is possible between Cyrene and petroleum-derived solvents. Indeed, today's production of dihydrolevoglucosenone chiefly takes place on a relatively small scale (few tonnes per year) at selected chemical companies in Asia and in Europe. The overall market was estimated at $180 million in 2024.20 At the time of writing, the solvent is sold at high selling prices varying between €143.5 per L (€574 for 4 L) on a small scale in Europe (from Merck)21 and $158.75 per L ($635 for 4 L) in North America (from Krackeler Scientific).22 In both cases, one can request 200 L of Cyrene with a price below €100 per L or $100 per L, with delivery after about 2 months, meaning that dedicated production would follow a customer order.
In brief, a critical analysis of the case of industrial uptake of dihydrolevoglucosenone confirms that customers will consider biobased alternatives only when the selling price of the biobased solvent is close to or just slightly higher than that of conventional petroleum-based solvent. Until then, the bioproduct will never be taken up on a large scale except for niche applications in which the superior performance of the bioproduct is a technical necessity.
3 The case of 2-methyltetrahydrofuran
As mentioned in the Introduction, 2-methyltetrahydrofuran is a solvent produced via two consecutive hydrogenations of furfural obtained from hemicellulose.4 Furfural undergoes hydrogenation in two steps to 2-methyltetrahydrofuran in one-pot over a dual solid catalyst based on packed Cu-affording 2-MeTHF in 97.1% yield at atmospheric pressure and 180 °C, thereby avoiding high H2 pressure and eliminating the intermediate product-separation step (Scheme 2).23 2-MeTHF is then recovered by distillation affording a product purity of >99.9%.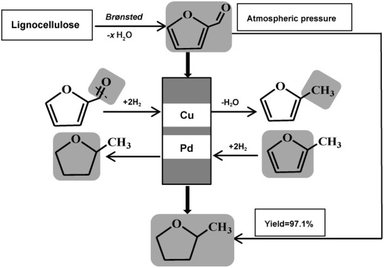 | ||
| Scheme 2 Two-step synthesis of 2-methyltetrahydrofuran from furfural (reproduced from ref. 23, CC BY-NC 3.0 Creative Commons License). | ||
Its technical properties are comparable to those of hexane, so its industrial use is possible with minimal modifications to existing extraction plants.4 Lipophilic solvent hexane, a petroleum-derived mixture whose main component is n-hexane, is widely used to extract oil and protein from oilseeds and other plant-based food sources and natural products subsequently employed as flavours, fragrances, and dyes.
Hexane is neurotoxic.24 Though being a severe irritant to the eyes and skin and able to cause respiratory issues if inhaled, 2-MeTHF is not neurotoxic. Furthermore, 2-MeTHF is neither carcinogenic nor mutagenic25 and is classified by the pharmaceutical industry as a low toxicity solvent (class 3, the same as ethanol). Accordingly, employed as extraction solvent for oil and protein from plant sources, 2-MeTHF does not raise a safety concern when used according to maximum residue limits in the extracted foods or food ingredients of 1 mg kg−1 in fat, oil, butter, foods and 10 mg kg−1 in defatted protein products, defatted flour and other defatted solid ingredients.25
Enabling extraction plants to transition to the use of 2-MeTHF as a substitute for hexane without significant changes to existing equipment, the aforementioned maximum permitted levels for 2-MeTHF are the same as those in EU countries for hexane. The reason for which it is being actively considered by industry for the replacement of hexane is that the bulk price of 2-MeTHF is comparable to that of hexane. The latter is sold in China at a bulk price of $860–880 per t,26 whereas 2-MeTHF can be purchased from the same platform at $800–1300 per t.27
Under these conditions, the higher (“premium”) price of 2-MeTHF can be borne by oilseed and natural product extraction companies who will sell their oils, food and cosmetic ingredients as “green” products certified by third party certification companies.
Indeed, advertising its “Ecomeo” solvent (tradename for 2-MeTHF) as solvent for simultaneous production of soybean oil, defatted meal and natural products, the France-based company that jointly developed the technology (obtaining approval for food applications in Europe, Australia, and New Zealand)28 informs customers that the solvent “allows you to produce COSMOS approved cosmetic ingredients or perfumes”.29
Developed first in 2013 and in its second version in 2020, the COSMetics Organic Standard (COSMOS) standard requires the use of ingredients that are physically processed or chemically processed agro-ingredients.30 The latter, furthermore, cannot come from live or slaughtered animals or from genetically modified plants and microorganisms, with at least 95% of physically processed ingredients of organic agricultural origin.
