Open Access Article
Open Access ArticleFirst sustainable synthesis of biobased ethyl and methyl formates by ecocatalysis
Arthur
Lasbleiz
,
Pierre-Alexandre
Deyris
,
Franck
Pelissier
,
Yves-Marie
Legrand
 ,
Claude
Grison
,
Claude
Grison
 * and
Claire M.
Grison
* and
Claire M.
Grison
Bio-inspired Chemistry and Ecological Innovations (ChimEco), UMR 5021 CNRS-University of Montpellier, Cap Delta, 1682 rue de la Valsière, 34790 Grabels, France. E-mail: claude.grison@cnrs.fr
First published on 31st October 2025
Abstract
We described the first synthesis of biobased ethyl and methyl formates. The synthetic strategy is based on the transesterification of natural geranyl and citronellyl formates derived from Pelargonium species essential oils with ethanol or methanol, promoted by an ecocatalyst®. Good yields (up to 80%) were obtained with an excellent selectivity.
Sustainability spotlightThe work described in this article goes beyond the boundaries of sustainable chemistry: first, it adheres to all the principles of green chemistry described by Anastas and Warner. This study quantitatively assesses, through life-cycle analysis (LCA), the possible environmental benefits of replacing organometallic complexes (Ru, Pd, Co, Cu–Zn, or Fe-based catalysts) with biosourced catalysts, called ecocatalysts. Ecocatalysts are natural, non-toxic, non-ecotoxic, efficient and abundant. Elimination of hazardous substances in production and use is demonstrated. No synthetic or petroleum-based inputs are used. The process is simple and straightforward. It generates no waste and improves the quality of the raw material, Pelargonium graveolens essential oil. Second, the proposed strategy involves massive harvesting of the world's most invasive plant species, exotic knotweeds. Using the cut parts of these plants contributes to management efforts for these plant species by weakening them. Their use in organic synthesis also supports the repeated harvesting of these plants. In other words, this article shows how sustainable chemistry can contribute to ecological solutions aimed at controlling the development of invasive plant species. This is an additional principle that can inspire future research in sustainable chemistry. |
Introduction
Ethyl and methyl formates (EF and MF, respectively) are small, polar and reactive molecules with ever-expanding applications. They are good solvents for polymers such as cellulose nitrate, cellulose acetate, and also for vegetable oils, fatty acids and resins.1 Their low boiling points, 54 °C for EF and 32 °C for MF, allow an easy removal during chemical processes and facilitate drying. Initially appreciated for their volatility and high vapor pressures, these formates are also known for their pleasant odor. EF is particularly present in raspberries.2 Its fruity odor is used in the food industry.3 With a relatively low toxicity to mammals4,5 and being recognized as safe by the Food and Drug Administration and the Occupational Safety and Health Administration of the United States,6 EF is also used as an insecticide to replace toxic compounds such as methyl bromide.7 Its fumigant insecticide activity is effective and used in Australia and China to protect dried fruits, seeds and cereals.8 EF and MF are also very useful in the domain of organic synthesis in which they are described as reactants of interest. They are particularly good formylation agents allowing the creation of N–CHO bonds from amines9–11 and amides.12 They also promote C–CHO bond formation by aldol condensation reactions13–15 or by attacking positive halogens in aromatic series.16,17 They can participate in cyanation reactions in the presence of phosphoryl oxytrichloride.18–20 More recently, EF and MF have been described as co-catalysts for the alkoxylation of inactivated aryl halides in the presence of cuprous halides.21–24 This is an elegant and eco-friendly alternative to O-arylation reactions promoted by expensive and toxic liganded palladium25–29 or cuprous30–32 catalysts.Different methods for the preparation of EF and MF are described in the literature. Industrially, they are derived from the carbonylation of ethanol or methanol.33 However, this carbonylation requires high-purity CO gas,34 which is difficult to obtain without any trace of CO2, which is an inhibitor of ruthenium-based catalysts used in the reaction.
