 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
SimpleBox4Planet: environmental fate modelling of PFASs and their alternatives via the Enalos Cloud Platform
Dimitris G.
Mintis
 ab,
Constantinos
Papavasiliou
ab,
Constantinos
Papavasiliou
 ac,
Dimitra-Danai
Varsou
ac,
Dimitra-Danai
Varsou
 bd,
Andreas
Tsoumanis
bd,
Andreas
Tsoumanis
 ab,
Georgia
Melagraki
ab,
Georgia
Melagraki
 e,
Johannes P.
Seif
e,
Johannes P.
Seif
 f,
Marc
Majó
f,
Marc
Majó
 g,
Alejandro J.
del Real
g,
Alejandro J.
del Real
 fh,
Tommaso
Serchi
fh,
Tommaso
Serchi
 i,
Roland
Hischier
i,
Roland
Hischier
 g,
Iseult
Lynch
g,
Iseult
Lynch
 bj and
Antreas
Afantitis
bj and
Antreas
Afantitis
 *abck
*abck
aNovaMechanics Ltd, Nicosia 1070, Cyprus. E-mail: afantitis@novamechanics.com; Tel: +357 99048039
bEntelos Institute, Larnaca 6059, Cyprus
cSchool of Chemical Engineering, National Technical University of Athens, Athens, Greece
dNovaMechanics MIKE, Piraeus 18545, Greece
eDivision of Physical Sciences and Applications, Hellenic Military Academy, Vari 16672, Greece
fIDENER, 41300 Sevilla, Spain
gTechnology & Society Laboratory, Empa – Swiss Federal Laboratories for Materials Science and Technology, Lerchenfeldstrasse 5, St. Gallen 9014, Switzerland
hDepartment of Systems and Automation, University of Seville, 41092 Seville, Spain
iEnvironmental Sustainability Assessment and Circularity (SUSTAIN) Unit, Luxembourg Institute of Science and Technology (LIST), Esch-sur-Alzette, Luxembourg
jSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham B15 2TT, UK
kDepartment of Pharmacy, Frederick University, Nicosia 1036, Cyprus
First published on 11th December 2025
Abstract
This work presents the development of SimpleBox4Planet, a user-friendly web application implementation of SimpleBox, and demonstrates its use in facilitating the assessment of the environmental fate of per- and polyfluoroalkyl substances (PFASs) as well as other chemicals of interest, with the aim of supporting research into safer chemical alternatives with lower environmental impact. The SimpleBox4Planet web application is freely accessible on the Enalos Cloud Platform (https://www.enaloscloud.novamechanics.com/proplanet/simplebox4planet/ and https://www.enaloscloud.novamechanics.com/chiasma/simplebox4planet/). The SimpleBox4Planet web application integrates the SimpleBox (version 4.04) multimedia mass balance model (based on a ‘Mackay type’ model), accommodating both steady-state (level III) and quasi-dynamic (level IV) computations of mass flows and chemical concentrations across three environmental scales: regional, continental and global, while also considering the chemical distributions at each scale across environmental compartments, including air, soil, water and sediment, thus streamlining the workflow and enhancing visualisation of the model outcomes. The complexities related to modelling SimpleBox through MS Excel spreadsheets are eliminated through the design of the user-friendly graphical user interface (GUI) provided by SimpleBox4Planet. This interface enables users to input the physicochemical properties of any chemical of interest (based on its CAS number) from the CompTox Chemicals Dashboard either directly or dynamically through application programming interfaces (APIs), to define emission rates, and to configure landscape settings. Both expert and non-expert users can efficiently perform complex multimedia fate modelling, significantly broadening the tool's applicability in regulatory, academic, and industrial contexts. Furthermore, the platform facilitates integration with other tools and models, including Life Cycle Impact Assessment (LCIA) frameworks, and can be used as an input to risk assessment, to support the evaluation of both ecotoxicological and human health impacts.
Sustainability spotlightThis work supports sustainability by providing an accessible computational tool to model how persistent chemicals like PFASs move and accumulate in the environment. Delivered as a web-based platform, SimpleBox4Planet reduces the need for resource-intensive laboratory testing and complex manual modeling, saving time and limiting environmental sampling. It enables the identification of safer chemical alternatives, supporting responsible consumption and production (SDG 12) and protecting human and environmental health (SDG 3). By being freely available and interoperable with other assessment tools, it fosters innovation and infrastructure for environmental protection (SDG 9). Through evaluating legacy PFASs and greener substitutes, SimpleBox4Planet informs sustainable policies and encourages the design of safer, sustainable-by-design materials. |
1 Introduction
Understanding the fate and transport of chemicals across multiple environmental scales is essential for evaluating their ecotoxicological risks and potential impacts on human health. Evaluating the environmental persistence of chemicals requires a comprehensive understanding of their transport and fate, including far from their emission sources over long distances in air, water, soil and sediment media. Additionally, a crucial consideration is the dispersion of these chemicals across distinct geographic scales, including regional, continental and global domains, which is essential for effectively assessing potential risks to ecosystems and human health. In response to these challenges, several multimedia environmental models have been developed,1 classified into 3 categories: fugacity-based compartmental models, integrated spatial-multimedia compartmental models (ISMCM) and linked spatial single-media models (LSSMM). These three categories differ in the treatment of spatial and temporal resolution, as well as in their respective complexity levels.2,3 Among these models, the SimpleBox model, a fugacity based multimedia mass balance model, is often used to model non-ionizable organics, ionizable organics and metals across regional, continental and global scales.4–8 On the regional and continental scales, SimpleBox consists of nine well-mixed homogeneous compartments including air, three soil compartments (natural, agricultural and other soil), three water compartments (freshwater, freshwater lakes and coastal seawater) and two sediment/vegetation compartments (fresh water sediment and coastal marine sediment). The global scale is further classified into three zones, moderate, arctic and tropical, each consisting of five well-mixed homogeneous compartments including air, soil, two water compartments (surface and deep ocean water) and ocean sediment.5The SimpleBox model (version 4.04) employs the level III ‘Mackay type’ model for steady-state modelling, where the mass in each compartment remains constant with time, and the level IV ‘Mackay type’ model for non-steady-state modelling, allowing the mass in each compartment to vary with time. However, unlike the fugacity-based approach adopted by Mackay and coworkers,9–11 the mass flows and concentrations of chemicals in the SimpleBox model are calculated using concentration-based ‘piston velocity’ type mass transfer coefficients (m s−1) rather than fugacity-based ‘conductivity’ type coefficients.6,12 The widespread acceptance of the SimpleBox model is highlighted by its integration into the risk policy tool ‘Chesar’ of the European Union, hosted by the European Chemicals Agency.5 The model is employed to demonstrate that chemicals can be managed safely, ensuring compliance with the EU's stringent Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulations by verifying that environmental impacts remain within acceptable limits defined by REACH.4,5 The SimpleBox model is also integrated within Life Cycle Impact Assessment models, such as the UseTox model,13 for modelling the fate of chemicals. While fugacity-based models like SimpleBox are widely favored due to their simplicity and ease of execution, they do suffer from limitations. Specifically, the accuracy and reliability of calculations rely on the quality and precision of input parameters, such as physicochemical properties and transformation rates in soil, air, surface and/or sediments. Also, the assumption of the uniform mixing of chemicals in the compartment becomes invalid when modelling the fate of chemicals quantitatively at smaller scales or in heterogeneous environments.1 Nonetheless, the SimpleBox model remains a powerful and practical approach for evaluating chemical safety, supporting informed environmental risk assessments, and facilitating regulatory compliance.
The objective of this work is to present the development of SimpleBox4Planet, a free-to-use web application that integrates the SimpleBox model (version 4.04) for performing both steady-state and dynamic (non-steady-state) simulations. The motivation behind developing SimpleBox4Planet is to provide a user-friendly interface that simplifies the execution of the SimpleBox model by removing the complexities of running simulations through Excel spreadsheets and making it accessible to users regardless of their technical and programming expertise. Additionally, SimpleBox4Planet includes Application Programming Interfaces (APIs) to facilitate integration with external software systems and tools, including regulatory and decision-support tools. It also provides users with the ability to dynamically retrieve physicochemical properties by entering a CAS Registry Number from the CompTox Chemicals Dashboard (EPA).14 Here, we demonstrate the capability of the SimpleBox4Planet web application to assess and compare the fate and transport of chemicals, using the family of PFASs and their alternatives, which are designed to be less persistent in the environment, although the web application also enables users to define and evaluate any chemical of interest.