Furthermore, in September 2024, the European Food Safety Authority (EFSA), following a request from the European Commission, published a report that concluded that hexane approval as a food extraction solvent should be reassessed.31 In other words, based on new health concerns including neurotoxicity, carcinogenicity, and endocrine disruption and the potential for impurities from technical hexane to transfer to food products, hexane will shortly undergo re-evaluation of its safety, which in western European countries was addressed by a scientific committee on food in 1996.
In June 2025 the EFSA published a call for data for the re-evaluation of technical hexane used as an extraction solvent in the preparation of food and food ingredients.32 In February 2025, based on scientific evidence indicating that n-hexane exposure in occupational settings may pose significant health risks, including neurotoxicity and reproductive toxicity, Slovenia asked the European Chemicals Agency (ECHA) to add n-hexane to the Substances of Very High Concern (SVHC) Candidate List under the REACH Regulation (EC) 1907/2006. Consultation started on September 1 and ended on October 16, 2025. The ECHA proposed adding n-hexane to the SVHC Candidate List.33
Should hexane be included in the SVHC list, industries in EU countries relying on n-hexane in foods, adhesives, coatings, lubricants, pharmaceuticals, and cosmetics will need to apply for authorization and begin exploring alternative solutions to ensure compliance.
In brief, based also on what happened in the past for other chemicals going through the same process, a likely consequence of this regulatory evolution will be a reduction in maximum residue limits of hexane in seed oils, or even significant usage restrictions, driving oilseed and other food companies to consider alternative extraction solvents,34 while minimizing the cost of switching solvent.
The substantially lower bulk price of 2-MeTHF in comparison to dihydrolevoglucosenone is due to the lower cost of the acid-catalyzed hydrolytic process needed to convert xylose, sourced via hydrolysis from hemicellulose, to furfural via dehydration at high temperatures and pressures, typically utilising sulphuric acid or phosphoric acid as catalysts, followed by furfural recovery via steam stripping (high-pressure steam is fed through the reactor's bottom in order to remove furfural from the reaction mixture avoiding resinification) and purification through azeotropic distillation.35
The cost of furfural is particularly low when the feedstock employed to source hemicellulose is sugarcane bagasse,35 as it happens in furfural plants such as those based in the Dominican Republic, or wheat straw.36
Contrary to levoglucosenone, production of furfural started in the mid-1950s when a company in the Dominican Republic (Romana By-Products) started to produce it from pentosan, a residue extracted from sugarcane bagasse and other agricultural waste such as corn cobs and peanut shells.37 In 1973, the company expanded the plant, achieving an annual production of 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes, making it the largest furfural plant in the world. Furfural made in the plant is chiefly used to produce furfuryl alcohol at another plant owned in Belgium by the company since 1995.
000 tonnes, making it the largest furfural plant in the world. Furfural made in the plant is chiefly used to produce furfuryl alcohol at another plant owned in Belgium by the company since 1995.