At the laboratory scale, these two esters have usually been prepared by esterification of formic acid.35 Nowadays, many research articles are devoted to the reduction of CO2 to small organic molecules. The O-formylation of methanol or ethanol using CO2 and Ru, Pd, Co, Cu–Zn or Fe-based catalysts has been reported36–38 but in each case, the yields remained moderate. Cannon et al. showed the importance of the pH effect in these reactions.39 Unlike the above syntheses performed in basic medium, the hydrogenation of CO2 in acidic medium, catalysed by phosphine-ligated Ru catalysts, favours the formation of MF.39 Electrochemical reduction of CO2 in acidic ethanol on Pb and Sn cathodes also leads to the production of EF. In this reaction, the formed formic acid serves as an autocatalyst to perform its own in situ Fischer esterification with ethanol and therefore produces EF with an interesting yield.40 Promising results were obtained with the Ni-catalyzed hydrosilylation of CO2 followed by an O-formylation of alcohols in the presence of HBF4.41 The benefit of these acidic conditions was also described when using boryl formate in the TsOH-catalysed O-formylation of benzylic alcohols.42 Similarly, the presence of triformatoborohydride, which is formed during the reaction of NaBH4 with CO2,43 could act as an O-formylation reagent with formic acid as a catalyst.44 These acidic conditions suggest that the O-formylation step proceeds via a transesterification reaction.40
All these new approaches to the synthesis of formates are driven by the efforts to decarbonize industrial activity coupled with the valorisation of CO2, which is largely responsible for the greenhouse effect. However, these approaches suffer from the use of expensive, toxic and/or ecotoxic complex metal catalysts. Additionally, hydrosilane or borohydride reducing agents lead to the formation of problematic wastes regulated by REACH.
In this article, we propose to develop the first fully biobased synthesis of EF and MF using essential oils (EOs) derived from Pelargonium species, which are rich in geranyl and citronellyl formates (GF and CF, respectively) and an ecocatalyst® acting as a biosourced base catalyst, in accordance with an eco-responsible approach.
Results and discussion
Choice of Pelargonium essential oil and strategies
EOs of Pelargonium species are known and exploited for their high content in citronellol and geraniol, which can reach 35 and 29%, respectively, depending on their origin.45 The global market for these EOs is substantial, with nearly 300 tons produced per year. Curiously, its abundance in CF and GF is less known and little exploited. While many hybrid cultivars (Hybridum cv) have been produced to optimise the levels of citronellol and geraniol, the highest levels are found in the native species Pelargonium graveolens, stemming from South Africa46 (Table 1).In this article, we propose to use the natural abundance of CF and GF contained in Pelargonium graveolens L’Hér. as a natural raw material to prepare EF and MF.
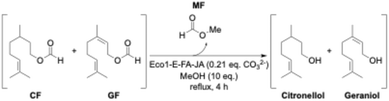
The synthetic strategy is based on the transesterification of these two formates with ethanol or methanol. The reaction is promoted by an ecocatalyst® (Eco1-E-FA-JA), a mineral catalyst of 100% plant origin. The selected plant species is Fallopia japonica, an invasive alien species (IAS) of wetlands. Its intensive harvesting helps weaken it and control its proliferation by exhaustion (Fig. 1).
Characterization of the ecocatalyst®
The aerial parts of Fallopia japonica collected in large quantities were crushed directly in their wet form. They then underwent a heat treatment under air at 550 °C (SI, part 1.6.1). The thermal residue, called ecocatalyst® Eco1-E-FA-JA, has an unusual mineral composition, which was analysed by Mass Plasma – Atomic Emission Spectroscopy (MP-AES) (Table 2 and SI part 1.2).| Element | Al | Ca | Fe | K | Mg | Na |
|---|---|---|---|---|---|---|
| wt% rsd | 0.10 ± 0.00 | 6.78 ± 0.08 | 0.15 ± 0.02 | 30.75 ± 0.13 | 3.64 ± 0.02 | 0.15 ± 0.00 |
The Mg content is 3.64 wt%, which is high in comparison to classical plants, but reasonable given the high chlorophyll content in the aerial parts. The K content (30.75 wt%) is remarkably high but falls within the main specificities of many IAS.