SimpleBox4Planet enables users to either select from predefined cases/scenarios or to customize emission rates across regional, continental and global scales, as well as landscape settings. One of its key features is a comparative tool that allows users to compute the total mass distribution of different chemicals under the same emission and landscape settings, thus providing valuable insights into which substances pose the least environmental threat. This is particularly relevant for per- and polyfluoroalkyl substances (PFASs), a diverse class of synthetic organofluorine compounds, with an estimated inventory exceeding 4000 individual substances.15 The European Commission (EC) has declared PFASs as emerging organic contaminants due to their environmental persistence and potential risks to human health and ecosystems.16–18 All PFASs share a similar molecular architecture – a hydrophobic fully fluorinated (perfluorinated) or partially fluorinated (polyfluorinated) carbon backbone paired with a hydrophilic functional headgroup, resulting in unique amphiphilic properties.18 An example illustrating the structural distinction between polyfluorinated and perfluorinated carboxylic acid substances is shown in Fig. 1, where the left panel depicts a perfluoroalkyl carboxylic acid (PFCA) with all hydrogen atoms on the alkyl chain replaced by fluorine atoms, and the right panel shows a polyfluoroalkyl carboxylic acid, in which some hydrogen atoms still remain on the alkyl chain. Regulatory agencies worldwide have expressed growing concern over long-chain perfluoroalkyl sulfonic acids (PFSAs) with more than six carbon-fluorine bonds and long-chain perfluorocarboxylic acids (PFCAs) containing more than seven carbon–fluorine bonds, due to their greater potential for bioaccumulation compared to their short-chain counterparts.19,20 Among these substances, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) are the most extensively studied and frequently cited in scientific research.16,17,21 Their short-chain alternatives include perfluorohexanoic acid (PFHxA) and perfluorobutane sulfonic acid (PFBS).15 The chemical structure of PFASs underpins their extreme thermodynamic and kinetic stability, conferred by the strength of the carbon–fluorine bond, which has a bond energy of 466 kJ mol−1,22 rendering them resistant to abiotic degradation processes (e.g., hydrolysis, photolysis, and oxidation) and biotic degradation pathways (e.g., microbial metabolism and enzymatic transformation).23,24 This has led to their classification as ‘forever chemicals’.25,26
Due to their outstanding resistance to heat and chemical breakdown, combined with their unique surfactant properties, PFASs have been extensively used in waterproof textiles, grease-resistant food packaging, non-stick cookware, semiconductor manufacturing and fire suppression systems.23,27 Their incorporation into textiles, paper-based packaging, electronics, and aqueous film-forming foams (AFFFs) is particularly favored due to their unique hydrophobic and oleophobic surface-modifying characteristics.17,28–30 However, their resistance to degradation, combined with their ability to partition between air, water, soil, and sediment, along with the atmospheric transport and oxidative transformation of volatile PFAS precursors, enhances their environmental mobility and facilitates their long-range atmospheric transport, enabling their deposition in and contamination of remote regions far from the primary emission sources.18,31–36 Epidemiological and toxicological evidence links PFAS exposure to hepatic toxicity, immune system dysregulation, endocrine disruption, and carcinogenicity,37,38 highlighting the critical need for comprehensive environmental monitoring and multimedia fate modelling to quantify the fate and transport of PFASs and assess their long-term impacts on ecosystems and human populations.15,27,39
Releases of PFASs originate from primary emissions, which represent direct emissions from industrial chemical manufacturing facilities, and secondary emissions, which represent indirect sources of emissions resulting from the environmental transformation of precursor compounds and the remobilization from contaminated environmental reservoirs.23 Primary emissions include direct sources of PFAS emissions such as fluoropolymer manufacturing facilities, electrochemical fluorination (ECF) plants, telomerization production sites, aqueous film-forming foam (AFFF) applications at firefighting training grounds, textile finishing plants, paper mills, semiconductor manufacturing operations, wastewater treatment plants (WWTPs) and landfills.18,40 In contrast, secondary emissions refer to indirect PFAS releases that arise through diffuse sources, including emissions from unknown origins, atmospheric transport and deposition of volatile precursors, leaching from PFAS-treated consumer products, and chemical or biological transformation of precursor compounds into stable PFASs.40–43 Once released into the environment, PFASs partition and distribute across all major environmental compartments, including atmospheric aerosols, the hydrosphere (surface water, groundwater, and seawater), the cryosphere (glacial and permafrost environments), the lithosphere (soils and sediments), and the biosphere (wildlife and human tissues).35,44
The partitioning dynamics between air, water, soil and sediment are governed by the unique physicochemical properties of PFASs, which consist of low vapor pressures, variable acid dissociation constants (pKa), and a broad spectrum of organic-carbon-based partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc) values.44,45 Long-chain PFASs, including perfluoroalkyl carboxylic acids (PFCAs) such as PFOA and perfluoroalkyl sulfonic acids (PFSAs) such as PFOS, preferentially sorb to sediments, suspended particulates, and biotic tissues (biological macromolecules such as proteins and phospholipid membranes), due to their strong hydrophobicity and low vapor pressure, contributing to their bioaccumulation and biomagnification in aquatic and terrestrial organisms.35,46 In contrast, short-chain PFASs (typically possessing fewer than six perfluorinated carbons), with lower hydrophobicity and weaker adsorption coefficients (Kd), persist predominantly in the aqueous phase, demonstrating enhanced advective transport through surface water and porous media.47
Koc) values.44,45 Long-chain PFASs, including perfluoroalkyl carboxylic acids (PFCAs) such as PFOA and perfluoroalkyl sulfonic acids (PFSAs) such as PFOS, preferentially sorb to sediments, suspended particulates, and biotic tissues (biological macromolecules such as proteins and phospholipid membranes), due to their strong hydrophobicity and low vapor pressure, contributing to their bioaccumulation and biomagnification in aquatic and terrestrial organisms.35,46 In contrast, short-chain PFASs (typically possessing fewer than six perfluorinated carbons), with lower hydrophobicity and weaker adsorption coefficients (Kd), persist predominantly in the aqueous phase, demonstrating enhanced advective transport through surface water and porous media.47
While shorter-chain PFASs have been introduced as replacements for long-chain PFASs, recent evidence suggests that their enhanced mobility increases their potential to contaminate groundwater and migrate through porous media, particularly in low organic carbon aquifers.47 Additionally, the volatilization and atmospheric transport of neutral and ionic PFAS precursors serve as key mechanisms for their global distribution. PFAS precursors, particularly fluorotelomer alcohols (FTOHs) and perfluoroalkyl sulfonamido ethanols (FOSEs), undergo long-range atmospheric transport in the gas phase due to their volatility,42 followed by oxidative degradation via hydroxyl radical attack to yield persistent terminal perfluoroalkyl acids such as PFOA and PFOS,48 which subsequently undergo atmospheric deposition processes, including precipitation (wet deposition) and aerosol particle adhesion (dry deposition).42 This differential environmental mobility, controlled by chain length, functional group chemistry, and local geochemical conditions (e.g., ionic strength, pH, and mineral composition), plays a critical role in determining localized versus long-range environmental dispersion.49
The environmental persistence and health risks associated with PFASs have prompted extensive research into safer alternatives across various industries. A comprehensive study identified 530 PFAS-free alternatives spanning 325 applications in 18 industries, including 162 alternative substances, 163 alternative materials, 128 alternative products, 37 alternative processes, and 40 alternative technologies, whereas for 83 applications of PFASs no alternatives have been identified as yet.50 For instance, advanced ceramics like silicon nitride and boron nitride have been proposed as replacements for PFASs in high-temperature applications such as cookware and industrial processes, offering thermal stability without releasing harmful substances.27 Additionally, fluorine-free firefighting foams (F3) are under development to match the efficacy of traditional PFAS-based foams, aiming to reduce environmental contamination from fire suppression activities.51 Despite these advancements, certain applications, particularly in industrial processes like plastic and rubber production, currently lack suitable PFAS alternatives, underscoring the need for ongoing research and development to address these gaps.50
The next sections outline the theory underpinning SimpleBox, present the development of the web application interface, provide an example of the practical application of the SimpleBox4Planet web application via a case study focusing on the environmental fate and transport of perfluorooctanoic acid (PFOA), a well-characterized and extensively studied PFAS known for its environmental persistence and mobility,17,40,44,52,53 and provide some future perspectives for further development of SimpleBox4Planet to enhance its integration into regulatory risk assessment and life cycle assessment approaches.
2 Methods and materials
2.1 SimpleBox model
The SimpleBox4Planet web application integrates the SimpleBox model (version 4.04), a nested multimedia environmental fate model, designed to simulate the transport and distribution of chemical substances across multiple homogeneous environmental compartments (represented by boxes) at different spatial scales.5 The model operates at regional, continental, and global scales, incorporating reversible mass transport processes between scales, highlighting the dynamic exchange of chemicals at these levels, as shown in Fig. 2. Within the global scale, three distinct climatic zones are defined: the arctic, moderate, and tropic zones, allowing for a refined representation of geographical variations in environmental fate processes.4,5At both the local (regional) and continental scales, SimpleBox 4.04 defines nine homogeneous environmental compartments to capture the behavior of chemicals in different environmental compartments. These include the atmospheric compartment (air), three soil compartments (natural soil, agricultural soil, and other soil), three aquatic compartments (freshwater, freshwater lakes, and coastal seawater) and two sediment compartments (freshwater sediment and coastal marine sediment). Fig. 3 illustrates the interconnected nature of these compartments, with mass exchange processes governing the transport and partitioning of chemical substances within and between these environmental compartments. This detailed compartmentalization reflects the diverse environmental media and land-use types typically found within regional and continental boundaries.4,5
At the global scale, the model structure adapts to accommodate climatic variability, consisting of the atmospheric compartment (air), the soil compartment, two oceanic compartments (surface ocean and deep ocean) and an ocean sediment compartment. The variation in the compartmental structure on the global scale is primarily due to differences in the dominant processes influencing chemical behavior. At the regional and continental scales, localized factors such as land use, soil composition, and the presence of inland water bodies significantly affect chemical fate, necessitating a more detailed compartmental approach. In contrast, on the global scale, the focus shifts to large-scale processes like atmospheric circulation and oceanic currents, which can be effectively represented with fewer, broader compartments (Fig. 4).4,5
The SimpleBox 4.04 model applies a mass balance approach to describe the fate and transport of chemicals in multiple environmental compartments at different spatial scales. It computes both the steady-state and quasi-dynamic chemical distributions, classifying it as a Mackay Level III/IV multimedia environmental fate model that operates under non-equilibrium conditions to simulate the distribution and transport of chemicals across multiple compartments. While most multimedia fate models adopt a fugacity-based approach that treats compartments as being in near-equilibrium and uses fugacity capacities (Z-values) to describe how readily chemicals partition between media, SimpleBox treats intermedia transport as a mass transfer process driven by bulk flow rather than by fugacity-driven diffusion.12 This piston-like conceptualization assumes that chemicals are physically transferred between compartments through advection, deposition, and sedimentation, with transfer rates determined by empirical environmental parameters (e.g., wind-driven deposition, river flow, and soil runoff), rather than strict thermodynamic equilibrium.1 This approach allows SimpleBox 4.04 to be more computationally efficient and applicable to complex regulatory scenarios, particularly when dealing with chemicals that do not rapidly equilibrate across compartments (e.g., persistent pollutants, heavy metals, or ionizing compounds). The movement of chemicals within each compartment is dictated by various mass flow processes that regulate their entry, transfer, and removal. Chemicals can enter a compartment through intermedia transport from another box inside the spatial scale or via emissions and import flows of air or water from boxes outside the spatial scale to which the box belongs. On the other hand, chemicals can leave a compartment through intermedia transport to other boxes in the same spatial system, export to a different spatial scale and degradation due to chemical transformation.