Today, nearly all furfural commercialized by industry is produced in China, followed by the Dominican Republic, South Africa and Belgium. The “Chinese Batch Process” has the largest market share due to its higher energy efficiency due to partial condensation of the reactor outlet to provide the heat at the boiler of the first distillation column.38
Again, in agreement with bioeconomy management guidelines,7 intense research efforts are aimed at developing a heterogeneously catalyzed synthetic process by which to substantially reduce the cost of production of 2-MeTHF by eliminating the need to separate the product from the catalyst (and the amount of wastewater)39 along with the possibility to intensify the process.35
Furthermore, the furfural hydrogenation is also being improved. Current hydrogenation is conducted in the liquid phase in a single reactor loaded with two non-noble catalysts: Co/SiO2 to convert furfural to 2-methylfuran (2-MeF) via hydrodeoxygenation and Ni/Al2O3 to hydrogenate 2-MeF to 2-MeTHF (affording an overall yield of 87% 2-MeTHF at 80 °C and 10 bar H2 pressure).40 The new hydrogenation of 2-MeF to 2-MeTHF with 99% yield will be conducted in the gas phase over low cost Ni/SiO2 under moderate reaction conditions (90 °C and 2 bar H2).41
Meanwhile, driven by its increasing use as a safer solvent than conventional solvents like tetrahydrofuran and dichloromethane across various sectors, including pharmaceutical ingredient production, electronics, oil, cosmetic and food sectors, the market for 2-MeTHF ($450 million in 2024) is growing at nearly a 10% annual growth rate to double at $900 million by 2033.42
4 Recommendations
By investigating the nexus between green chemistry and the bioeconomy, we lately showed that access to an economically viable green chemistry production route is necessary but not sufficient for economically successful bioproduction.43 Access to cheap and abundant biobased raw materials and a relatively high bioproduct selling price are also required. The outcomes of the study of the commercial uptake of dihydrolevoglucosenone (tradenamed “Cyrene”) and 2-methyltetrahydrofuran (tradenamed “Ecomeo”) confirm the validity of these principles concerning biobased production.The overly high price of dihydrolevoglucosenone, due to the intrinsic high cost of its production from cellulose, in 2024 led to closure of the first company that attempted large-scale commercialization of its production in France. The selling price of the solvent was not €2 per kg as reported in a chemistry research article published in 2020,17 but rather $59 per kg.15
On the other hand, the substantially lower price of 2-MeTHF derived from furfural, in turn produced on the industrial scale from sugarcane bagasse hemicellulose since 1955, created a market for 2-MeTHF that in the early 2020s already amounted to 4500 tonnes.44 Accordingly, another company based in France is targeting the huge hexane oilseed and natural product extraction market (1.1 million t per year),45 and not the markets of DMF or NMM oil-derived industrial solvents which are far more expensive than hexane. Hexane is traded on the marketplace at a bulk price of $860–880 per t, whereas 2-MeTHF can be purchased from the same platform at $800–1300 per t.
This fact, inter alia, points to the need for bioeconomy company managers to access reliable information from independent (third party) sources and not from manufacturers or from authors directly or indirectly funded by industry. As lately highlighted in the aforementioned study on green chemistry and the bioeconomy,43 competition in the bioeconomy area of chemical manufacturing is between existing and new manufacturers of valued chemical products.
The France-based company that jointly pioneered the use of 2-MeTHF as oilseed and natural product lipophilic extraction solvent has already obtained approval for the use of the biobased cyclic ether as oilseed and food ingredient extraction solvent in EU countries, Australia and New Zealand. When and if hexane is classified as a substance of very high concern in EU countries, the demand for 2-MeTHF will suddenly expand leading to deployment of new production plants using intensified production processes, while sourcing hemicellulose from low-cost feedstocks not competing with food production such as sugarcane bagasse.
Hence, increasing the industrial uptake of green solvents such as MeTHF and Cyrene requires finding new cost-effective routes to produce both oxygenate molecules.
Lowering the cost of dihydrolevoglucosenone requires lowering the cost of its production. According to Huber and co-workers, its production, based on the use of sulfuric acid as a catalyst and a mixture of tetrahydrofuran (THF) with water as solvent, might afford LGO at $3500 per t production cost if LGO is produced at scales of >30 kton per year using pure cellulose as a feedstock, with cellulose cost being the primary driver (67.1%) for production cost followed by the dehydration reaction (14.3% of the overall cost).46
However, the optimum cellulose loading cannot be higher than 5%, inevitably leading to a large sized plant. Energy-intensive dehydration of cellulose requiring pyrolysis in phosphoric acid (at 330–350 °C) affords LGO in moderate (40%) yield and requires plentiful steps for recovering the solvent (sulfolane), separating biochar, and treating acidic waste,47 making Cyrene production uneconomic in countries and regions where energy is expensive (such as in western Europe).