Ion chromatography analyses give the concentration of the anions from the ecocatalyst® soluble in water. Phosphate PO43−, sulfate SO42−, carbonate CO32− and chloride Cl− anions were detected in significant quantities (Table 3 and SI part 1.4 & 2.2).
| Anion | PO43− | SO42− | CO32− | Cl− |
|---|---|---|---|---|
| wt% (g of anion/100 g ecocatalyst) ± rsd | 1.90 ± 0.02 | 7.20 ± 0.11 | 11.10 ± 0.10 | 5.40 ± 0.09 |
The amount of the analysed sulfates is particularly high (4 times higher than phosphates). Chlorides and carbonates are also formed in significant quantities. Given the high carbonate content, the pH of the solution was measured and found to be 11.9 (200 mg of the ecocatalyst® in 10 mL of water). This basicity is higher than the pKa2 of a simple carbonate, which makes the ecocatalyst® interesting and appropriate to be used in organic synthesis.
X-Ray Powder Diffraction (XRPD) studies were carried out to determine the nature of the combinations between the different mineral elements in the crystalline state (Table 4 and SI part 1.5 & 2.1).
| Name | Formula |
|---|---|
| Sylvite | KCl |
| Potassium calcium phosphate | KCaPO4 |
| Potassium sulfate | K2SO4 |
| Potassium carbonate sesquihydrate | K2CO3(H2O)1,5 |
| Fairchildite | K2Ca(CO3)2 |
| Calcite | CaCO3 |
| Periclase | MgO |
During heat treatment, the self-assembly of the mineral elements led to the formation of two unusual but interesting salts, which are rarely used in organic synthesis:
- a double carbonate of potassium and calcium, fairchildite, K2Ca(CO3)2
- a mixed phosphate of potassium and calcium, KCaPO4.
The presence of carbonates, and fairchildite (K2Ca(CO3)2) in particular, was expected in Eco1-E-FA-JA, as it has been found previously in other ecocatalysts® derived from F. japonica.50 Likewise, the presence of mixed phosphates was consistent with previous ecocatalysts® derived from F. japonica.50 However, the phosphate species differed from only one cation from previous work, as KCaPO4 was observed here, instead of KMgPO4. This slight difference might be attributed to the harvesting period of F. japonica and hence its physiological composition.
Moreover, another crystalline species with basic properties was detected: MgO, despite the modest temperature of the heat treatment. The presence of MgO is advantageous since this salt brings a dual character to the ecocatalyst®: the presence of oxide confers Brønsted basic properties while the magnesium shows Lewis acid properties.51
Regarding these analyses, Eco1-E-FA-JA exhibits advantageous properties to act as an excellent catalyst for transesterification reactions.
Synthesis of ethyl and methyl formates
The reaction was first studied with ethanol as a model nucleophile (Scheme 1).Taking as a reference the total quantity of GF and CF present in the native Pelargonium graveolens EO, 10 eq. of ethanol were used. A detailed study of the impact of the catalytic charge on CF and GF conversion rates was carried out over 28 h (Fig. 2). While the reaction without a catalyst only gave 10% conversion of CF and GF, the reaction was studied between 0.04 and 0.21 equivalents of carbonates relative to both citronellyl and geranyl formates. According to the ion chromatography results, the ecocatalyst comprises 42% soluble carbonates, and therefore, 42% of the potassium salts are in the form of carbonates. Thus, 0.5 equivalents of potassium correspond to 0.21 equivalents of potassium carbonates (including fairchildite and potassium carbonate).
 | ||
| Fig. 2 Effects of different Eco-1-E-FA-JA loads (0.04 to 0.21 eq. soluble carbonates) on the conversion of citronellyl (a) and geranyl (b) formates versus time. | ||
The monitoring was performed by GC-FID using external quantification of the two esters (see SI, part 1.3). Comparing the curves depicted in Fig. 2(a and b), the conversion times for the same catalytic load follow the same trend, suggesting that GF and CF exhibit a comparable reactivity.