To solve the system of mass balance equations in the SimpleBox model, matrix algebra is employed. For a system of n environmental compartments, the mass balance can be expressed in the matrix form as6
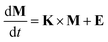 | (1) |
 , E is the vector of emission rates into each compartment, measured in kg
, E is the vector of emission rates into each compartment, measured in kg  , and K is the matrix of transfer rate coefficients between compartments, measured in per day
, and K is the matrix of transfer rate coefficients between compartments, measured in per day  . In the fully nested implementation (regional, continental and three global zones), K is a 33 × 33 model matrix that also includes the inter-scale exchange terms between regional, continental and global boxes. Assuming a steady-state condition (no accumulation dM/dt = 0), the mass balance equation can be solved as
. In the fully nested implementation (regional, continental and three global zones), K is a 33 × 33 model matrix that also includes the inter-scale exchange terms between regional, continental and global boxes. Assuming a steady-state condition (no accumulation dM/dt = 0), the mass balance equation can be solved as| M = −K−1 × E | (2) |
| FF = −K−1 | (3) |
2.2 The SimpleBox4Planet web application
The SimpleBox4Planet web application, hosted on the Enalos Cloud Platform,55 is accessible at https://www.enaloscloud.novamechanics.com/proplanet/simplebox4planet/ and https://www.enaloscloud.novamechanics.com/chiasma/simplebox4planet/. The backend of SimpleBox4Planet is implemented in the Java programming language and is designed to run the SimpleBox 4.04 Excel model, integrating user-defined input parameters from the web-based interface built with the ZK framework, an open-source Ajax web application framework implemented in Java.56For quasi-dynamic simulations, a fourth-order Runge–Kutta numerical integration scheme is employed to solve the first-order differential mass balance equations governing the environmental fate of chemicals in SimpleBox4Planet. To solve this system dynamically, the Apache Commons Math library is employed, specifically utilizing the Classical Runge–Kutta Integrator to perform a fourth-order Runge–Kutta (RK4) integration. The simulation was executed in two distinct phases: an emission-driven buildup phase, followed by a decay phase, where emissions were set to zero, allowing natural dissipation. Thorough validation of the output generated between the SimpleBox 4.04 Excel and the SimpleBox4Planet web application is carried out to verify the output from the steady-state and dynamic calculations, as shown in Sections S1–S3 in the SI. An excellent agreement is found between the outputs generated by the SimpleBox 4.04 Excel file and the outputs generated by the SimpleBox4Planet web application, thus ensuring the robustness and accuracy of the web application implementation presented here.
As shown in Fig. 5, the Graphical User Interface (GUI) of SimpleBox4Planet is structured into four distinct sections to enhance clarity and facilitate user input. Specifically, the interface includes the Chemical Substance Input Section, the Emission Settings Input Section, the Landscape Parameters Input Section and the Dynamic Simulation Parameters Input Section. Within the Chemical Substance Input Section, users can select from a predefined list of PFASs and their alternatives. The various physicochemical properties of the predefined PFASs and their alternatives were retrieved from the CompTox Chemicals Dashboard (EPA).14 Specifically, the full list of PFASs (∼4000 substances) integrated within SimpleBox4Planet along with their CAS numbers is retrieved from the study of Mudlaff et al.57 Physicochemical properties such as pKa, Mw, Tm, Pvap, solubility and partition coefficients are retrieved in a consistent manner via an API from the EPA CompTox Chemicals Dashboard14 to avoid cross-source discrepancies. For degradation rates and environmental half-lives, the default QSAR-based chemical-class parameters defined in the SimpleBox 4.04 model are used (neutral organics, acids, bases, hydrophobics, phenols, anilines, esters, triazines, etc.). Additionally, the interface offers a custom definition option, allowing users to manually input the physicochemical properties of a chemical of interest. When selecting the custom option, an interactive pop-up window (Fig. 6) is triggered, enabling users to enter detailed physicochemical parameters directly or indirectly via their CAS numbers. Within the interactive pop-up window for defining a custom chemical substance (Fig. 6), users have the option to define a chemical by its Chemical Abstracts Service (CAS) registry number and retrieve its physicochemical properties automatically. By clicking the ‘API CAS’ button, the system queries the CompTox Chemicals Dashboard (EPA)14 to extract key properties, including molecular weight, acid dissociation constant (pKa), melting temperature, vapor pressure, solubility, and octanol–water partitioning coefficient (Kow). This feature enhances the usability of SimpleBox4Planet, allowing users to efficiently incorporate accurate physicochemical data into their environmental fate modelling simulations. Each selected chemical can be classified into predefined chemical classes based on the original SimpleBox 4.04 model, which integrates Quantitative Structure–Activity Relationships (QSARs) to estimate the solid–water partitioning coefficient.5 The available chemical classes include neutral compounds, acids, bases, metals, hydrophobics, non-hydrophobics, phenols, anilines, benzonitriles, nitrobenzenes, acetanilides, carbamates, esters, phenylureas, phosphates, triazines, triazoles, uracils, alcohols, organic acids, amides, and dinitroanilines.
 | ||
| Fig. 6 An interactive pop-up window for defining a custom chemical substance in SimpleBox4Planet to manually input physicochemical properties. Additionally, users can enter a CAS number and utilize the ‘API CAS’ button to automatically retrieve physicochemical data from the CompTox Chemicals Dashboard (EPA) if such data are available.14 | ||
Within the Emission Settings Input Section, users can specify emission values for different environmental compartments directly through the GUI, enabling users to define emissions at the desired scale for the corresponding compartment. The SimpleBox 4.04 model does not permit direct emissions to sediment compartments, and therefore, the GUI does not provide an emission input option for sediments in SimpleBox4Planet. In the Landscape Settings Input Section, users can configure landscape parameters by either selecting from default settings or choosing from a set of predefined landscapes available in the SimpleBox 4.04 database. These include the standard EUSES landscape,58,59 17 sub-continental landscapes,5 and a default landscape that resembles the environmental characteristics of the Northern Hemisphere. Additionally, users have the option to manually define landscape parameters by selecting the custom option, which opens an interactive pop-up window (Fig. 7), allowing for precise user-defined landscape configurations. Within the Dynamic Simulation Parameters Input Section, users can manually specify the desired simulation duration in years for executing the dynamic simulation.
When users select the ‘Execute Steady-State Simulation’ button, a new window opens displaying the simulation outputs. This window provides a brief description of the steady-state simulation, followed by a comprehensive table presenting the steady-state masses and concentrations for each scale and compartment. The output generated in this window is shown in Section S4 of the SI. Users also have the option to export the results in Excel format by clicking the ‘Export Results’ button. Below the table, a graphical representation of the mass distribution across different scales is displayed, illustrating both the mass within each scale and the total mass calculated across all scales. For further analysis, next to each mass calculation, a ‘Show’ button is available, allowing users to visualize the mass distribution within each scale across individual compartments through interactive graphical outputs. Additionally, a second table provides detailed information on steady-state mass flows at each scale and for each compartment. This includes mass transfer processes such as emission, inflow, outflow, removal, degradation, air–water exchange, water–air exchange, air–soil exchange, soil–air exchange, soil–water exchange, water–sediment exchange, and sediment–water exchange. The table also includes a mass balance verification column, ensuring that input flows equal output flows, thereby confirming the accuracy of the steady-state calculations. Users can export these results using the ‘Export Mass Flows’ button, allowing for in-depth data analysis and reporting in Excel format.
The ‘Execute Dynamic Simulation’ option can be selected only if the user has predefined the simulation duration (in years) within the GUI. Once this button is activated, a new window opens, initially providing a brief description of the quasi-dynamic simulation process. The interface then presents a graphical representation of mass distribution across different scales, allowing users to explore the dynamic evolution of mass over time. By selecting the ‘Show’ button for a specific scale, users can visualize the time-dependent changes in mass, normalized by the steady-state mass (normalized mass), as a function of the user-defined simulation duration, as shown in Fig. 8. The graphical output depicts the mass evolution within each compartment for the selected scale, offering insights into the temporal behavior of chemical fate and transport. A comprehensive representation of this output window is provided in Section S5 of the SI.
The ‘Calculate Fate Factors’ button initiates the computation of fate factors based on the user-defined environmental scenario. This computation is only enabled when the user specifies an emission into a single compartment at the continental scale. If the user selects more than one compartment for emission or defines the scenario at the regional or global scale, the application will disable the fate factor computation, as the calculation is only valid and meaningful for single-compartment emissions at the continental level. This constraint ensures consistency with the theoretical assumptions underlying the fate matrix derivation in the model.54 The output of the fate factor computation is shown in Section S6 of the SI, where users can also define the time (in years) over which they wish to evaluate the fate behavior of the chemical.
SimpleBox4Planet also features a comparative analysis tool that allows users to evaluate multiple chemicals under identical emission and landscape settings. This tool facilitates the assessment of total mass distribution across environmental scales and compartments, enabling the identification of chemicals that exhibit the lowest overall environmental burden. By comparing mass distributions, users can systematically assess potential alternatives to PFASs, aiding in the selection of more environmentally sustainable chemical substitutes. When users opt to compare chemicals, a new window opens, where they can select the specific chemicals for analysis. Upon clicking ‘Execute’, the system generates a bar chart (Fig. 9), visually comparing the total mass distributed across environmental compartments for each selected chemical. Users also have the flexibility to select all available chemicals for a comprehensive comparative analysis. This feature provides a data-driven approach to chemical substitution, supporting informed decision-making in regulatory assessments and sustainable materials design.