For the biosolvent to reach a price comparable to that of petroleum-based solvents, the second guideline of bioeconomy management companies suggests that a heterogeneously catalyzed continuous process (including continuous recovery of the product) efficiently and flexibly conducted in small flow reactors needs to be developed.7 Indeed, this is what the team of Kudo based in Japan first conceived has already partly achieved48 and is in the process of achieving its full potential in collaboration with researchers in China.49
5 Conclusions
In conclusion, the study of the commercial uptake of biobased, green solvents dihydrolevoglucosenone (Cyrene) and 2-methyltetrahydrofuran (Ecomeo) to replace lipophilic oil-derived solvents in different industrial applications presents two remarkable case studies in the emerging bioeconomy whose outcomes confirm the validity of management guidelines concerning successful biobased production.Customers will consider biobased, green solvents only when the selling price of the biobased solvent is close to, or just slightly higher than, that of conventional petroleum-based solvents. Until then, the bioproduct will never be taken up on a large scale except for niche applications in which the superior performance of the bioproduct is a technical necessity. This explains why the first companies trying to commercialize dihydrolevoglucosenone either closed or had to curb production volumes originally planned having to face a lack of customer demand due to the overly high cost and low yield (40%) of the synthetic process based on catalytic pyrolysis of cellulose using phosphoric acid in sulfolane followed by catalytic hydrogenation of LGO.
On the other hand, 2-MeTHF is being actively considered by industry for the replacement of hexane as oilseed extraction solvent (and for numerous other applications) because its bulk price ($800–1300 per t) is comparable to that of hexane ($860–880 per t).
The substantially lower bulk price of 2-MeTHF in comparison to dihydrolevoglucosenone is due to the lower cost of the acid-catalyzed hydrolytic process to convert xylose to furfural via dehydration, followed by furfural hydrogenation over a dual catalyst. When, and if, the commercial use of 2-MeTHF as solvent for lipophilic extraction of oilseed and other food ingredients significantly increases, production from furfural will correspondingly increase, driving substantial cost reduction via industrialization of the more efficient, intensified production processes for the two-step production of 2-MeTHF.
Conflicts of interest
There are no conflicts of interest to declare.Data availability
No new data have been generated for this perspective study.Acknowledgements
R. C. and M. P. thank Ministero dell'Università e della Ricerca for funding under “FutuRaw. Le materie prime del futuro da fonti non-critiche, residuali e rinnovabili” project, Fondo Ordinario Enti di Ricerca, 2022 (CUP B53C23008390005).References
- M. Pagliaro, Gen. Chem., 2020, 6, 200007, DOI:10.21127/yaoyigc20.
- F. G. Calvo-Flores, M. J. Monteagudo-Arrebola and J. A. Dobado, et al. , Top. Curr. Chem., 2018, 376, 18, DOI:10.1007/s41061-018-0191-6.
- J. Sherwood, M. De bruyn and A. Constantinou, et al. , Chem. Commun., 2014, 50, 9650, 10.1039/c4cc04133j.
- V. Rapinel, O. Claux and M. Abert-Vian, et al. , Molecules, 2020, 25, 3417, DOI:10.3390/molecules25153417.
- Commission Directive (EU) 2023/175 of 2023 is an amendment to the Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 that adds the solvent 2-methyloxolane to the list of permitted extraction solvents for use in food production. See: Commission Directive (EU) 2023/175 of 26 January 2023 amending Directive 2009/32/EC of the European Parliament and of the Council as regards 2-methyloxolane, https://eur-lex.europa.eu/eli/dir/2023/175/oj/eng, accessed December 9, 2025.
- Toxicological data provided by F. Hoffmann La Roche Ltd to Circa Group Ltd, manufacturer of Cyrene. See: J. Zhang, G. B. White and M. D. Ryan, , et al., ACS Sustainable Chem. Eng., 2016, 4(12), 7186–7192, DOI:10.1021/acssuschemeng.6b02115.
- R. Ciriminna, L. Albanese and F. Meneguzzo, et al. , J. Clean. Prod., 2022, 366, 132851, DOI:10.1016/j.jclepro.2022.132851.
- G. R. Court, C. H. Lawrence, W. D. Raverty and A. J. Duncan, Method for converting lignocellulosic materials into useful chemicals, US Pat., US20120111714A1, 2012 Search PubMed.
- N. A. Stini, P. L. Gkizis and C. G. Kokotos, Green Chem., 2022, 24, 6435–6449, 10.1039/d2gc02332f.