The catalytic loading had a significant impact on the kinetics of the reaction. Indeed, using 0.04 or 0.08 eq. CO32− did not allow the reaction to reach full conversion after 28 h. While 0.13 and 0.17 eq. CO32− provided 98% conversion of both formates in 24 and 20 h, respectively, 0.21 eq. CO32− allowed the same conversion to be reached in 8 h. It is also important to note that the other terpenes contained in the EO of P. graveolens were not affected by the reaction and no byproducts were observed, highlighting the selectivity of the reaction at the same time (see SI, part 3.2 and Fig. 2).
The efficiency of the reaction was evaluated by recovering the formed EF by distillation of the reaction medium. Indeed, its boiling point at atmospheric pressure is 54 °C, which allows its separation from the EO and solvent by fractional distillation. This process yielded EF at 80% as a colourless liquid, and the excess of ethanol was recovered by increasing the bath temperature. The difference between the optimum yield and the conversion of the substrates is explained by losses of materials during distillation, linked to the fact that the product has a very low boiling point. However, this result remains very satisfactory and shows that the transesterification of the formates naturally present in the EO is a good method for producing EF. The remaining EO collected in the distillation residue was separated from the catalyst by simple filtration. Its composition was evaluated by GC-FID and compared to the starting EO (Fig. 3).
A comparison of the activity of the ecocatalyst Eco1-E-FA-JA with common basic catalysts and reagents was carried out (Table 5). This comparative study was carried out under the best conditions obtained with the ecocatalyst (0.21 equivalents of soluble carbonates (K2Ca(CO3)2 + K2CO3), 6 h at ethanol reflux). Eco1-E-FA-JA was first compared to KOH, a reagent classically used in transesterification reactions. Initially, 0.21 equivalents of KOH were used to mimic the 0.21 equivalents of soluble carbonates present in Eco1-E-FA-JA (see ion chromatography analyses, Table 3). The conversion rates of citronellyl and geranyl formates were low (46 and 30%, respectively) instead of 90 and 90% with Eco1-E-FA-JA. By introducing excess KOH (0.87 equiv. then 1 equiv.), the conversion rates of citronellyl and geranyl formates reached 88–89% and then 95–97%. However, treatment of the aqueous phase (see SI Section 3.3) revealed the formation of potassium formate (23 and 91%, respectively, see SI Fig. 14 and 15), resulting from the hydrolysis of citronellyl and geranyl formates. The ecocatalyst Eco1-E-FA-JA is therefore much more advantageous than KOH; it is both more reactive (90% conversion rate) and does not lead to any secondary hydrolysis reactions.