The SimpleBox4Planet web application features a Representational State Transfer (REST) application programming interface (API) designed to facilitate integration with external systems, thereby enhancing interoperability and extending its utility in various environmental modelling and regulatory contexts. These APIs include two GET endpoints (https://enaloscloud.novamechanics.com/proplanet/apis/sb4p/scenarios or https://enaloscloud.novamechanics.com/chiasma/apis/sb4p/scenarios and https://enaloscloud.novamechanics.com/proplanet/apis/sb4p/substances or https://enaloscloud.novamechanics.com/chiasma/apis/sb4p/substances) that retrieve the chemical substance and landscape and emission settings defined in the SimpleBox4Planet and one POST endpoint (https://enaloscloud.novamechanics.com/proplanet/apis/sb4p/results/ or https://enaloscloud.novamechanics.com/chiasma/apis/sb4p/results/) that enables users to submit simulation parameters and receive output results of the steady-state calculations. To demonstrate the functionality and accessibility of these APIs, the SI includes detailed documentation of the API interactions using Postman in Section S7 of the SI. Incorporating RESTful APIs not only enhances the flexibility, scalability and interoperability of environmental modelling applications but also aligns with the FAIR (Findable, Accessible, Interoperable and Reusable) and FAIR4ResearchSoftware (FAIR4RS) principles,60,61 ensuring that data and computational models can be shared, reused and integrated across platforms. By enabling standardized data exchange, these APIs facilitate the integration of environmental models into broader decision-support frameworks, thereby strengthening evidence-based policy-making and advancing sustainable-by-design (SSbD) materials development. Furthermore, the integration of the SimpleBox4Planet web application within the Enalos Cloud Platform enhances its FAIR compliance by allowing users to find, access, and interact with other hosted free-to-use web applications within the nano-informatics framework (e.g., LungDepo,62 MicroPlasticFate,63 NanoBioAccumulate,64 NanoTube Construct,65 NanoXtract,66 NInChI,67 NanoConstruct,68 EcoTox,69 and SafeNanoScope70) and beyond.63–73
Although SimpleBox4Planet can, in principle, be parameterised for any substance for which the required input data are available, its current implementation follows the generic SimpleBox model (version 4.04) and is therefore most appropriate for neutral and ionizable organic chemicals.4,5,12 For chemical classes, such as metals, whose environmental fate is dominated by complex speciation and redox-dependent processes, the use of bulk partition coefficients and first-order degradation rates may not fully capture mechanistic behaviour, and results should be interpreted as screening-level indicators unless supported by additional metal-specific speciation modelling.74
3 Case study using SimpleBox4Planet
In this section, the practical application of the SimpleBox4Planet web application is demonstrated through a case study focusing on the environmental fate and transport of perfluorooctanoic acid (PFOA), a well-characterized and extensively studied PFAS that belongs to the class of perfluoroalkyl carboxylic acids (PFCAs),75 known for their environmental persistence and mobility.17,40,44,52,53 This case study shows how SimpleBox4Planet can be used to analyze and compare the distribution of PFOA across different environmental compartments (air, water, soil, sediment, etc.) and across multiple spatial scales (continental, regional, and global). The objective is to support science-based decision-making regarding the environmental behavior and potential harm of PFOA, as well as its alternatives, by evaluating the total mass distribution and their potential impact across environmental media. A statistical analysis is also conducted to highlight the effect of physicochemical properties on the transport and fate (behavior) of PFOA. Notably, the SimpleBox4Planet platform supports a wide range of predefined PFAS compounds, offering users the flexibility to explore the fate and transport of numerous emerging PFASs beyond the one covered in this section.3.1 Environmental transport of PFOA
The physicochemical properties used in the SimpleBox4Planet web application to model the steady-state environmental transport of perfluorooctanoic acid (PFOA) are presented in Fig. S12 in the SI. These properties have been obtained from previous studies.75–78 According to numerous studies,40,79–81 long-range environmental transport of PFOA occurs primarily via atmospheric and oceanic pathways, while soil-mediated transport is negligible. To investigate this transport behaviour, model simulations were conducted using the SimpleBox4Planet platform under three distinct emission scenarios, each assuming a direct source of 1000 tonnes per year on a continental scale: (i) emissions to air only, (ii) emissions to seawater only, and (iii) emissions to natural soil environments (e.g., forested landscapes). These emission values were selected for comparative purposes and are intended to qualitatively explore the relative importance of different environmental pathways. The simulations were executed using the default continental-scale environmental settings of the original SimpleBox model, which are designed to reflect environmental conditions in the Northern Hemisphere.5 For users seeking to perform more quantitatively robust assessments, the platform allows the incorporation of real-world emission estimates, such as those reported by Armitage et al.79,82 who used the BETR Global and GloboPOP fate models to model the global transport and fate of PFOA and PFO in which they have considered direct emissions to range from 2700 to 5140 tonnes between 1950 and 2004, and from 475 to 932 tonnes between 2005 and 2050 (the lower values here being consistent with PFOA production being phased out in the US since 2015 and banned in the EU from 2020) under the POPs Regulation.83Model simulations performed using the SimpleBox4Planet web application under the scenario of direct emissions of PFOA to air (1000 tonnes per year) reveal distinct patterns of spatial (and compartmental) distribution across environmental scales. As shown in Fig. 10, approximately 48% of the total mass remains within the continental scale, while only a minor fraction (∼0.5%) is transported to the regional scale. A substantial portion, nearly 50%, is subject to long-range environmental transport to the global scale, with approximately 30% distributed to the moderate zone, 20% to the arctic, and ∼1% to the tropical zone. It is interesting to note that if emissions were considered on the regional scale, a similar behavior is observed, as shown in Fig. S13 in the SI, where around 15% of total mass remains on the regional scale, 42% is on the continental scale and around 44% on the global scale with more in the moderate and arctic zones and almost negligible in the tropical zone. Comparable behavior is also evident in the scenario where emissions are released directly to seawater (Fig. S14). Here, approximately 56% of the distributed mass remains within the continental scale, with minimal transfer to the regional scale. The remainder is distributed globally, primarily in the moderate and arctic zones, with only a negligible fraction reaching the tropical zone. In contrast, when emissions occur directly to natural soils (e.g., forested areas), as seen in Fig. S15, the model predicts a higher degree of mass retention at the continental scale, approximately 76%, with the remaining mass again following the established trend: highest transfer to the moderate zone, followed by the arctic, and minimal transport to the tropics. These findings collectively emphasize the consistency of PFOA's long-range transport behavior across different emission scenarios, with enhanced transport toward cooler climatic zones (moderate and arctic), consistent with previous studies.40,79,82
Additionally, results regarding the total mass of PFOA distributed across the environment vary significantly depending on the initial emission compartment, despite using an equivalent emission load of 1000 tonnes per year in each scenario. Specifically, direct emissions to seawater result in the highest environmental burden, with approximately 672 tonnes of PFOA (Fig. S14) remaining distributed across environmental compartments. In comparison, direct emissions to natural soil result in a slightly lower but still substantial distribution of around 606 tonnes (Fig. S15), while emissions to air yield the lowest total distributed mass, at approximately 344 tonnes (Fig. 10). These findings suggest that direct discharges to aquatic systems, particularly seawater, may pose the greatest risk of widespread environmental contamination, primarily due to PFOA's high water solubility, low degradability, and strong persistence in marine environments. Emissions to soil also result in considerable long-term distribution, highlighting the potential for leaching and subsequent transport to groundwater or surface waters. In contrast, emissions to air, while still significant, are comparatively less persistent due to atmospheric dispersion and eventual deposition, leading to lower cumulative environmental mass. Overall, these results underscore the importance of controlling point-source discharges, especially to aquatic and terrestrial compartments, to mitigate the long-range transport and accumulation of persistent pollutants such as PFOA.
The compartmental mass distribution patterns of PFOA are visually summarized in Fig. 11, which illustrates the environmental partitioning of PFOA across air, water, soil, and sediment compartments within the continental scale and the moderate and arctic zones of the global scale. It is revealed that under a direct emission scenario of 1000 tonnes per year of PFOA released to air at the continental scale, the majority of the retained mass (∼84%) partitions into aquatic compartments. Within this domain, coastal seawater emerges as the dominant sink, accounting for approximately 74.5% of the total mass. A relatively small fraction (∼2.6%) remains in the atmospheric compartment, while around 13% distributes into soil compartments. Sediment compartments accumulate only negligible amounts, consistent with the physicochemical behavior of PFOA, including its high water solubility, low vapor pressure, limited sorption to organic matter, and low partitioning tendency to particulates, all of which contribute to its preferential distribution in the aqueous phase. In contrast, on the global scale, particularly within the moderate and arctic zones, the distribution is even more strongly toward the aquatic environment. Between 96% and 99% of the transported PFOA mass is found in water compartments, with over 77% specifically residing in deep ocean waters. The contributions of air and sediment compartments are minimal, and only 1–3% of the mass is retained in soil. These findings underscore PFOA's high mobility and persistence in the hydrosphere, as well as its propensity for long-term accumulation in marine environments, particularly coastal seawater at the continental level and deep ocean waters in the global zones. A similar compartmental distribution pattern is observed when direct emissions at the continental scale are released into seawater (see Fig. S16). In this scenario, nearly 100% of the emitted PFOA mass remains within aquatic compartments across all spatial scales. At the continental scale, the entirety of the retained mass is found in coastal seawater. At the global scale, both the moderate and arctic zones exhibit strong partitioning into deep ocean waters, with approximately 80% and 92%, respectively, of the transported mass residing in these compartments. The remaining fractions are distributed in surface ocean waters, with negligible amounts present in atmospheric, soil, or sediment compartments. In the case of direct emissions to natural soil at the continental scale (see Fig. S17), the distribution pattern differs slightly. Here, approximately 34% of the total mass is retained in soil compartments, while the remaining 66% partitions into aquatic media. Despite the differences in initial compartmental allocation, long-range transport dynamics to the global scale remain consistent across emission scenarios. For both the moderate and arctic zones, regardless of whether the initial emission occurs to air, water, or soil at the continental scale, the transported PFOA mass is found exclusively in aquatic compartments. In these zones, more than 78% of the mass accumulates in deep ocean waters, with the remainder residing in surface ocean layers. These results further highlight the dominant role of aquatic pathways in the global-scale transport and environmental persistence of PFOA, reinforcing its classification as a highly mobile and persistent organic pollutant with a strong tendency for long-term marine accumulation. It is important to note, however, that the results presented here should be interpreted as screening-level conceptual outputs, as parameter uncertainties (e.g., degradation rates and partition coefficients) limit accurate and reliable quantitative prediction of environmental concentrations at the global scale.
3.2 Environmental dynamics and fate of PFOA
Following the analysis of the steady-state transport of PFOA across environmental scales and compartments, this section extends the investigation to assess the dynamic behavior and environmental fate of PFOA at the compartmental level within the continental scale using the SimpleBox4Planet web application. The objective is to quantify the persistence of PFOA in individual environmental media by calculating the fate factor (see eqn (3)), a critical metric for evaluating a chemical substance's potential to persist and be transported across environmental compartments. Similar to the previous section, three discrete emission scenarios were modeled, each assuming a direct emission of 1000 tonnes per year into a specific environmental compartment at the continental scale: (i) air, (ii) seawater, and (iii) natural soil to evaluate the fate of PFOA.Fig. 12 presents the results of the dynamic simulation conducted over an 8-year period, using the SimpleBox4Planet web application to assess the time-dependent behavior of PFOA at the continental scale. The simulation assumes constant direct emissions to air at a rate of 1000 tonnes per year for the first 4 years, followed by zero emissions for the subsequent 4 years, to analyze both the buildup and decay phases of PFOA mass in each environmental compartment. The y-axis displays the normalized mass (i.e., the ratio of dynamic mass to steady-state mass), allowing for the clear identification of the time to steady-state, as a normalized value of 1 indicates that equilibrium has been reached. The corresponding time to reach the steady state, expressed in days, is also shown within the figure. As described by the first-order differential equation governing mass transport and degradation (see eqn (1)), the time required for a compartment to reach the steady state under constant emissions is equivalent to the time required for the mass to decay to negligible levels following cessation of emissions. The results for emissions directed to seawater and natural soil are shown in Fig. S18 and S19 in the SI, respectively. In all three scenarios, PFOA concentrations across compartments reach steady-state in less than 4 years.