- Euronext, Circa Group AS Decision to File for Bankruptcy, 7 October 2024, https://live.euronext.com/en/products/equities/company-news/2024-10-07-circa-group-decision-file-petition-bankruptcy, accessed December 9, 2025.
- Circa Group, Circa Group Announces Price Increases For Levoglucosenone And Bio-Solvent Cyrene, Oslo, 28 February 2022, https://circa-group.com/news/circa-group-announces-price-increases-for-levoglucosenone-and-bio-solvent-cyrene/, accessed December 9, 2025.
- A. Marathianos, E. Liarou and E. Hancox, et al. , Green Chem., 2020, 22, 5833–5837, 10.1039/d0gc02184a.
- University of York, Cyrene production to increase, 1 March 2021, https://www.york.ac.uk/chemistry/research/green/news/newsarchive/2021/cyrene-production/, accessed December 9, 2025.
- Circa Group, Circa and Merck Sign OEM Agreement for Supply and Sale of Cyrene, Oslo, 25 April 2024, https://circa-group.com/news/circa-and-merck-sign-oem-agreement-for-supply-and-sale-of-cyrene/, accessed December 9, 2025.
- The Massachusetts Toxics Use Reduction Institute, Lowell, Cyrene, 2025, https://doss.turi.org/solvent_preview.php?id=53716-82-8, accessed December 9, 2025.
- IMARC Group, Dimethylformamide (DMF) Prices, Trend, Chart, Demand, Market Analysis, News, Historical and Forecast Data Report 2025 Edition, Noida (India), 2025, https://www.imarcgroup.com/dimethylformamide-pricing-report, accessed December 9, 2025.
- T. Brouwer and B. Schuur, ACS Sustainable Chem. Eng., 2020, 8, 14807–14817, DOI:10.1021/acssuschemeng.0c04159.
- S. H. Krishna, K. Huang and K. J. Barnett, et al. , AIChE J., 2018, 64, 1910–1922, DOI:10.1002/aic.16172.
- S. Kudo, X. Huang and S. Asano, et al. , Energy Fuels, 2021, 35, 9809–9824, DOI:10.1021/acs.energyfuels.1c01062.
- Growth Market Reports, Cyrene Market Research Report 2033, Pune, 2025, https://growthmarketreports.com/report/cyrene-market, accessed December 9, 2025 Search PubMed.
- Merck, Cyrene, 2025, https://www.sigmaaldrich.com/IT/it/product/sial/807796, accessed December 9, 2025.
- Krackeler Scientific, Cyrene, 2025, https://krackeler.com/catalog/sigma/SIAL/8077962025, accessed December 9, 2025.
- F. Dong, Y. Zhu and G. Ding, et al. , ChemSusChem, 2015, 8, 1534–1537, DOI:10.1002/cssc.201500178.
- C. Zhang, L. Hou and J. Yang, et al. , Cell Death Dis., 2018, 9, 60, DOI:10.1038/s41419-017-0091-7.
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids, EFSA J.. 2022, 20, 7138. doi: DOI:10.2903/j.efsa.2022.7138.
- Made-in-China, China Iso Hexane price, 2025, https://www.made-in-china.com/products-search/hot-china-products/China_Iso_Hexane_Price.html, accessed September 5, 2025.
- Made-in-China, CAS: 96-47-9 Hot Industrial Grade High Quality High Purity 2-Methyltetrahydrofuran Quantity Discount, 2025, https://dexiangchemical.en.made-in-china.com/product/NQGRCMyjrKrI/China-CAS-96-47-9-Hot-Industrial-Grade-High-Quality-High-Purity-2-Methyltetrahydrofuran-Quantity-Discount.html, accessed September 5, 2025.
- For example, the process to extract an oil rich in polyphenols, starting from biological substrates, using 2-methyloxolane and water oil: V. Rapinel, N. Patouillard, F. Chemat, A.-S. Fabiano Tixier, K. Ruiz and L. Jacques, Process for producing oils and defatted meal by means of solid/liquid extraction, WO2020128307, 2020.
- EcoXtract, Ecomeo, Dunkerque, 2025, https://ecoxtract.com/our-solvents/, accessed September 5, 2025 Search PubMed.
- The COSMOS standard was developed by COSMOS-standard AISBL (an international non-profit association registered in Belgium founded by Bdih, Germany, Cosmebio & Ecocert, France, Icea, Italy, and Soil Association, Great Britain). For more information on the role of certification in current regulatory and market frameworks in green cosmetics, see: A. Bozza, C. Campi and S. Garelli, et al. , Sust. Chem. Pharm., 2022, 30, 100851, DOI:10.1016/j.scp.2022.100851.
- European Food Safety Authority, EFSA Supporting Publ., 2024, 21(9), 9001, DOI:10.2903/sp.efsa.2024.EN-9001.
- European Food Safety Authority, Call for data for the re-evaluation of technical hexane used as extraction solvent in the preparation of food and food ingredients, 22 June 2025, https://www.efsa.europa.eu/en/call/call-data-re-evaluation-technical-hexane-used-extraction-solvent-preparation-food-and-food, accessed December 9, 2025.
- European Chemicals Agency, Registry of SVHC intentions until outcome, n-hexane, 2025, https://echa.europa.eu/registry-of-svhc-intentions/-/dislist/details/0b0236e18b416b7a/, accessed December 9, 2025.
- P. Carré, S. Berthold and T. Piofczyk, et al. , OCL, 2025, 32, 3, DOI:10.1051/ocl/2024034.
- L. Pierrat and P. García-Triñanes, Chem. Eng. Res. Des., 2024, 212, 261–280, DOI:10.1016/j.cherd.2024.10.035.
- G. Contreras-Zarazúa, M. Martin-Martin and E. Sánchez-Ramirez, et al. , Chem. Eng. Res. Des., 2022, 171, 108569, DOI:10.1016/j.cep.2021.108569.
- Central Romana Corporation, Central Romana Manufacturing, 2025, https://centralromana.com.do/en/corporate-structure/manufacturing/, accessed December 95, 2025.
- W. Adhami, A. Richel and C. Len, Mol. Catal., 2023, 545, 113178, DOI:10.1016/j.mcat.2023.113178.
- J. Blanco-Cejas, I. Agirre and I. Gandarias, et al. , RSC Sustain., 2025, 3, 2899–2914, 10.1039/d5su00106d.
- T. Kabwe, J. Louw and J. F. Gorgens, Ind. Crop. Prod., 2025, 228, 120890, DOI:10.1016/j.indcrop.2025.120890.
- Y. Zhang, W. Zhang and J. Wang, et al. , Chem. Eng. J., 2024, 498, 154955, DOI:10.1016/j.cej.2024.154955.
- Verified Market Reports, Global 2-Methyl Tetrahydrofuran Market Size By Application (Pharmaceuticals, Fuels and Lubricants), By End-User Industry (Automotive, Pharmaceutical), By Product Type (Analytical Reagent Grade, Industrial Grade), By Distribution Channel (Online Marketplaces, Physical Retail Stores), By Formulation (Pure 2-MTHF, Blended Solvents), By Geographic Scope and Forecast, Pune (India), 2025 Search PubMed.
- R. Ciriminna, G. Angellotti and R. Luque, et al. , Biofuels, Bioprod. Biorefin., 2024, 18, 347–355, DOI:10.1002/bbb.2585.
- C. Cravotto, O. Claux, M. Bartier and A.-S. Fabiano-Tixier, et al. , Molecules, 2023, 28, 5973, DOI:10.3390/molecules28165973.
- C. Cravotto, A.-S. Fabiano-Tixier and O. Claux, et al. , Foods, 2022, 11, 3412, DOI:10.3390/foods11213412.
- J. He, M. Liu, K. Huang and C. T. Maravelias, et al. , Green Chem., 2017, 19, 3642–3653, 10.1039/c7gc01688c.
- D. E. Richardson and W. Raverty, Appita J., 2016, 6, 344–351 Search PubMed.
- S. Kudo, N. Goto and J. Sperry, et al. , ACS Sustainable Chem. Eng., 2017, 5, 1132–1140, DOI:10.1021/acssuschemeng.6b02463.
- X. Huang, S. Kudo and J.-I. Hayashi, Fuel Process. Technol., 2019, 191, 29–35, DOI:10.1016/j.fvuproc.2019.03.014.
| This journal is © The Royal Society of Chemistry 2026 |