| Catalyst or reagent | Formate equivalent | Citronellyl formate conversion (%) | Geranyl formate conversion (%) |
|---|---|---|---|
| a 0.21 equiv. K2CO3 + 0.16 equiv. MgO + 0.43 equiv. CaSO4 + 0.17 equiv. KCl + 0.02 equiv. Ca10(PO4)6(OH)2. b 0.21 equivalents of soluble carbonates. | |||
| KOH | 0.21 | 46 | 30 |
| KOH | 0.87 | 89 | 88 |
| KOH | 1 | 97 | 95 |
| CaSO4 | 0.21 | 33 | 17 |
| CaCO3 | 0.21 | 2 | 19 |
| MgO | 0.21 | 47 | 29 |
| MgO | 1 | 88 | 72 |
| Ca10(PO4)6(OH)2 | 0.21 | 2 | 20 |
| K2CO3 | 0.21 | 83 | 77 |
| K2CO3 | 0.44 | 84 | 84 |
| K2CO3 | 1 | 100 | 92 |
| Synthetic mixture | 0.21a | 86 | 82 |
| Eco1-E-FA-JA | 0.21b | 90 | 90 |
Each mineral species of Eco1-E-FA-JA (MgO, CaCO3, CaSO4, K2CO3, K2Ca(CO3)2, KCaPO4) and a synthetic mixture of these salts were tested under the best conditions obtained with the ecocatalyst (6 h at ethanol reflux, Table 5). K2CO3 and Ca10(PO4)6(OH)2 were used to mimic K2Ca(CO3)2 and KCaPO4, respectively, because the two salts are unavailable and cannot be synthesized. As expected, conversion rates of citronellyl formate and geranyl formate were very low with CaSO4 (33–17%) and the three poorly soluble bases in EtOH, CaCO3 (19–2%), MgO (42–49%) and Ca10(PO4)6(OH)2 (2–20%). Only K2CO3 led to satisfactory conversion rates (83–77%). However, they remained slightly lower than those of the ecocatalyst. It was necessary to introduce 1 equivalent of K2CO3 to exceed the efficiency of 0.21 equivalents of soluble carbonates in Eco1-E-FA-JA (100–92% instead of 90–90%).
Finally, a synthetic mixture reconstituted from the constituent salts (0.21 equiv. K2CO3 + 0.16 equiv. MgO + 0.43 equiv. CaSO4 + 0.17 equiv. KCl + 0.02 equiv. Ca10(PO4)6(OH)2) has been studied. This composition corresponds to the results of ionic chromatography for each soluble salt (sulfate, chloride, phosphate, carbonate) and the MP-AES analysis for MgO. The conversion rates (86–82%) were slightly higher than those of K2CO3 (83–77%) and slightly lower than those of the ecocatalyst (90–90%). We can therefore conclude that the ecocatalyst Eco1-E-FA-JA is a very good transesterification catalyst. It is more active than simple petroleum-based carbonates. Its natural composition in mixed salts reinforces its reactivity.
A comparison with a catalyst frequently used in ester transesterification reactions, Ti(OiPr)4, was also performed.52 The reaction of transesterification of Pelargonium graveolens EO with EtOH and 4 mol% of this catalyst was set up in order to reproduce the conditions described by Corsi et al.52 After 8 h of reaction at reflux of EtOH, the conversions of CF and GF were measured at 42 and 58%, respectively. These conversions were far lower than the proposed ecocatalysts. The GC-FID analyses are added in part 3.4 of the SI.
Compared to the initial EO, the chemical composition of the remaining EO is significantly enriched in citronellol (40% instead of 28%) and geraniol (25% instead of 18%). This result is of significant interest, because these two terpenes are used in various applications as repellents for arthropods that carry infectious diseases,53 in fragrances54,55 and as key intermediates in organic synthesis.56,57 Moreover, the pH of the remaining EO was measured at 7, while the value of the initial EO was assessed at 2.7. The ecocatalyst® is thus able to perform quantitative transesterification reactions and also to neutralise the acidity of the initial EO in order to obtain a citronellol- and geraniol-enriched EO more suitable for human skin applications. These very encouraging results led us to extend the principle of this reaction to the transesterification of formates present in the P. graveolens EO with methanol (Scheme 2). More reactive than ethanol, methanol allows a total conversion in 4 hours.
Considering the gap between MF and methanol boiling points (32 °C and 65 °C, respectively), the distillation step took place at the same time as the reaction. While MF was directly distilled after its formation, the equilibrium of the reaction was shifted, providing a drastic reduction of the reaction duration. MF was successfully obtained with 75% yield as a colourless liquid. The excess methanol was then distilled in a subsequent fraction to be recycled. The same advantages as previously can be noted:
- Enrichment of the EO in citronellol and geraniol (SI, part 3.2 and Fig. 12 and 13).