The fate factor is next calculated using the year 4 time point, at which the steady state was confirmed, and by determining the residence time of PFOA in each compartment under the different emission scenarios. The results are summarized in Table 1. Under the air emission scenario, coastal seawater exhibited the highest persistence, with an estimated fate factor of approximately 45 days, followed by agricultural soil (∼6 days) and freshwater (∼5 days). Lower persistence was observed in air (∼1.6 days), lakes and natural soil (∼1 day each), while other compartments exhibited negligible retention. In the scenario of direct emissions to seawater, coastal seawater again demonstrated dominant retention, with an exceptionally high persistence of approximately 136 days, while no meaningful persistence was observed in any other compartment. This finding reinforces the strong affinity of PFOA for the aqueous phase. In the natural soil emission scenario, the highest fate factors were observed in both natural soil and coastal seawater, with residence times of approximately 54 days. Notably, freshwater also showed high persistence (∼47 days) with freshwater lakes showing moderate persistence (∼11 days), while agricultural soil retained PFOA for a shorter duration (∼2 days), and air again contributed negligibly. These patterns agree with the steady-state simulations, where transport to aquatic systems remained dominant, even when emissions originated from terrestrial compartments. Overall, these dynamic simulations further highlight that PFOA, regardless of the emission scenario, rapidly partitions into aquatic compartments where it exhibits high environmental persistence, especially in coastal ocean waters. The integration of time-dependent modelling provides a more comprehensive perspective on compartment-specific residence times, offering valuable insights for regulatory risk assessments and the development of mitigation strategies for persistent organic pollutants like PFOA.
| Air | Seawater | Natural soil | |
|---|---|---|---|
| Air | 1.6 | 0.0 | 0.5 |
| Fresh water lake | 1.1 | 0.0 | 10.6 |
| Fresh water | 4.6 | 0.0 | 46.5 |
| Coastal seawater | 45.3 | 136.4 | 53.6 |
| Natural soil | 1.4 | 0.0 | 54.3 |
| Agricultural soil | 6.1 | 0.0 | 2.1 |
| Other soil | 0.5 | 0.0 | 0.2 |
While multimedia fate modelling provides essential information on persistence, long-range transport and environmental distribution, Safe-and-Sustainable-by-Design (SSbD) assessments, such as those outlined in the European Commission's SSbD framework and the OECD guidance on safer chemical alternatives, also require integration with toxicological and ecotoxicological evidence, including hazard characterisation, bioaccumulation potential and mechanistic toxicity endpoints.84SimpleBox4Planet therefore serves as the environmental exposure component of SSbD evaluation and should be complemented with substance-specific hazard data and life-cycle considerations to support comprehensive, safe-and-sustainable chemical substitution.84 A key advantage of SimpleBox4Planet is that its output format is suited for direct upload into other tools in the Enalos Cloud Platform such as LungDepo62 and NanoBioAccumulate.64
3.3 Alternatives to PFOA
The SimpleBox4Planet web application offers comparative environmental assessments by allowing users to input and simulate the environmental behavior of a wide range of chemicals, including PFAS substitutes, under identical emission scenarios. Specifically, users can compare the total mass distributed, which is particularly valuable in the context of assessing replacements such as short-chain PFASs (e.g., perfluorobutanesulfonic acid, PFBS), fluorine-free or bio-based alternatives such as starch (corn starch), sodium alginate, chitosan, glycerol and acetic acid, or other synthetic hydrophobic agents such as methyltrimethoxysilane and trimethoxyphenylsilane, which are currently being explored in the literature as potential alternatives to legacy compounds like PFOA.85,86 Although 530 PFAS-free alternatives were already identified in the literature,50 the objective here was not to screen all possible alternatives, but rather to demonstrate the comparative capability of the SimpleBox4Planet web application using a representative subset of alternatives. The selected alternatives were chosen based on their chemical diversity, enabling illustration of the model's behaviour across bio-based materials, short-chain PFASs, and synthetic hydrophobic agents, the availability of complete physicochemical property data and their industrial and environmental relevance.Fig. 13 presents the total environmental mass distribution for PFOA and its proposed alternatives across all environmental scales under three distinct continental-scale emission scenarios: (i) direct emission to air (1000 tonnes per year), (ii) direct emission to seawater (1000 tonnes per year), and (iii) direct emission to natural soil (1000 tonnes per year). The results reveal clear distinctions in environmental mass distribution depending on both the chemical identity and the emission pathway. Under the air emission scenario, sodium alginate shows a higher total environmental mass than PFOA, with methyltrimethoxysilane closely matching PFOA in magnitude. All other alternatives, including corn starch, PFBS, chitosan, glycerol, and acetic acid, exhibit significantly lower environmental retention, suggesting reduced persistence and transport. For emissions to seawater, PFOA, sodium alginate, and methyltrimethoxysilane show similar mass distribution patterns. Meanwhile, corn starch, PFBS, chitosan, and trimethoxyphenylsilane display moderately lower mass but still remain environmentally significant. Notably, acetic acid and glycerol have the lowest environmental mass, indicating rapid degradation or limited intermedia transport capacity. In the case of emissions to natural soil, corn starch displays a total environmental mass approximately twice that of PFOA, with sodium alginate slightly exceeding PFOA's value. Methyltrimethoxysilane remains within a comparable range, while all other compounds including PFBS, chitosan, glycerol, and acetic acid, demonstrate substantially lower environmental persistence. Of particular interest, acetic acid and glycerol consistently exhibit the lowest total environmental mass across all emission scenarios, suggesting that they may be among the most environmentally benign alternatives to PFOA.
The behaviour of PFBS, while often regarded as a lower-impact short-chain PFAS, appears highly dependent on the emission scenario. Although it shows lower mass distribution in the emission to air and soil cases, its environmental persistence remains significant under seawater emission, indicating that chain length alone does not guarantee environmental safety. These simulations suggest that acetic acid and glycerol represent promising environmentally friendly alternatives to PFOA, given their minimal persistence and transport potential. However, it is essential to complement these multimedia fate assessments with toxicological evaluations, including bioaccumulation, ecotoxicity, and human health impact studies, to ensure their overall safety. The SimpleBox4Planet platform proves to be a valuable regulatory support tool, offering quantitative assessments of how chemical emissions influence environmental distribution, thereby aiding in the prevention, mitigation, and informed substitution of harmful substances within regulatory and industrial frameworks without the need for advanced mathematical skills.
3.4 Statistical significance of the physicochemical properties of PFOA
One of the key advantages of the SimpleBox4Planet platform is its capability to explore the impact of numerous emission and environmental scenarios with minimal manual effort. In this section, we leverage this capability to investigate the influence of physicochemical property variability on the total environmental mass distribution of PFOA, specifically following direct emissions to seawater at the continental scale, a scenario previously identified as producing the highest environmental burden. To systematically assess the sensitivity and significance of selected physicochemical parameters, a Plackett–Burman (DoE) design, a type of fractional factorial design that assumes interactions can be completely ignored and thus that the main effects can be calculated with a reduced number of experiments, was employed using the Isalos Data Analytics Platform.87–89 A total of 20 experimental combinations were simulated based on the Plackett–Burman design that varied key properties within their defined minimum and maximum bounds (see Table 2). The physicochemical properties explored included the soil–water partition coefficient (Ksw), air–water partition coefficient (Kaw), octanol–water partition coefficient (Kow), acid dissociation constant (pKa), melting temperature (Tm), vapour pressure at 25 °C (Pvap25) and solubility at 25 °C (Sol25). The range of values selected for each property reflects bounds relevant to PFOA's speciation, encompassing both its neutral and anionic forms.| T m (°C) | pKa | P vap25 (Pa) | Sol25 (mg L−1) | K aw | K ow | K sw | |
|---|---|---|---|---|---|---|---|
| Min | 40 | 0 | 0.1 | 500 | 8.08 × 10−8 | 83 | 1 |
| Max | 60 | 3.8 | 12 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.00 × 10−6 | 3981 | 100 |
Fig. 14 presents the Pareto analysis (the 80/20 rule to determine the strongest contributing factors), and Table 3 summarizes the results of the analysis of variance (ANOVA)-based regression modelling, both computed using the Isalos Data Analytics Platform based on simulation data generated by SimpleBox4Planet for the total mass of PFOA distributed across the environment (see Table S8 in the SI). It is important to note that the Pareto chart plays a prioritization role following the Pareto principle (80/20 rule), where the parameters (factors) whose cumulative contribution reaches approximately 80% variability are considered the most influential, whereas those contributing the remaining 20% are treated as less important. The red dotted threshold line in Fig. 14 visually marks this 80% cut-off, clearly distinguishing the dominant parameters, thereby identifying those factors that most strongly drive PFOA's environmental fate. Prior to discussing this statistical analysis, it is worth noting that the total environmental mass of PFOA distributed across compartments under the 20 Plackett–Burman design scenarios ranges narrowly from 675.49 tonnes to 679.49 tonnes. This illustrates that even modest changes within realistic ranges still yield consistently high environmental retention. This observation confirms that, while PFOA's distribution behaviour remains generally robust, small parameter variations can influence its multimedia fate and should not be overlooked. The results of the Pareto chart and ANOVA analysis both reveal that Ksw, the soil–water partition coefficient, has the most significant influence on the total environmental mass distribution of PFOA. Additionally, Kow (the octanol–water partition coefficient) is identified as a statistically significant contributor. The rest of the physicochemical properties, including Kaw, Pvap25, Sol25, and Tm, are found not to be statistically important, within the tested parameter space. These findings underscore a critical modelling insight into the application of multimedia environmental fate models such as SimpleBox: the accurate specification of partition coefficients, particularly those governing water–soil and water–organic phase interactions, is critical to ensuring the reliability of model predictions. Given PFOA's strong intermedia mobility, hydrophilicity, and persistence, small inaccuracies in these parameters can propagate through simulations and meaningfully affect the outcomes. As such, great care should be taken when sourcing, estimating, or experimentally determining these properties during model setup, especially in the context of regulatory assessments or comparative evaluations of chemical alternatives.
| DF | Adjusted SS | Adjusted MS | F-Value | P-Value | |
|---|---|---|---|---|---|
| T m | 1 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041 041 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041 041 |
0.60 | 0.453 |
| pKa | 1 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 897 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 897 |
0.60 | 0.454 |
| P vap25 | 1 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 057 057 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 057 057 |
0.60 | 0.453 |
| Sol25 | 1 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 955 955 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 955 955 |
0.60 | 0.454 |
| K aw | 1 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 628 628 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 628 628 |
0.60 | 0.455 |
| K ow | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255 255 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255 255 |
15.00 | 0.002 |
| K sw | 1 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 286 286![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 286 286![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
735.16 | 0.000 |
4 Discussion and conclusion
This work presents the development and implementation of SimpleBox4Planet, a web-based environmental fate modelling tool that integrates the functionality of the SimpleBox 4.04 fugacity-based multimedia model within a user-friendly graphical interface and facilitates further analysis of factors driving environmental fate via Design of Experiments using the Isalos platform. SimpleBox4Planet is a freely accessible web application hosted on the Enalos Cloud platform (https://www.enaloscloud.novamechanics.com/proplanet/simplebox4planet/or https://www.enaloscloud.novamechanics.com/chiasma/simplebox4planet/), for quantifying the environmental fate and transport of chemical substances, demonstrated here via a case study on persistent and emerging pollutants such as per- and polyfluoroalkyl substances (PFASs) and their alternatives. The web application supports both steady-state and dynamic modelling, allowing users to investigate time-dependent chemical behaviour and fate factor computation. Through its incorporation of RESTful APIs and automated CAS-based property retrieval via the EPA CompTox Dashboard,14SimpleBox4Planet enhances FAIR compliance and ensures interoperability with other environmental and regulatory tools. Its comparative analysis capability further provides a decision-support mechanism for identifying more sustainable alternatives to persistent chemicals such as PFASs, thereby addressing urgent regulatory and public health challenges. Overall, in comparison to prior SimpleBox-based tools, such as SimpleBox,5 SimpleBox4Nano90 and SimpleBox4Plastic,91 the SimpleBox4Planet web application eliminates the need for complex Excel workbooks and external R scripts by providing a single, browser-based platform that performs steady-state, quasi-dynamic, and fate-factor calculations. It enables fast, transparent parameterization through a clean, sectioned GUI, providing direct controls for emissions and landscape settings instead of multi-tab spreadsheets, and offers multi-scale visualization (regional, continental, and global zones) with clear tabular and graphical outputs. A PFAS baseline (∼4000 substances) is included with physicochemical properties retrieved from CompTox to support comparative assessments of PFASs and non-PFAS alternatives. SimpleBox4Planet exposes REST APIs to integrate results into regulatory and SSbD workflows, which was not directly achievable in prior spreadsheet-based implementations.The case study on PFOA presented in this work demonstrated the capability of SimpleBox4Planet in characterizing environmental distribution patterns across different emission scenarios in air, seawater and soil. Consistent with the literature,40,79,82 PFOA showed high mobility and long-term persistence in aquatic systems, with dominant partitioning into coastal and deep ocean waters and a marked tendency for long-range transport toward arctic and moderate climatic zones. Dynamic simulations further verified the environmental persistence of PFOA, particularly in aqueous compartments, with coastal seawater exhibiting the highest residence times across all emission pathways. The comparative analysis of the proposed alternatives, including PFBS, chitosan, acetic acid, and glycerol, showed that fluorine-free alternatives such as acetic acid and glycerol exhibited significantly lower total environmental burdens than PFOA, providing new insights into environmentally preferable substitutes. Moreover, sensitivity analyses based on a Plackett–Burman design, facilitated through integration of SimpleBox4Planet and Isalos Analytics Platform, emphasized the critical influence of soil–water and octanol–water partition coefficients on overall environmental distribution, highlighting the importance of accurately defining key physicochemical parameters during fate modelling. Collectively, the findings validate SimpleBox4Planet as a scientifically robust and policy-relevant platform, with enhanced user-friendliness and extended analysis capabilities relative to SimpleBox, for supporting chemical safety assessments and regulatory decision-making.
Future work will focus on extending the platform's capabilities by coupling it with Life Cycle Impact Assessment (LCIA) frameworks such as USEtox13,92 to facilitate comprehensive evaluations of both environmental and human health risks, bridging the gap between multimedia fate modelling and impact quantification. Such an integration has been demonstrated previously for modelling the environmental fate of nanomaterials (using SimpleBox4Nano), in which two main adaptations were made: (i) merging of the compartments of air and rain and (ii) accounting for the sum of the free, aggregated, and attached species in the receiving compartments, via which it was extended to estimate characterisation factors (CF) for nano-TiO2 for the impact category of freshwater ecotoxicity in life cycle assessment (LCA).92 To improve the parameterization process for chemical inputs, SimpleBox4Planet will be coupled with automated workflows that incorporate Quantitative Structure–Activity/Property Relationships (QSAR/QSPR), machine learning (ML) algorithms, and quantum mechanical (QM) computations.57,93 This integrative approach will enable the rapid and accurate estimation of critical physicochemical properties (such as partition coefficients, degradation kinetics, solubilities, melting temperatures and vapor pressures), thereby increasing the model's predictive accuracy. The platform's scope will also be expanded to support the modelling of emerging contaminants, particularly engineered nanomaterials (ENMs) and micro- and nanoplastics (MNPs), using the existing extensions of SimpleBox for nanomaterials (SimpleBox4Nano),90 and the web application of this, https://enaloscloud.novamechanics.com/nanosolveit/simplebox4nano/. These advances will collectively transform SimpleBox4Planet into a comprehensive, next-generation environmental fate modelling platform—capable of supporting regulatory compliance, and the development of safe and sustainable-by-design (SSbD) chemicals, materials, and products across their entire life cycles.
Author contributions
Conceptualization: D. M. and A. A.; methodology: D. M.; software: D. M., C. P., and A. T.; supervision: G. M., J. S., R. H., I. L., and A. A.; writing—original draft preparation: D. M.; writing—review and editing: D. M., C. P., D.-D. V., A. T., G. M., J. S., M. M., A. R., T. S., R. H., I. L., and A. A.; funding acquisition: J. S., A. R., T. S., R. H., I. L., and A. A.Conflicts of interest
There are no conflicts to declare.Data availability
Supplementary information (SI): comprehensive analysis of the outcomes generated by using the SimpleBox4Planet web-tool. See DOI: https://doi.org/10.1039/d5su00622h.Acknowledgements
This research has received funding from the European Union's HORIZON Europe PROPLANET project (GA 101091842) for the steady-state fate modelling approach development. The implementation of dynamic simulations and the comparative assessment feature have been completed within the European Union's HORIZON Europe CHIASMA project (GA 101137613) with UoB's funding for participation in CHIASMA coming from UKRI/Innovate UK under the Horizon Europe Guarantee Fund (Project No. 10101594) and Empa's funding for participation in CHIASMA coming from the Swiss State Secretariat for Education, Research and Innovation (SERI – Project No. 24.00029).References
- Z. Rong-Rong, Z. Che-Sheng, H. Zhong-Peng and S. Xiao-Meng, Review of environmental multimedia models, Environ. Forensics, 2012, 13(3), 216–224 Search PubMed.
- C. Hsieh and J. Ouimette, Comparative study of multimedia modeling for dynamic partitioning of fossil fuels-related pollutants, J. Hazard. Mater., 1994, 37(3), 489–505 CrossRef CAS.
- Y. Cohen and E. J. Cooter, Multimedia environmental distribution of toxics (Mend-Tox). I: Hybrid compartmental-spatial modeling framework, Practice Periodical of Hazardous, Toxic, and Radioactive Waste Management, 2002, vol. 6, iss. 2, pp. 70–86 Search PubMed.
- M. Schoorl, A. Hollander and D. van de Meent, SimpleBox 4.0. A Multimedia Mass Balance Model for Evaluating the Fate of Chemical Substances, RIVM Report 2015, vol. 161 Search PubMed.
- A. Hollander, M. Schoorl and D. van de Meent, SimpleBox 4.0: Improving the model while keeping it simple, Chemosphere, 2016, 148, 99–107 CrossRef CAS.
- L. Brandes and H. Den Hollander, SimpleBox 2.0: a Nested Multimedia Fate Model for Evaluating the Environmental Fate of Chemicals, 1996 Search PubMed.
- H. Den Hollander, J. Van Eijkeren and D. Van de Meent, SimpleBox 3.0: Multimedia Mass Balance Model for Evaluating the Fate of Chemicals in the Environment, National Institute for Public Health and the Environment (RIVM), Bilthoven (NL), 2004, Report, 601200003 Search PubMed.
- D. Meent, SimpleBox: A Generic Multimedia Fate Evaluation Model, National Institute of Public Health and Environmental Protection, 1993 Search PubMed.
- D. Mackay, Finding fugacity feasible, Environ. Sci. Technol., 1979, 13(10), 1218–1223 CrossRef CAS.
- D. Mackay and S. Paterson, Calculating fugacity, Environ. Sci. Technol., 1981, 15(9), 1006–1014 Search PubMed.
- D. Mackay and S. Paterson, Fugacity revisited, Environ. Sci. Technol., 1982, 16(12), 654A–660A CAS.
- C. Su, H. Zhang, C. Cridge and R. Liang, A review of multimedia transport and fate models for chemicals: Principles, features and applicability, Sci. Total Environ., 2019, 668, 881–892 CrossRef CAS.
- R. K. Rosenbaum, T. M. Bachmann, L. S. Gold, M. A. Huijbregts, O. Jolliet, R. Juraske, A. Koehler, H. F. Larsen, M. MacLeod and M. Margni, USEtox—the UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment, Int. J. Life Cycle Assess., 2008, 13, 532–546 CrossRef CAS.
- A. J. Williams, C. M. Grulke, J. Edwards, A. D. McEachran, K. Mansouri, N. C. Baker, G. Patlewicz, I. Shah, J. F. Wambaugh and R. S. Judson, The CompTox Chemistry Dashboard: a community data resource for environmental chemistry, J. Cheminf., 2017, 9, 1–27 Search PubMed.
- N. M. Brennan, A. T. Evans, M. K. Fritz, S. A. Peak and H. E. von Holst, Trends in the regulation of per-and polyfluoroalkyl substances (PFAS): a scoping review, Int. J. Environ. Res. Public Health, 2021, 18(20), 10900 CrossRef PubMed.
- S. Brendel, É. Fetter, C. Staude, L. Vierke and A. Biegel-Engler, Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH, Environ. Sci. Eur., 2018, 30, 1–11 CrossRef CAS PubMed.
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. De Voogt, A. A. Jensen, K. Kannan, S. A. Mabury and S. P. van Leeuwen, Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins, Integr. Environ. Assess. Manage., 2011, 7(4), 513–541 Search PubMed.
- S. Kurwadkar, J. Dane, S. R. Kanel, M. N. Nadagouda, R. W. Cawdrey, B. Ambade, G. C. Struckhoff and R. Wilkin, Per-and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution, Sci. Total Environ., 2022, 809, 151003 CrossRef CAS.
- J. W. Martin, S. A. Mabury, K. R. Solomon and D. C. Muir, Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss), Environ. Toxicol. Chem., 2003, 22(1), 189–195 CrossRef CAS.
- J. M. Conder, R. A. Hoke, W. d. Wolf, M. H. Russell and R. C. Buck, Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds, Environ. Sci. Technol., 2008, 42(4), 995–1003 Search PubMed.