- Neutralization of the acidity of the EO.
- Other compounds stayed untouched, and no byproducts were observed.
Conclusions
Here we described the first preparation of biobased EF and MF by using an ecocatalyst® derived from the invasive alien species Fallopia japonica. The conception of this new type of catalyst contributes to supporting the control of the proliferation of this plant. The characterization of this biobased catalyst highlighted the presence of carbonates and phosphates of potassium and calcium, as well as MgO, which is known for its Lewis acid character and its Brønsted basicity. This ecocatalyst® proved to be highly efficient in the transesterification of CF and GF contained in the Pelargonium graveolens EO without the use of any additive or solvent, improving the green potential of the reaction. Good yields (up to 80%) of EF and MF were obtained with an excellent selectivity. To the best of our knowledge, the application of this catalytic system to this specific reaction has never been described. Finally, this study highlighted the triple advantage of this methodology:- Highly efficient synthesis of biobased MF and EF.
- Production of a Pelargonium graveolens EO with higher citronellol and geraniol contents.
- Neutralisation of EO acidity.
The current international market of P. graveolens EO is substantial, with nearly 300 tons produced per year. The projection of the ecocatalytic process application to this annual production could lead to form more than 18 tons of biobased EF or MF.
Author contributions
AL: investigation, formal analysis, data curation. PAD: investigation, formal analysis, data curation, writing and reviewing. FP: formal analysis, validation, data curation. YML: formal analysis, validation, data curation. CG: writing, reviewing and editing, supervision, funding acquisition, formal analysis, methodology, conceptualization. CMG: formal analyses, validation, data curation, funding acquisition, writing, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI) and are also available at https://osf.io/g24y7. Supplementary information is available. See DOI: https://doi.org/10.1039/d5su00739a.Notes and references
- J. Hietala, A. Vuori, P. Johnsson, I. Pollari, W. Reutemann and H. Kieczka, Formic Acid, DOI:10.1002/14356007.a12013.pub3, accessed 26 November 2024.
- Redaction, Framboise : qu’est-ce que c’est ?, https://www.futura-sciences.com/planete/definitions/botanique-framboise-7571/.
- Redaction, Global Ethyl Formate Market By Type (Superior Grade, First Grade), By Application (Solvent, Pharmaceutical Intermediates), By Geographic Scope And Forecast, https://www.verifiedmarketreports.com/en/product/ethyl-formate-market/.
- Redaction, Occupational Safety and Health Administration Chemical Database Ethyl Formate, https://www.osha.gov/chemicaldata/106.
- Environment and Climate Change Canada, Screening assessment formic acid and formates substance group, 2023.
- International Symposium on Controlled Atmosphere Grain Storage, Controlled Atmosphere and Fumigation in Grain Storages: Proceedings of an International Symposium ‘Practical Aspects of Controlled Atmosphere and Fumigation in Grain Storages’ Held from 11 to 22 April 1983 in Perth Western Australia, ed. B. E. Ripp and H. J. Banks, Elsevier, Amsterdam New York, 2011 Search PubMed.
- V. S. K. Bharathi and D. S. Jayas, J. Stored Prod. Res., 2024, 106, 102280, DOI:10.1016/j.jspr.2024.102280.
- A. Zaitoon, L.-T. Lim and C. Scott-Dupree, J. Agric. Food Chem., 2019, 67, 13914–13921, DOI:10.1021/acs.jafc.9b06335.
- B. Kapuku and D. S. Bohle, Molecules, 2024, 29, 1084, DOI:10.3390/molecules29051084.
- M. Liu and X. Wang, CN118388452, 2024.
- H. Suzhou, WO2024149370, 2024.
- Y. Li, Z. Shi, Y. Pei, L. Chen, T. Shu, P. Wang, S. Shi, L. He, P. Tang, C. Zhang and P. Yan, WO2024017294, 2023.
- Q. Gao, S. Liu and Z. Kang, CN118420581, 2023.
- V. V. Levterov, Y. Panasiuk, O. Shablykin, O. Stashkevych, K. Sahun, A. Rassokhin, I. Sadkova, D. Lesyk, A. Anisiforova, Y. Holota, P. Borysko, I. Bodenchuk, N. M. Voloshchuk and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2024, 63, e202319831, DOI:10.1002/anie.202319831.
- R. Bouzammit, I. Lakkab, M. El Fadili, Y. Kanzouai, M. Chalkha, A. Nakkabi, B. El Bali, S. Obbade, L. Jouffret, M. Lachkar and G. Al Houari, J. Mol. Struct., 2024, 1312, 138582, DOI:10.1016/j.molstruc.2024.138582.
- X. Ke, Q. Fang and Q. Sun, WO2024169773, 2024.
- F. Zhu and J. Huang, CN117776973, 2023.
- H. Zhao, V. D. Cuomo, J. A. Rossi-Ashton and D. J. Procter, Chem, 2024, 10, 1240–1251, DOI:10.1016/j.chempr.2024.01.020.
- Z. Jiao, K. T. Jaunich, T. Tao, O. Gottschall, M. M. Hughes, A. Turlik and A. W. Schuppe, Angew. Chem., Int. Ed., 2024, 63, e202405779, DOI:10.1002/anie.202405779.
- Y. Wu, W. Cai, L. Yu, J. Chang and C. Song, Org. Chem. Front., 2024, 11, 89–93, 10.1039/D3QO01454A.
- Y. Guo, X. Fan, M. Nie, H. Liu, D. Liao, X. Pan and Y. Ji, Eur. J. Org Chem., 2015, 2015, 4744–4755, DOI:10.1002/ejoc.201500500.
- X. Zhang, W. Li, Y. Yu, M. Luo, H. Bai, L. Shi and H. Li, Green Chem., 2024, 26, 2207–2212, 10.1039/D3GC04070D.
- S. S. Huang, Z. J. Zheng, Y. M. Cui, Z. Xu, K. F. Yang and L. W. Xu, Synthesis, 2019, 51, 4249–4252, DOI:10.1055/s-0039-1690617.
- Y. Guo, Y.-D. Li, C. Chen, J.-H. Zhao, H.-W. Liu, D.-H. Liao and Y.-F. Ji, Res. Chem. Intermed., 2016, 42, 2525–2537, DOI:10.1007/s11164-015-2165-4.
- S. Gowrisankar, A. G. Sergeev, P. Anbarasan, A. Spannenberg, H. Neumann and M. Beller, J. Am. Chem. Soc., 2010, 132, 11592–11598, DOI:10.1021/ja103248d.
- K. W. Anderson, T. Ikawa, R. E. Tundel and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 10694–10695, DOI:10.1021/ja0639719.
- X. Wu, B. P. Fors and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 9943–9947, DOI:10.1002/anie.201104361.
- K. E. Torraca, X. Huang, C. A. Parrish and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 10770–10771, DOI:10.1021/ja016863p.
- A. V. Vorogushin, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 8146–8149, DOI:10.1021/ja050471r.
- S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449, DOI:10.1002/anie.200300594.
- G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054–3131, DOI:10.1021/cr8002505.
- F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954–6971, DOI:10.1002/anie.200804497.
- Y. Han, D. Jia, Z. Guo and Y. Zhang, CN116688896, 2023.
- E. Gérard, PhD thesis, University of Lille 1, 1994.
- L. Rong, Z. Xu, J. Sun and G. Guo, J. Energy Chem., 2018, 27, 238–242, DOI:10.1016/j.jechem.2017.07.015.
- B. An, Y. Meng, Z. Li, Y. Hong, T. Wang, S. Wang, J. Lin, C. Wang, S. Wan, Y. Wang and W. Lin, J. Catal., 2019, 373, 37–47, DOI:10.1016/j.jcat.2019.03.008.
- J. He, M. Wen, Y. Zhu, Z. An, X. Xiang, H. Song and X. Shu, CN113908849, 2020.
- W. Zhang, Y. Yao, S. Xie, K. Gubsch, Y. Yang, X. Lan and H. Lin, Catal. Today, 2021, 374, 53–60, DOI:10.1016/j.cattod.2020.12.027.
- A. T. Cannon and C. T. Saouma, Polyhedron, 2021, 207, 115375, DOI:10.1016/j.poly.2021.115375.
- D. T. Hofsommer, I. R. Zamborini, S. S. Uttarwar, C. A. Phipps, M. Gautam, F. Nkurunziza, C. A. Grapperhaus and J. M. Spurgeon, ACS Sustainable Chem. Eng., 2024, 12, 882–892, DOI:10.1021/acssuschemeng.3c05853.
- L. González-Sebastián, M. Flores-Alamo and J. J. García, Organometallics, 2013, 32, 7186–7194, DOI:10.1021/om400876j.
- B. Zhang, G. Du, W. Hang, S. Wang and C. Xi, Eur. J. Org Chem., 2018, 2018, 1739–1743, DOI:10.1002/ejoc.201800320.
- I. Knopf and C. C. Cummins, Organometallics, 2015, 34, 1601–1603, DOI:10.1021/acs.organomet.5b00190.
- G. Gastelu, D. Savary, M. Hulla, D. Ortiz, J. G. Uranga and P. J. Dyson, ACS Catal., 2023, 13, 2403–2409, DOI:10.1021/acscatal.2c06218.
- B. Blerot, S. Baudino, C. Prunier, F. Demarne, B. Toulemonde and J.-C. Caissard, Phytochem. Rev., 2016, 15, 935–960, DOI:10.1007/s11101-015-9441-1.
- K. Ghedira and P. Goetz, Phytothérapie, 2015, 13, 197–201, DOI:10.1007/s10298-015-0955-x.
- B. Blerot, PhD thesis, University of Lyon, 2016.
- N. S. Ravindra and R. N. Kulkarni, Sci. Hortic., 2015, 184, 31–35, DOI:10.1016/j.scienta.2014.12.023.
- M. N. Boukhatem, A. Kameli and F. Saidi, Food Control, 2013, 34, 208–213, DOI:10.1016/j.foodcont.2013.03.045.
- A. Lasbleiz, F. Pelissier, J.-H. Renault, C. M. Grison, Y.-M. Legrand and C. Grison, C. R. Chim., 2024, 27, 85–97, DOI:10.5802/crchim.273.
- L. Liénart, Y.-M. Legrand, F. Pelissier, P. Gaveau, P. Hesemann, E. Petit, C. Grison and C. M. Grison, Appl. Catal. B Environ. Energy, 2024, 349, 123888, DOI:10.1016/j.apcatb.2024.123888.
- M. Corsi, S. Magnolfi and F. Machetti, Eur. J. Org Chem., 2020, 2020, 1720–1726, DOI:10.1002/ejoc.202000050.
- C. Grison and A. Stanovych, WO2021005204A1, 2021.
- W. Chen and A. M. Viljoen, J. S. Afr. Bot., 2010, 76, 643–651, DOI:10.1016/j.sajb.2010.05.008.
- A. Lapczynski, S. P. Bhatia, C. S. Letizia and A. M. Api, Food Chem. Toxicol., 2008, 46, S110–S113, DOI:10.1016/j.fct.2008.06.056.
- P. A. Grieco, J. Chem. Soc., Chem. Commun., 1972, 8, 486, 10.1039/c39720000486.
- E. J. Lenardão, G. V. Botteselle, F. De Azambuja, G. Perin and R. G. Jacob, Tetrahedron, 2007, 63, 6671–6712, DOI:10.1016/j.tet.2007.03.159.
| This journal is © The Royal Society of Chemistry 2026 |