- C. F. Kwiatkowski, D. Q. Andrews, L. S. Birnbaum, T. A. Bruton, J. C. DeWitt, D. R. Knappe, M. V. Maffini, M. F. Miller, K. E. Pelch and A. Reade, Scientific basis for managing PFAS as a chemical class, Environ. Sci. Technol. Lett., 2020, 7(8), 532–543 CrossRef CAS PubMed.
- L. H. Weinstein and A. Davison, Fluorides in the Environment: Effects on Plants and Animals, Cabi, 2004 Search PubMed.
- Z. Wang, I. T. Cousins, M. Scheringer and K. Hungerbuehler, Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions, Environ. Int., 2015, 75, 172–179 CrossRef CAS.
- E. M. Sunderland, X. C. Hu, C. Dassuncao, A. K. Tokranov, C. C. Wagner and J. G. Allen, A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects, J. Exposure Sci. Environ. Epidemiol., 2019, 29(2), 131–147 CrossRef CAS PubMed.
- M. Kotthoff and M. Bücking, Four chemical trends will shape the next decade's directions in perfluoroalkyl and polyfluoroalkyl substances research, Front. Chem., 2018, 6, 103 Search PubMed.
- K. Sznajder-Katarzyńska, M. Surma and I. Cieślik, A review of perfluoroalkyl acids (PFAAs) in terms of sources, applications, human exposure, dietary intake, toxicity, legal regulation, and methods of determination, J. Chem., 2019, 1, 2717528 Search PubMed.
- J. Glüge, M. Scheringer, I. T. Cousins, J. C. DeWitt, G. Goldenman, D. Herzke, R. Lohmann, C. A. Ng, X. Trier and Z. Wang, An overview of the uses of per-and polyfluoroalkyl substances (PFAS), Environ. Sci.: Processes Impacts, 2020, 22(12), 2345–2373 RSC.
- E. Kissa, Fluorinated Surfactants and Repellents, CRC Press, 2001 Search PubMed.
- R. E. Banks, B. E. Smart and J. Tatlow, Organofluorine Chemistry: Principles and Commercial Applications, Springer Science & Business Media, 2013 Search PubMed.
- Z. Wang, J. C. DeWitt, C. P. Higgins and I. T. Cousins, A Never-Ending Story of Per-And Polyfluoroalkyl Substances (PFASs)?, ACS Publications, 2017 Search PubMed.
- X. Li, Y. Wang, J. Cui, Y. Shi and Y. Cai, Occurrence and fate of per-and polyfluoroalkyl substances (PFAS) in atmosphere: Size-dependent gas-particle partitioning, precipitation scavenging, and amplification, Environ. Sci. Technol., 2024, 58(21), 9283–9291 CrossRef CAS.
- B. Sha, J. H. Johansson, P. Tunved, P. Bohlin-Nizzetto, I. T. Cousins and M. E. Salter, Sea spray aerosol (SSA) as a source of perfluoroalkyl acids (PFAAs) to the atmosphere: field evidence from long-term air monitoring, Environ. Sci. Technol., 2021, 56(1), 228–238 CrossRef.
- M. Sörengård, J. Kikuchi, K. Wiberg and L. Ahrens, Spatial distribution and load of per-and polyfluoroalkyl substances (PFAS) in background soils in Sweden, Chemosphere, 2022, 295, 133944 Search PubMed.
- N. Quinete, Q. Wu, T. Zhang, S. H. Yun, I. Moreira and K. Kannan, Specific profiles of perfluorinated compounds in surface and drinking waters and accumulation in mussels, fish, and dolphins from southeastern Brazil, Chemosphere, 2009, 77(6), 863–869 CrossRef CAS PubMed.
- L. Ahrens and M. Bundschuh, Fate and effects of poly-and perfluoroalkyl substances in the aquatic environment: A review, Environ. Toxicol. Chem., 2014, 33(9), 1921–1929 CrossRef CAS.
- W. A. Gebbink and S. P. van Leeuwen, Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands, Environ. Int., 2020, 137, 105583 Search PubMed.
- C. Lau, K. Anitole, C. Hodes, D. Lai, A. Pfahles-Hutchens and J. Seed, Perfluoroalkyl acids: a review of monitoring and toxicological findings, Toxicol. Sci., 2007, 99(2), 366–394 CrossRef CAS.
- M. Liu, G. Zhang, L. Meng, X. Han, Y. Li, Y. Shi, A. Li, M. E. Turyk, Q. Zhang and G. Jiang, Associations between novel and legacy per-and polyfluoroalkyl substances in human serum and thyroid cancer: a case and healthy population in Shandong Province, East China, Environ. Sci. Technol., 2021, 56(10), 6144–6151 Search PubMed.
- I. T. Cousins, G. Goldenman, D. Herzke, R. Lohmann, M. Miller, C. A. Ng, S. Patton, M. Scheringer, X. Trier and L. Vierke, The concept of essential use for determining when uses of PFASs can be phased out, Environ. Sci.: Processes Impacts, 2019, 21(11), 1803–1815 Search PubMed.
- K. Prevedouros, I. T. Cousins, R. C. Buck and S. H. Korzeniowski, Sources, fate and transport of perfluorocarboxylates, Environ. Sci. Technol., 2006, 40(1), 32–44 Search PubMed.
- I. T. Cousins, J. H. Johansson, M. E. Salter, B. Sha and M. Scheringer, Outside the safe operating space of a new planetary boundary for per-and polyfluoroalkyl substances (PFAS), Environ. Sci. Technol., 2022, 56(16), 11172–11179 CrossRef CAS PubMed.
- J. P. Benskin, D. C. Muir, B. F. Scott, C. Spencer, A. O. De Silva, H. Kylin, J. W. Martin, A. Morris, R. Lohmann and G. Tomy, Perfluoroalkyl acids in the Atlantic and Canadian Arctic oceans, Environ. Sci. Technol., 2012, 46(11), 5815–5823 CrossRef CAS PubMed.
- B. J. Ruyle, H. M. Pickard, D. R. LeBlanc, A. K. Tokranov, C. P. Thackray, X. C. Hu, C. D. Vecitis and E. M. Sunderland, Isolating the AFFF signature in coastal watersheds using oxidizable PFAS precursors and unexplained organofluorine, Environ. Sci. Technol., 2021, 55(6), 3686–3695 CrossRef CAS.
- L. Ahrens, M. Shoeib, T. Harner, S. C. Lee, R. Guo and E. J. Reiner, Wastewater treatment plant and landfills as sources of polyfluoroalkyl compounds to the atmosphere, Environ. Sci. Technol., 2011, 45(19), 8098–8105 CrossRef CAS PubMed.
- J. L. Guelfo and C. P. Higgins, Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites, Environ. Sci. Technol., 2013, 47(9), 4164–4171 CrossRef CAS PubMed.
- C. A. Ng and K. Hungerbühler, Bioconcentration of perfluorinated alkyl acids: how important is specific binding?, Environ. Sci. Technol., 2013, 47(13), 7214–7223 CrossRef CAS PubMed.
- M. L. Brusseau, Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface, Sci. Total Environ., 2018, 613, 176–185 CrossRef PubMed.
- L. Vierke, A. Möller and S. Klitzke, Transport of perfluoroalkyl acids in a water-saturated sediment column investigated under near-natural conditions, Environ. Pollut., 2014, 186, 7–13 Search PubMed.
- P. Zareitalabad, J. Siemens, M. Hamer and W. Amelung, Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater–A review on concentrations and distribution coefficients, Chemosphere, 2013, 91(6), 725–732 Search PubMed.
- R. Figuière, L. T. Miaz, E. Savvidou and I. T. Cousins, An Overview of Potential Alternatives for the Multiple Uses of Per-and Polyfluoroalkyl Substances, Environ. Sci. Technol., 2025, 59(4), 2031–2042 CrossRef.
- F. T. Jahura, N.-U.-S. Mazumder, M. T. Hossain, A. Kasebi, A. Girase and R. B. Ormond, Exploring the Prospects and Challenges of Fluorine-Free Firefighting Foams (F3) as Alternatives to Aqueous Film-Forming Foams (AFFF): A Review, ACS Omega, 2024, 9(36), 37430–37444 CrossRef CAS PubMed.
- G. B. Post, P. D. Cohn and K. R. Cooper, Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature, Environ. Res., 2012, 116, 93–117 Search PubMed.
- B. O. Clarke and S. R. Smith, Review of ‘emerging’organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids, Environ. Int., 2011, 37(1), 226–247 Search PubMed.
- R. K. Rosenbaum, M. Margni and O. Jolliet, A flexible matrix algebra framework for the multimedia multipathway modeling of emission to impacts, Environ. Int., 2007, 33(5), 624–634 CrossRef.
- D.-D. Varsou, A. Tsoumanis, A. Afantitis and G. Melagraki, Enalos Cloud Platform: Nanoinformatics and Cheminformatics Tools, Ecotoxicological QSARs, 2020, pp. 789–800 Search PubMed.
- M. Stäuble and H.-J. Schumacher, ZK Developer's Guide, Packt Publishing Ltd, 2008 Search PubMed.
- M. Mudlaff, A. Sosnowska, L. Gorb, N. Bulawska, K. Jagiello and T. Puzyn, Environmental impact of PFAS: Filling data gaps using theoretical quantum chemistry and QSPR modeling, Environ. Int., 2024, 185, 108568 CrossRef CAS PubMed.
- T. Vermeire, D. Jager, B. Bussian, J. Devillers, K. Den Haan, B. Hansen, I. Lundberg, H. Niessen, S. Robertson and H. Tyle, European union system for the evaluation of substances (EUSES). Principles and structure, Chemosphere, 1997, 34(8), 1823–1836 CrossRef CAS PubMed.
- T. Vermeire, M. Rikken, L. Attias, P. Boccardi, G. Boeije, D. Brooke, J. De Bruijn, M. Comber, B. Dolan and S. Fischer, European union system for the evaluation of substances: the second version, Chemosphere, 2005, 59(4), 473–485 Search PubMed.
- M. D. Wilkinson, M. Dumontier, I. J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J.-W. Boiten, L. B. da Silva Santos and P. E. Bourne, The FAIR Guiding Principles for scientific data management and stewardship, Sci. Data, 2016, 3(1), 1–9 Search PubMed.
- M. Barker, N. P. Chue Hong, D. S. Katz, A.-L. Lamprecht, C. Martinez-Ortiz, F. Psomopoulos, J. Harrow, L. J. Castro, M. Gruenpeter and P. A. Martinez, Introducing the FAIR Principles for research software, Sci. Data, 2022, 9(1), 622 CrossRef.
- D. G. Mintis, D.-D. Varsou, P. D. Kolokathis, A. Tsoumanis, G. Melagraki, J. P. Seif, A. J. del Real, I. Lynch and A. Afantitis, LungDepo: modelling the regional particle deposition in the human lung via the Enalos Cloud Platform, Environ. Sci.: Nano, 2025, 12(8), 3921–3937 RSC.
- C. Papavasiliou, D. G. Mintis, A. Tsoumanis, A. Karaoli, I. Lynch, S. Krause, D. D. Varsou, G. Melagraki, M. Kavousanakis and A. Afantitis, MicroPlasticFate web application: Multimedia environmental fate modelling of microplastic particles via the enalos cloud platform, Bioresour. Technol. Rep., 2025, 102157 CrossRef CAS.
- D. G. Mintis, N. Cheimarios, A. Tsoumanis, A. G. Papadiamantis, N. W. van den Brink, H. J. van Lingen, G. Melagraki, I. Lynch and A. Afantitis, NanoBioAccumulate: Modelling the uptake and bioaccumulation of nanomaterials in soil and aquatic invertebrates via the Enalos DIAGONAL Cloud Platform, Comput. Struct. Biotechnol. J., 2024, 25, 243–255 CrossRef CAS PubMed.
- P. D. Kolokathis, D. Zouraris, N. K. Sidiropoulos, A. Tsoumanis, G. Melagraki, I. Lynch and A. Afantitis, NanoTube Construct: A web tool for the digital construction of nanotubes of single-layer materials and the calculation of their atomistic descriptors powered by Enalos Cloud Platform, Comput. Struct. Biotechnol. J., 2024, 25, 230–242 CrossRef CAS PubMed.
- D. D. Varsou, A. Afantitis, A. Tsoumanis, A. Papadiamantis, E. Valsami-Jones, I. Lynch and G. Melagraki, Zeta-potential read-across model utilizing nanodescriptors extracted via the nanoxtract image analysis tool available on the enalos nanoinformatics cloud platform, Small, 2020, 16(21), 1906588 CrossRef CAS PubMed.
- I. Lynch, A. Afantitis, T. Exner, M. Himly, V. Lobaskin, P. Doganis, D. Maier, N. Sanabria, A. G. Papadiamantis and A. Rybinska-Fryca, Can an InChI for nano address the need for a simplified representation of complex nanomaterials across experimental and nanoinformatics studies?, Nanomaterials, 2020, 10(12), 2493 Search PubMed.
- P. D. Kolokathis, D. Zouraris, E. Voyiatzis, N. K. Sidiropoulos, A. Tsoumanis, G. Melagraki, K. Tämm, I. Lynch and A. Afantitis, NanoConstruct: A web application builder of ellipsoidal nanoparticles for the investigation of their crystal growth, stability, and the calculation of atomistic descriptors, Comput. Struct. Biotechnol. J., 2024, 25, 81–90 Search PubMed.
- D.-D. Varsou, L.-J. A. Ellis, A. Afantitis, G. Melagraki and I. Lynch, Ecotoxicological read-across models for predicting acute toxicity of freshly dispersed versus medium-aged NMs to Daphnia magna, Chemosphere, 2021, 285, 131452 CrossRef CAS.
- D.-D. Varsou, P. D. Kolokathis, M. Antoniou, N. K. Sidiropoulos, A. Tsoumanis, A. G. Papadiamantis, G. Melagraki, I. Lynch and A. Afantitis, In silico assessment of nanoparticle toxicity powered by the Enalos Cloud Platform: Integrating automated machine learning and synthetic data for enhanced nanosafety evaluation, Comput. Struct. Biotechnol. J., 2024, 25, 47–60 CrossRef CAS.
- P. D. Kolokathis, E. Voyiatzis, N. K. Sidiropoulos, A. Tsoumanis, G. Melagraki, K. Tämm, I. Lynch and A. Afantitis, ASCOT: a web tool for the digital construction of energy minimized Ag, CuO, TiO2 spherical nanoparticles and calculation of their atomistic descriptors, Comput. Struct. Biotechnol. J., 2024, 25, 34–46 CrossRef CAS.
- P. Tsiros, N. Cheimarios, A. Tsoumanis, A. Ø. Jensen, G. Melagraki, I. Lynch, H. Sarimveis and A. Afantitis, Towards an in silico integrated approach for testing and assessment of nanomaterials: from predicted indoor air concentrations to lung dose and biodistribution, Environ. Sci.: Nano, 2022, 9(4), 1282–1297 RSC.
- N. Cheimarios, B. Pem, A. Tsoumanis, K. Ilić, I. V. Vrček, G. Melagraki, D. Bitounis, P. Isigonis, M. Dusinska and I. Lynch, An in vitro dosimetry tool for the numerical transport modeling of engineered nanomaterials powered by the Enalos RiskGONE Cloud Platform, Nanomaterials, 2022, 12(22), 3935 CrossRef CAS.
- S. P. Bhavsar, N. Gandhi, M. L. Diamond, A. S. Lock, G. Spiers and M. C. A. de la Torre, Effects of estimates from different geochemical models on metal fate predicted by coupled speciation-fate models, Environ. Toxicol. Chem., 2008, 27(5), 1020–1030 CrossRef CAS PubMed.
- D. C. Burns, D. A. Ellis, H. Li, C. J. McMurdo and E. Webster, Experimental p K a determination for perfluorooctanoic acid (PFOA) and the potential impact of p K a concentration dependence on laboratory-measured partitioning phenomena and environmental modeling, Environ. Sci. Technol., 2008, 42(24), 9283–9288 CrossRef CAS PubMed.
- P. Meng, S. Deng, Z. Du, B. Wang, J. Huang, Y. Wang, G. Yu and B. Xing, Effect of hydro-oleophobic perfluorocarbon chain on interfacial behavior and mechanism of perfluorooctane sulfonate in oil-water mixture, Sci. Rep., 2017, 7(1), 44694 CrossRef PubMed.
- M. Zhang, K. Yamada, S. Bourguet, J. Guelfo and E. M. Suuberg, Vapor pressure of nine perfluoroalkyl substances (PFASs) determined using the Knudsen Effusion Method, J. Chem. Eng. Data, 2020, 65(5), 2332–2342 CrossRef CAS.
- J. Wang, L. N. Tran, J. Mendoza, K. Chen, L. Tian, Y. Zhao, J. Liu and Y.-H. Lin, Thermal transformations of perfluorooctanoic acid (PFOA): Mechanisms, volatile organofluorine emissions, and implications to thermal regeneration of granular activated carbon, J. Hazard. Mater., 2024, 479, 135737 CrossRef CAS PubMed.
- J. M. Armitage, M. MacLeod and I. T. Cousins, Modeling the global fate and transport of perfluorooctanoic acid (PFOA) and perfluorooctanoate (PFO) emitted from direct sources using a multispecies mass balance model, Environ. Sci. Technol., 2009, 43(4), 1134–1140 CrossRef CAS.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbühler, Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources, Environ. Int., 2014, 70, 62–75 CrossRef CAS.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbühler, Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: the remaining pieces of the puzzle, Environ. Int., 2014, 69, 166–176 CrossRef CAS PubMed.
- J. Armitage, I. T. Cousins, R. C. Buck, K. Prevedouros, M. H. Russell, M. MacLeod and S. H. Korzeniowski, Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources, Environ. Sci. Technol., 2006, 40(22), 6969–6975 Search PubMed.
- A. R. Bock and B. E. Laird, PFAS Regulations: Past and Present and Their Impact on Fluoropolymers, 2022 Search PubMed.
- E. Abbate, A. I. Garmendia, G. Bracalente, L. Mancini, D. Tosches, K. Rasmussen, M. J. Bennett, H. Rauscher and S. Sala, Safe and Sustainable by Design Chemicals and Materials-Methodological Guidance, 2024 Search PubMed.
- Y. Lu, Y. Liang, Z. Zhou, Y. Wang and G. Jiang, Possible Fluorinated Alternatives of PFOS and PFOA: Ready to Go?, ACS Publications, 2019 Search PubMed.
- W. Shi, Z. Zhang, M. Li, H. Dong and J. Li, Reproductive toxicity of PFOA, PFOS and their substitutes: A review based on epidemiological and toxicological evidence, Environ. Res., 2024, 250, 118485 Search PubMed.
- D.-D. Varsou, A. Tsoumanis, A. G. Papadiamantis, G. Melagraki and A. Afantitis, Isalos Predictive Analytics Platform: Cheminformatics, Nanoinformatics, and Data Mining Applications, in Machine Learning and Deep Learning in Computational Toxicology, Springer, 2023, pp. 223–242 Search PubMed.
- P. Adamou, E. Harkou, S. Bellomi, I. Barlocco, D. Mintis, A. Afantitis, J. J. Delgado, X. Chen, G. Manos and N. Dimitratos, H2 Production from Ammonia Borane: Integrating Experiments, Computational Fluid Dynamics, and Statistical Analysis for Predicting and Optimizing Process and Reactor Design, ChemCatChem, 2025, e00615 Search PubMed.
- A. Michael, P. Adamou, E. Harkou, C. Christodoulou, I. Barlocco, D. Mintis, A. Afantitis, J. J. Delgado, X. Chen and G. Manos, Application of CFD, statistical analysis and DoE for optimising H2 generation through ammonia borane catalytic hydrolysis: A novel approach for multiobjective optimisation, Chem. Eng. J., 2025, 170772 CrossRef CAS.
- J. A. Meesters, A. A. Koelmans, J. T. Quik, A. J. Hendriks and D. van de Meent, Multimedia modeling of engineered nanoparticles with SimpleBox4nano: model definition and evaluation, Environ. Sci. Technol., 2014, 48(10), 5726–5736 Search PubMed.
- J. Quik, J. Meesters and A. Koelmans, A multimedia model to estimate the environmental fate of microplastic particles, Sci. Total Environ., 2023, 882, 163437 CrossRef CAS.
- B. Salieri, R. Hischier, J. T. Quik and O. Jolliet, Fate modelling of nanoparticle releases in LCA: An integrative approach towards “USEtox4Nano”, J. Cleaner Prod., 2019, 206, 701–712 CrossRef CAS.
- A. Sosnowska, M. Mudlaff, L. Gorb, N. Bulawska, S. Zdybel, M. Bakker, W. Peijnenburg and T. Puzyn, Expanding the applicability domain of QSPRs for predicting water solubility and vapor pressure of PFAS, Chemosphere, 2023, 340, 139965 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |













