 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Moisture sorption of cellulose-based porous media containing co-solvents and its impact on pore-fiber transport rates of co-solvent solutions
Sajjad
Karimnejad
and
Anton A.
Darhuber
 *
*
Fluids & Flows Group, Department of Applied Physics, Eindhoven University of Technology, Postbus 513, 5600MB Eindhoven, The Netherlands. E-mail: a.a.darhuber@tue.nl
First published on 23rd December 2025
Abstract
Water-based inkjet inks typically contain non-volatile, polar compounds – referred to as co-solvents – such as glycerol and ethylene glycol oligomers, which constitute approximately 5–50 wt% of the total ink. The hygroscopic nature of both paper and co-solvents makes their interplay with atmospheric moisture a critical factor in controlling the penetration and drying dynamics of ink, as well as the long-term mechanical and morphological stability of the printed paper. In this study, we systematically investigate how co-solvent deposition influences equilibrium moisture uptake and how the ambient humidity influences the ink absorption dynamics into cellulose fibers. We find that co-solvent addition substantially increases moisture uptake and eliminates the sorption hysteresis present in paper. The moisture sorption of co-solvent-infused paper is well-predicted by the mass-weighted average of the individual, single-material sorption isotherms of paper and co-solvent. The rate of pore-fiber transport of co-solvents was observed to depend sensitively on ambient humidity, the presence of predeposited liquids as well as the addition of surfactants and divalent salts.
1. Introduction
Inkjet printing is a non-contact digital deposition technique widely used in both graphic applications and functional device manufacturing due to its high resolution, scalability, and flexibility regarding ink composition.1–3 To ensure optimum performance, critical factors such as ink properties, ink–substrate interactions, and environmental conditions must be carefully controlled. Specifically, fluctuations in ambient relative humidity (RH) and temperature can significantly impact print quality.4–8Cellulose-based paper, exhibiting biocompatibility and mechanical flexibility, constitutes the default substrate for a range of printing applications. Paper is a hygroscopic material. Its moisture content (defined as the ratio of the moisture mass absorbed by a paper sheet to its dry mass) responds dynamically to variations in ambient relative humidity and temperature.9–15 Moisture uptake strongly affects the mechanical properties of paper such as its stiffness.14,16–19 Moreover, paper is an anisotropic material, which exhibits non-uniform hygroexpansion.20–22 Spatial gradients in moisture content can induce morphological instabilities such as curl, cockling, and multi-directional sheet distortions.23–29
Water-based inks are widely used in inkjet printing.30 The main ingredients are water (30–90 wt%) and a co-solvent such as glycerol or ethylene glycol oligomers (EGOs, 5–50 wt%), along with several other components such as surfactants and colorants.31 Co-solvents are polar, hygroscopic liquids with low vapor pressure that are added to prevent nozzle clogging in inkjet systems.32
After ink deposition and drying, the presence of residual co-solvent within the paper matrix can significantly alter its moisture sorption behavior. The affinity of co-solvents for the cellulose fibers potentially modifies their capacity to absorb and retain moisture. As a result, the sorption isotherm, which represents the equilibrium relation between moisture content and relative humidity, will change. The impact of EGOs on the hygroscopic response of wood – a closely related material system – was found to depend on the molecular weight of the EGO molecules and their specific location within the wood matrix.33 Shomali et al. observed that addition of EGOs reduces the overall moisture sorption capacity.34 In contrast, Saberi et al. found that starch films containing glycerol absorbed more moisture at all RH levels.35
While the moisture sorption properties of paper and co-solvents have been studied independently,10,12,36,37 the behavior of co-solvent-infused paper regarding moisture uptake remains largely unexplored. Given the strong dependence of paper properties on the moisture content and the hygroscopic nature of co-solvents, it is critical to understand the response of the two-component system to varying humidity conditions relevant to inkjet printing. In this study, we address this gap by investigating two key aspects: (1) the effect of the presence of co-solvents in the paper matrix on the overall moisture sorption capacity and (2) the influence of relative humidity on the transport rates of co-solvents from the interfiber into the intrafiber pores ('pore-fiber transport').28,38–40 These factors are central to understanding the drying dynamics and to controlling print quality and its long-term stability in inkjet printing applications.
We built two experimental setups to study these aspects under controlled environmental conditions. To ensure the generality of our results, two different commercial printing papers and one laboratory-grade filter paper were used. We propose a model that reproduces the measured moisture sorption behavior of the two-component system of paper/co-solvents well over a broad range of water activities, for different co-solvents and different paper types. Furthermore, we systematically investigated the influence of the initial moisture and the initial co-solvent content on the pore-fiber transport dynamics of model inks. In addition to inkjet printing, our results are relevant to chromatography applications using paper substrates,41 paper-based sensors,42 paper microfluidics,43 absorbent technology,44 and the conservation of wood using co-solvents.34
2. Materials and methods
2.1. Materials and material properties
As co-solvents we used ethylene glycol (EG), tetra(ethylene glycol) (TEG), poly(ethylene glycol) with an average molecular weight (MW) of 300 (PEG300), and glycerol. PEG300 and glycerol were specifically selected for the sorption isotherm experiment due to their low vapor pressures of ≤14 µPa (PEG300)45 and (13 ± 5) mPa (glycerol) at 20 °C,46 which render them essentially non-volatile at room temperature. All chemicals were purchased from Sigma-Aldrich and used as received. The relevant material properties of the pure co-solvents are listed in Table S1 in the SI. Deionized water (Millipore, Direct-Q3 R) is used for rehydration and the preparation of aqueous co-solvent solutions.28,38 For some experiments we used the anionic surfactant sodium dodecyl sulfate (SDS, Sigma-Aldrich, 436143) and the divalent metal salt MgCl2 (Sigma-Aldrich, M8266).As substrates we used the following uncoated, uncalendered and unsized paper types:
• Paper 'A': DNS high-speed inkjet natural feel paper (Mondi, grammage gA = 80 g m−2 and thickness dsub = 104 µm),
• Paper 'B': Z-Plot 650 paper (Ziegler, gB = 90 g m−2 and dsub = 116 µm),
• Paper 'C': Whatman filter paper, grade 44, diameter = 70 mm, gC = 80 g m−2, dsub = 180 µm, made from cotton linters, ashless.
Papers A and B contain approximately 20 wt% and 12 wt% of CaCO3 filler particles, respectively. Paper C is filler-free. Hereafter, the term 'untreated' refers to paper in its as-received condition.
2.2. Equilibrium moisture sorption – sample preparation
The sample dimensions for papers A and B are 20 × 10 cm. For Paper C, three circular pieces with a diameter of 7 cm are used. For experiments involving co-solvent-free papers, the samples are utilized without any additional treatment. For the preparation of co-solvent-infused papers, an atomizer (Timbertech, Airbrush Kit ABPST06) is employed to spray the co-solvent evenly across the samples. After spraying, the papers are placed on a holder for a minimum of one hour to allow for homogenization of the co-solvent distribution. This is followed by two sequential rehydration steps using deionized water to facilitate complete pore-fiber transport of the co-solvent.28,38 The quantity of water sprayed at each rehydration step corresponds approximately to half of the weight of the paper sample. The time interval between the rehydration steps was (50 ± 10) min to allow for drying. The measurements started no sooner than 90 min after the final rehydration step was completed.2.3. Equilibrium moisture sorption – experimental setup
Fig. 1 shows a sketch of the experimental setup. The prepared paper sample is placed on a metal wire basket that is suspended from a precision balance (Mettler Toledo, XSR225DU) by a thin metallic wire inside a humidity-controlled chamber. The paper weight is recorded every 10 minutes using a PC connected to the balance. A humidifier (Cellkraft, P-10) controls the relative humidity in the chamber. A humidity meter (Extech, SDL500) logs the temperature and humidity in the chamber. The temperature is usually (21 ± 0.2) °C and is constant throughout the course of an experiment. Care was taken when transferring the paper to prevent co-solvent loss, e.g., onto the wire-frame basket, on which the sample was placed. Adsorption isotherms were measured by incrementally increasing the relative humidity, followed by stepwise reduction of RH to record desorption isotherms.2.4. Equilibrium moisture sorption – data analysis
To measure the mass of the dry paper mp and the mass of the deposited co-solvent mcs, samples are placed in the measurement chamber at zero humidity for a minimum of 48 hours. These parameters are determined using the following equations:| mp = mass(paper + basket + wire) − mb | (1) |
| mcs = mass(cs + paper + basket + wire) − mb − mp | (2) |
 | (3) |
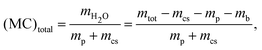 | (4) |
2.5. Paper swelling – sample preparation
For this experiment, samples of papers A and C with dimensions of 25 × 25 mm are used. For experiments without co-solvents, the samples are used as received. For the preparation of co-solvent-infused papers, an atomizer is employed to spray the co-solvent evenly across the samples. Subsequently, the papers are placed on a holder for a minimum of two hours to allow for homogenization of the co-solvent distribution. To prepare samples with an initial co-solvent content θcs,ini below or above 0.05 g g−1, one or two sequential rehydration steps are performed using deionized water, respectively. The time interval between the rehydration steps was (30 ± 10) min to allow for drying. The measurements started no sooner than 2 h after the final rehydration step was completed.2.6. Paper swelling – experimental setup
Fig. 2 shows a schematic of the experimental setup adapted from the study by Karimnejad et al.40 A sheet of paper is maintained in an upright orientation by sandwiching it between two blackened metal holders underneath an upright microscope (Olympus BX50) with a 10× objective. The microscope is equipped with a CCD camera (Basler piA1000-60gm) and records the cross-sectional view of a paper sheet to monitor its thickness as a function of time. At the top of the metal holders, there is a recess (dimensions 5 × 5 mm2), through which droplets of a model ink can be deposited onto the paper substrate using a digital syringe (Hamilton). All experiments were carried out at room temperature, (295 ± 2) K. The paper sample is enclosed in a chamber, the humidity of which is maintained at a constant level by a constant inflow of air from a humidifier (Cellkraft, P-10). A humidity meter logs the temperature and humidity in the chamber (Extech, SDL500).3. Theoretical models
3.1. Equilibrium moisture sorption of untreated paper
The Guggenheim–Anderson–de Boer (GAB) isotherm | (5) |
 | (6) |
3.2. Equilibrium moisture sorption of co-solvents
The water activity aw as a function of the composition of aqueous co-solvent solutions can usually be represented very accurately by the Van Laar equation48,49 | (7) |
| Co-solvent | A 12 | A 21 |
|---|---|---|
| PEG300 | −4.43 | −0.5 |
| Glycerol | −0.7 | −0.3 |
From the mole fractions xw and xcs, we can obtain the weight fraction of the co-solvent
 | (8) |
 | (9) |
 | ||
| Fig. 3 (a) Equilibrium co-solvent concentration of glycerol and PEG300 as a function of water activity aw for a temperature of 25 °C.50 (b) Equilibrium moisture content as a function of aw for glycerol (blue solid line) and PEG300 (purple dashed line). The green dotted line is a GAB fit for paper A. | ||
3.3. Equilibrium moisture sorption of the two-component system paper + co-solvent
There are three conceivable scenarios for equilibrium moisture sorption in the two-component system of co-solvent-infused paper:1. The two materials do not interact in terms of moisture sorption, i.e. each material absorbs the same amount of water as if it were present in an isolated state. In this case, the sorption isotherm of the combined system is the linear superposition of the isotherms of the isolated systems.
2. The presence of co-solvent reduces the moisture sorption of cellulose, because a part of the polar surface sites of cellulose available for hydrogen-bonding with water are blocked by co-solvent molecules. In this case, the sorption capacity of the combined system is lower than the superimposed capacities of the isolated materials.
3. The presence of co-solvent plasticizes the paper substrate and causes significant swelling, which makes more sites available that can bind water molecules. In this case, the sorption capacity of the combined system is larger than the superimposed capacities of the isolated materials.
Furthermore, it is plausible that the location of the deposited co-solvent, i.e. whether it is contained in the micron-scale inter-fiber or the nano-scale intra-fiber pores,28,38–40 makes a difference in which scenario is more likely to occur. Since moisture sorption in paper occurs primarily inside the cellulose fibers, scenario (1) is likely to occur if the co-solvent is contained in the inter-fiber pores. This is the case shortly after co-solvent deposition. In contrast, scenario (2) or (3) is potentially relevant to the equilibrium case, i.e. when the pore-fiber transfer of the co-solvent is complete and the co-solvent resides in the intra-fiber pores.
 | (10) |
4. Experimental results
4.1. Equilibrium moisture sorption and swelling of untreated paper
For every value of the water activity, 24 hours are allotted for moisture equilibration. The corresponding equilibrium moisture sorption isotherms for adsorption and desorption are shown in Fig. 4 as the filled and open symbols, respectively. A representative sorption cycle is provided in Fig. S2 in the SI. To illustrate the reproducibility of the experiment, two independent datasets are shown for untreated paper A in blue and green. We performed GAB fits using eqn (5) of both the adsorption and desorption data, which are shown as the solid and dashed lines, respectively. Table 2 lists the numerical values of the corresponding GAB fit parameters. For all paper types, the MC on the adsorption curve (measured in the direction of increasing aw levels) is always lower than that on the desorption curve (decreasing aw levels). The moisture sorption is thus history-dependent and exhibits a small degree of hysteresis.| M 0 | C | K | |
|---|---|---|---|
| Adsorption | 0.031 | 4.50 | 0.85 |
| Desorption | 0.042 | 3.60 | 0.75 |
Fig. 5 shows the thickness hygroexpansion strain
 | (11) |
4.2. Equilibrium moisture sorption and swelling of co-solvent-infused paper
Fig. 6(a and c) shows the equilibrium moisture sorption isotherms for co-solvent-infused paper A. The filled and open symbols denote the adsorption and desorption branches, respectively. The solid and dashed lines represent eqn (10), i.e. the theoretical model according to scenario (1), where we substituted the adsorption/desorption GAB fits of Fig. 4 for (MC)paper. Equivalent datasets for papers B and C, with the corresponding fit parameter values, are presented in Fig. S3 and Table S2 of the SI. The addition of co-solvents significantly increases the equilibrium moisture content and removes the hysteresis. Fig. 6(b) presents the scaled hysteresis amplitude | (12) |
 | ||
| Fig. 6 Moisture sorption isotherms for paper A containing (a) PEG300 and (c) glycerol. Solid and open symbols denote the adsorption and desorption branches, respectively. Solid and dashed lines represent eqn (10), i.e. the theoretical model according to scenario (1). (b) Scaled hysteresis amplitude ΔMC/〈MC〉 of the paper A + PEG300 isotherms at aw = 0.5 as a function of PEG300 content. The solid line is a guide to the eye. | ||
We also determined the influence of co-solvents on the correlation between the swelling amplitude and water activity aw. Fig. S4(b) in the SI illustrates that – similar to the removal of moisture sorption hysteresis in Fig. 6(a and b) – the presence of approximately 0.2 g g−1 PEG300 suppresses the considerable expansion strain hysteresis observed with untreated paper A in Fig. 5.
4.3. Impact of initial moisture/co-solvent content on pore-fiber transport rates of aqueous co-solvent solutions
Following the study by Karimnejad et al.,40 we monitored the swelling of a paper sheet in the thickness direction to characterize the pore-fiber transport of co-solvents. A paper sample was first equilibrated to a certain water activity for 24 h inside a humidity-controlled chamber at room temperature, after which we deposited a droplet (volume V = 1 µL) of an aqueous co-solvent solution. Fig. 7(a) shows typical results of the increment of the thickness expansion strain | (13) |
 | ||
| Fig. 7 (a) Increment of the thickness expansion strain ΔεTD of untreated paper A as a function of time after deposition of a droplet (V = 1 µL) of 60 wt% TEG for different values of aw. The solid lines are fits according to the Berens and Hopfenberg model.54 To aid visualization, the curves for aw = 0.55 and 0.68 were shifted by 0.1 and 0.2 along the ordinate axis, respectively. (b) Corresponding diffusion and relaxation timescales td (triangles) and tr1,2 (circles and squares) of untreated paper A as a function of initial water activity aw. (c) Overall characteristic swelling timescale t0.63 for solutions of TEG as a function of aw. (d) Ratio of the strain amplitude factors Ar1/Ad and Ar2/Ar1. The solid lines in (b–d) are guides to the eye. | ||
A droplet of pure water (V = 1 µL) was added to the sample at t ≈ 800 s during the experiment for aw = 0.31 to demonstrate the effect of rehydration.38 The added water increases the local moisture content, decreases viscosity and acts as a plasticizer, promoting pore-to-fiber transport of the co-solvent and enabling the system to reach a higher value of εTD, i.e. closer to equilibrium.
Fig. 7(b) shows the resulting timescales td and tr1,2 after deposition of a droplet of a 60 wt% TEG solution on paper A as a function of the initial water activity aw. Interestingly, tr1 strongly decreases with increasing aw, whereas td and tr2 show much less variation with aw. Fig. 7(c) presents the corresponding overall characteristic swelling timescale t0.63 for solutions of TEG as a function of the initial water activity aw. t0.63 is defined as the time required for ΔεTD to reach 63% of its maximum value.40 As t0.63 does not result from curve-fitting, it is a more robust and universal characterization of the swelling dynamics. In contrast, td and tr1,2 can be linked to different mechanisms or processes and thus contain more information, but their relevance is dependent on the success of the BH model in fitting the ΔεTD(t) data. In Fig. 7(d), we plot the ratios of the strain amplitude factors Ar1/Ad and Ar2/Ar1 as functions of the initial water activity aw.
Fig. 8(a) shows the increment of the thickness expansion strain ΔεTD of untreated paper A after deposition of a 1 µL droplet of pure EG as a function of the water activity. Fig. 8(b) presents the corresponding short diffusive timescale td and the longer relaxation timescale tr1 obtained from BH fits of the experimental data. Fig. 8(c) presents the corresponding overall characteristic swelling timescale t0.63 as a function of water activity aw. In contrast to the behavior of 60 wt% TEG in Fig. 7(b), td and t0.63 for pure EG strongly and monotonically decrease with increasing aw.
 | ||
| Fig. 8 (a) Increment of the thickness expansion strain ΔεTD as a function of time after deposition of pure EG droplets (V = 1 µL) on untreated paper A for different values of aw. Solid lines are fits according to the BH model.54 To aid visualization, the curves for aw = 0.55 and 0.69 were shifted by 0.1 and 0.3 along the ordinate axis, respectively. (b and c) Corresponding diffusion and relaxation timescales td and tr1 and overall characteristic swelling timescale t0.63 as functions of aw. Solid lines in (b,c) are guides to the eye. | ||
Fig. 9 shows the diffusion and relaxation timescales of paper A after deposition of a droplet (V = 1 µL) of pure EG as a function of the initial EG content of the paper sheet. This experiment mimics the case of inkjet printing a second time onto paper that already contains pre-existing ink patterns. Similar to Fig. 8(b), the swelling time for pure EG strongly and monotonically decreases with increasing θcs,ini.
Fig. 9(c) shows the overall characteristic swelling timescale t0.63 as a function of initial co-solvent (EG and glycerol) content θcs,ini at fixed water activities of aw = 0.22 (open squares) and 0.28 (circles). A relatively minor increment in water activity Δaw = 0.06 causes a significant reduction of t0.63.
4.4. Impact of adding salt or surfactant on pore-fiber transport rates of aqueous co-solvent solutions
Fig. 10 shows t0.63 of paper A after deposition of 60 wt% TEG containing the surfactant sodium-dodecyl-sulfate (SDS) or the divalent metal salt MgCl2 at different water activities aw. The addition of SDS induces a marked reduction of t0.63 for all water activities aw [Fig. 10(a)], reaching a nearly constant value above about 0.5 wt% SDS. In contrast, the addition of MgCl2 causes a strong increase of t0.63 for all water activities aw studied [Fig. 10(b)]. For both SDS and MgCl2, higher relative humidity values significantly slow down the swelling dynamics.5 Discussion
5.1. Equilibrium moisture sorption of untreated paper
Water vapor primarily interacts with hydroxyl groups on the surfaces of cellulose microfibrils or within non-crystalline domains, while those located in crystalline regions remain largely inaccessible due to intra- and intermolecular hydrogen bonding.55,56 According to Mihranyan et al.57 and Ioelovich and Leykin,58 the fraction of amorphous cellulose, i.e. its degree of crystallinity, has a major impact on its moisture sorption capacity, as it impacts the accessibility of polar groups such as hydroxyl and ether groups to water molecules.36 Therefore, the composition and the details of the manufacturing process of paper have a significant influence on its moisture sorption capacity. Parker et al. showed that the moisture sorption capacity of pulp increases with beating and even more so with bleaching.59 Bedane et al. showed that calendered paper types can have a 30% smaller moisture sorption capacity than uncalendered papers.60 CaCO3 filler particles are commonly added to printing paper, e.g., to increase opacity and reduce cost. They tend to sorb much less water than cellulose.61,62Although papers A and B contain approximately 12–20 wt% filler particles, their sorption capacities are higher than that of paper C, which is made purely from cotton linters. Since cotton contains a high level of crystalline cellulose and low levels of amorphous polysaccharide and nophenolic content,36,63 it exhibits fewer accessible OH groups compared to other fibers, which likely explains the lower moisture uptake of paper C.
5.2. Moisture sorption hysteresis
Sorption hysteresis refers to the fact that the moisture content (MC) of a material depends not only on the current value of aw but also on its preceding time history aw(t). As a consequence, the MC after moisture uptake (adsorption) differs from the MC after moisture loss (desorption), when an identical final aw level is considered. This commonly results in the occurrence of a hysteresis loop when plotting MC against aw,64,65 as shown, e.g., in Fig. 4.Sorption hysteresis is widely encountered with, e.g., natural fibers36 and synthetic polymers.66 Chen et al.53 used molecular dynamics (MD) simulations and swelling experiments to show that hysteresis in cellulose arises from history-dependent hydrogen bond distributions during adsorption and desorption. Chen et al.67 performed MD simulations that suggest that the presence of crystalline domains in amorphous cellulose reduces the sorption hysteresis. Salmén and Larsson68 investigated sorption hysteresis in cellulose and a chemically partially modified form. They found that with increasing degree of modification, the softening temperature Tg decreased. Concurrently, moisture sorption hysteresis was reduced, disappearing entirely in the most modified samples. Similarly, increasing temperature reduced hysteresis. Weinmüller et al. showed that sorption hysteresis in hydrogels disappears when the rubber-to-glass transition temperature Tg falls below the sample temperature.66 Zou et al. studied the interrelation of hysteresis and sorption-induced deformation.69
Fig. 6(b) shows that the sorption hysteresis of paper A disappears when a quantity of PEG300 with θcs ≳ 0.2 g g−1 is added. This quantity is comparable to the moisture content of paper A at very high humidity aw ≈ 1, see Fig. 3(b). Due to the non-volatile nature of glycerol and PEG300, the co-solvents remain in the paper for the entire duration of the experiment and induce a persistent strain28 on the order of ΔεTD ≳ 0.12, which is much larger than the hysteresis in εTD observed in Fig. 5. We hypothesize therefore that the co-solvents permanently occupy the adsorption sites in the cellulose fibers, the filling and emptying of which by water would otherwise give rise to hysteresis. Sala and Tomka observed an analogous disappearance of the sorption hysteresis in starch after addition of 10 wt% of glycerol, which reduced the glass transition temperature far below room temperature.70
5.3. Equilibrium moisture sorption of pure co-solvents
Glycerol has three hydroxyl groups (one per carbon atom), resulting in a higher number of hydrophilic sites per unit mass compared to EGOs (one ether group per two carbon atoms plus two terminal hydroxyl groups).71 As the molecular weight of an EGO increases, the relative importance of its hydroxyl endgroups decreases. Hence, higher molecular weight EGOs are less hydrophilic and exhibit a lower aqueous solubility.72 The different densities of polar groups explain why glycerol is able to absorb approximately twice as much water per unit mass as PEG300, as shown in Fig. 3(b).5.4. Equilibrium moisture sorption of the two-component system paper + co-solvent
Adding 10 wt% glycerol to starch, which is chemically very similar to cellulose, can reduce the equilibrium MC,73,74 although the MC of glycerol exceeds the MC of starch for all aw values. In wood, the addition of EGOs can strongly reduce the moisture sorption capacity due to pore-filling and the much smaller expansion that is restricted by the structural rigidity of wood.34 These systems are examples of scenario (2). In contrast, in all our experiments using glycerol and PEG300, the moisture sorption capacity of the two-component system did not fall measurably below that of the papers studied. The theoretical sorption model according to scenario (1) [lines in Fig. 6(a)] represents the experimental data for paper A + PEG300 very well; however, it slightly overpredicts the data for paper A + glycerol in Fig. 6(c). We hypothesize that the two effects ascribed to scenarios (2) and (3), i.e. the blockage of sorption sites by co-solvents and the availability of additional sorption sites due to the substantial swelling, might compensate each other, thereby explaining the unexpected success of scenario (1).5.5. Pore-fiber transport rates of solutions
The characteristic swelling timescale t0.63 for 40 wt% TEG in Fig. 7(c) is independent of aw. This is attributed to the fact that the pore-fiber transport of the co-solvent is primarily influenced by the high initial water content of the solution.40 A higher initial water concentration reduces the viscosity of the solution and, at the same time, efficiently plasticizes the fiber walls, thus promoting more expeditious pore-fiber transport.
The characteristic swelling timescale t0.63 for 60 wt% TEG in Fig. 7(c) is roughly constant until aw = 0.5 and then increases by a factor of 2. This seems to be inconsistent with all timescales remaining constant or decreasing in Fig. 7(b). The key is that above a water activity of 0.5, two separate relaxation times are required for an accurate BH fit of the experimental data and that the strain amplitude ratio Ar2/Ar1 increases strongly with aw, as shown in Fig. 7(d). This increases the relative importance of the slowest timescale tr2 and thus causes the increase of t0.63. In contrast, a monotonic decrease of t0.63(aw) is observed for EG in Fig. 8(c). The origin of this qualitative difference is unclear.
We note that the drying time tdry, i.e. the time required for the excess water to evaporate, depends on aw and is thus different for each of the experiments, as shown in Fig. 7. Prior to every experiment, we deposited a droplet of pure water (V = 1 µL) on a separate paper sample to determine its drying time tdry,H2O. Fig. S7(a) in the SI shows a plot of tdry,H2O as a function of aw. In all cases, tdry,H2O exceeds 100 s. Fig. S7(b) shows that tdry for aqueous EGO solutions is only very weakly dependent on the EGO concentration and the EGO MW. Therefore, we conclude that td < tr2 < tdry holds for all experiments in Fig. 7, whereas tr1 < tdry for aw ≳ 0.55 and tr1 > tdry for aw ⪅ 0.55. Karimnejad et al.40 measured the effect of the co-solvent concentration on timescales td and tr1 at a fixed water activity. An approximately exponential dependence of td on the co-solvent concentration was found. Consequently, the values of td and tr2 in Fig. 7 are representative of the initial TEG concentration. However, the large values of tr1 for aw ⪅ 0.55 are strongly affected by the evaporation of excess water and the concomitant increase in the TEG concentration.
The diffusion timescale of 60 wt% TEG in Fig. 7(b) at low water activities aw < 0.25 is a factor of 4–8 faster than that for pure EG in Fig. 8(b), despite that MW(TEG) ≫ MW (EG) and that the viscosity µ (60 wt% TEG) is only about half of µ (EG). We interpret this to be a consequence of the plasticization of the fibers induced by the water present in the aqueous TEG solution.75
The diffusion timescale decreases by a factor of 3 in all EG experiments when approximately 0.04 g g−1 of liquid is present, as a consequence of either moisture sorption [Fig. 8(b)] or co-solvent deposition [Fig. 9(a)]. In both cases, an increase in the initial liquid content will increase the relaxation timescale tr1. These observations suggest that the presence of moisture or EG both accelerate pore-fiber transport in an analogous fashion.
Fig. 9(c) shows that t0.63 decreases with increasing θcs,ini for both EG and glycerol, indicating faster overall characteristic swelling due to co-solvent-induced softening of cellulose. A higher water activity further accelerates the response. EG induces faster swelling due to its lower viscosity, smaller MW and weaker hydrogen bonding with cellulose. These properties enhance its diffusion and plasticization efficiency within the fiber network.
Wang and Darhuber introduced a phenomenological model for pore-fiber transport of co-solvents in paper.39 They assumed that the pore-fiber transport rate depends on the solution viscosity, the effective permeability of the fiber wall, the difference in capillary pressures of pores and fibers and an empirical, concentration-dependent retardation factor f(c). The data presented in Fig. 7–9 will be used to test the validity of their model and determine the values of the corresponding transport parameters.
Divalent metal salt solutions (so-called primer solutions) are frequently deposited on paper immediately before ink deposition to induce rapid flocculation of the colorant and latex particles suspended in the ink.77,78Fig. 10(b) shows that the addition of MgCl2 strongly decreases the pore-fiber transport rates of 60 wt% TEG solutions by approximately a factor of 3. The same retardation effect has been observed by Stamm and Tarkov for imbibition of a saturated MgCl2 solution into wood sections.79 Andreasson et al. observed a decrease in the nanoscale pore radii of the fiber walls with increasing salt concentration, which they attributed to a decreased electrostatic repulsion.80 Karlsson et al. reported that addition of salt has a significant impact on the water uptake capacity of cellulose nanofibrils.81 We found that an important contribution to the retardation effect is the high sensitivity of the solution viscosity to cMgCl2, which increases by more than 60% in the concentration range studied, as illustrated in Fig. S9 of the SI. Analogous to the case of ternary solutions containing SDS above, higher values of aw again significantly increase t0.63. This behavior also persists for a quaternary aqueous solution of TEG, SDS and MgCl2 (see Fig. S8 of the SI).
6. Conclusions
This manuscript provides a systematic evaluation of how water activity and the presence of co-solvents like glycerol and EGOs affect moisture uptake and the pore-to-fiber transport dynamics of model inks within cellulose-based papers. We infused papers with controlled amounts of co-solvent and measured their equilibrium moisture sorption for a wide range of water activities. This revealed that co-solvents increase the moisture uptake compared to untreated paper. Although co-solvents have a high affinity for paper, a non-interactive model based on a direct superposition of their individual sorption capacities represents the experimental data well. Interestingly, the deposition of co-solvents with a mass fraction of θcs ≳ 0.2 g g−1 eliminates the sorption hysteresis observed for untreated paper. Furthermore, we found that increasing the ambient humidity or the initial co-solvent content accelerates the pore-to-fiber transport of the model inks in an analogous fashion.6.1. Relevance to inkjet printing technology and beyond
Our results indicate that the co-solvent content and water activity have direct implications for print quality, ink-drying, sorption induced deformations of the paper substrate, and the long-term stability of inkjet-printed patterns. In inkjet printing, the quantity of ink deposited per area is typically smaller than the fiber holding capacity of the paper sheet. Therefore, co-solvents are not homogeneously distributed across a paper sheet, but are primarily located in the top part. As the regions containing co-solvents pick up more water than dry paper upon moisture sorption, this likely aggravates curl formation.29An increase of the pore-fiber transport rate with increasing ambient humidity implies that the ink penetration depth into the paper is expected to increase for low humidity, because the water-co-solvent mixture remains in the inter-fiber pores for a longer time. By means of rapid drying, this undesirable effect can be reduced, as the water is removed. However, the remaining co-solvent may impart mobility to ink colorants, thus potentially inducing long-term color changes. The use of a volatile co-solvent such as EG would reduce this effect, at the expense of it being less effective in preventing inkjet nozzle clogging.
The findings of this study are relevant not only to inkjet printing but also for microfluidic systems and lab-on-a-chip technologies. In these applications, precise control of fluid transport through porous substrates or narrow channels is essential for achieving reliable diagnostics, chemical reactions, or biological assays.82 The insights into how multicomponent solutions interact with hygroscopic porous media, such as cellulose fibers, and how ambient humidity modulates sorption and transport dynamics can inform the design of paper-based microfluidic devices. Moisture sorption can serve as an additional mechanism to enhance fluid control. By understanding the impact of moisture uptake, developers of low-cost, portable diagnostic tools can improve their reproducibility and stability under varying environmental conditions – challenges that are critical in point-of-care settings.
Author contributions
S. Karimnejad: investigation, data curation, methodology, visualization, formal analysis, writing – original draft, and writing – review and editing. A. A. Darhuber: conceptualization, investigation, methodology, formal analysis, supervision, funding acquisition, and writing – review and editing.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article are included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sm00847f.Acknowledgements
This work is part of an Industrial Partnership Program (i43-FIP) of the Foundation for Fundamental Research on Matter (FOM), which is part of the Netherlands Organisation for Scientific Research (NWO). This research programme is co-financed by Canon Production Printing, University of Twente, Eindhoven University of Technology, and the “Topconsortia voor Kennis en lnnovatie (TKI)” allowance from the Ministry of Economic Affairs. The authors are grateful to Nicolae Tomozeiu for the fruitful discussions.References
- S. D. Hoath, Fundamentals of inkjet printing: the science of inkjet and droplets, John Wiley & Sons, 2016 Search PubMed.
- P. J. Smith and A. Morrin, Reactive Inkjet Printing: A Chemical Synthesis Tool, Royal Society of Chemistry, 2017 Search PubMed.
- W. Zapka, Handbook of Industrial Inkjet Printing: A Full System Approach, Wiley-VCH, 2018 Search PubMed.
- M. McCormick-Goodhart and H. Wilhelm, IS&T'S NIP16: Int. Conf. Digital Printing Technol., 2000, 74–77 Search PubMed.
- O. Högberg, M. Talaskivi and G. Ström, IS&T'S NIP17: Int. Conf. Digital Printing Technol., 2001, 874–877 Search PubMed.
- M. McCormick-Goodhart and H. Wilhelm, IS&T'S NIP17: Int. Conf. Digital Printing Technol., 2001, 179–185 Search PubMed.
- B. Rat, K. Možina, S. Bračko and A. Podlesek, J. Imaging Sci. Technol., 2011, 55, 50607 CrossRef.
- A. Fricker, A. Hodgson, J. Townsend and C. Woods, e-Preservation Sci., 2012, 9, 60–66 CAS.
- L. Salmén and E. Back, Trans. VIth Fund. Res. Symp., 1977, 683–690 Search PubMed.
- B. V. Ramarao, Studies in Surface Science and Catalysis, Elsevier, 1999, 120, 531–560 Search PubMed.
- H. W. Haslach, Mech. Time-Depend. Mater., 2000, 4, 169–210 CrossRef CAS.
- C. Fellers, Paper Products Physics and Technology, de Gruyter, Berlin, 2009, pp. 109–143 Search PubMed.
- L. Solhi, V. Guccini, K. Heise, I. Solala, E. Niinivaara, W. Xu, K. Mihhels, M. Kröger, Z. Meng and J. Wohlert, et al. , Chem. Rev., 2023, 123, 1925–2015 CrossRef CAS PubMed.
- B. Lin, J. Auernhammer, J.-L. Schäfer, T. Meckel, R. Stark, M. Biesalski and B.-X. Xu, Cellulose, 2022, 29, 1129–1148 Search PubMed.
- M. A. Hubbe, B. Sjöstrand, M. Lestelius, H. Håkansson, A. Swerin and G. Henriksson, BioResources, 2024, 19, 6859–6945 CAS.
- K. C. Yeh, J. M. Considine and J. C. Suhling, Proc. Tappi Int. Pap. Phys. Conf., 1991, 695–711 Search PubMed.
- M. Lee, S. Kim, H.-Y. Kim and L. Mahadevan, Phys. Fluids, 2016, 28, 042101 CrossRef.
- J.-W. Rhim, Food Sci. Biotechnol., 2010, 19, 243–247 CrossRef CAS.
- W. J. Cousins, Wood Sci. Technol., 1978, 12, 161–167 CrossRef CAS.
- T. Uesaka, J. Mat. Sci., 1994, 29, 2373–2377 CrossRef.
- H. Nanko and Y. Tada, Proc. Int. Pap. Phys. Conf., 1995, 159–171 Search PubMed.
- A.-L. Erkkilä, T. Leppänen, M. Ora, T. Tuovinen and A. Puurtinen, Nord. Pulp Pap. Res. J., 2015, 30, 326–334 CrossRef.
- W. Lu and L. A. Carlsson, Mech. Time-Depend. Mater., 2001, 5, 79–100 Search PubMed.
- T. Leppänen, J. Sorvari, A.-L. Erkkilä and J. Hämäläinen, Model. Simul. Mat. Sci. Eng., 2005, 13, 841–850 CrossRef.
- P. Lipponen, T. Leppänen, J. Kouko and J. Hämäläinen, Int. J. Solids Struct., 2008, 45, 3596–3609 CrossRef.
- S. Douezan, M. Wyart, F. Brochard-Wyart and D. Cuvelier, Soft Matter, 2011, 7, 1506–1511 Search PubMed.
- E. Reyssat and L. Mahadevan, Europhys. Lett., 2011, 93, 54001 Search PubMed.
- C.-L. Wong, S. Wang, S. Karimnejad, M. Wijburg, H. Mansouri and A. Darhuber, Soft Matter, 2023, 19, 1202–1211 Search PubMed.
- A. Maass and U. Hirn, Mater. Des., 2024, 237, 112593 CrossRef CAS.
- S. Magdassi, The chemistry of inkjet inks, World scientific, 2009 Search PubMed.
- C. Schmid, The chemistry of inkjet inks, World Scientific, Singapore, 2009, ch. 7 Search PubMed.
- J. Vonasek, M. Tuniuns and K. Rydinge, Method of preventing clogging of nozzles, US Pat. 5,781,214A, 1998.
- J. Ljungdahl and L. A. Berglund, Holzforschung, 2007, 61, 279–284 CAS.
- A. Shomali, C. Zhang, B. Coasne, E. J. Schofield, B. Chabbert, D. Derome and J. Carmeliet, Int. J. Bio. Macromol., 2025, 285, 137661 CrossRef CAS.
- B. Saberi, Q. V. Vuong, S. Chockchaisawasdee, J. B. Golding, C. J. Scarlett and C. E. Stathopoulos, Foods, 2016, 5, 1 CrossRef.
- C. A. S. Hill, A. Norton and G. Newman, J. Appl. Polym. Sci., 2009, 112, 1524–1537 Search PubMed.
- L. Ninni, M. S. Camargo and A. J. A. Meirelles, Thermochim. Acta, 1999, 328, 169–176 CrossRef CAS.
- M. G. Wijburg, S. Wang and A. A. Darhuber, Colloids Surf., A, 2023, 656, 130268 CrossRef CAS.
- S. Wang and A. Darhuber, Colloids Surf., A, 2024, 682, 132839 Search PubMed.
- S. Karimnejad, E. Gonnet, S. Wang, H. Mansouri, N. Tomozeiu and A. A. Darhuber, Langmuir, 2024, 40, 19528–19537 CrossRef CAS PubMed.
- B. Spangenberg, C. F. Poole and C. Weins, Quantitative Thin-Layer Chromatography, Springer-Verlag, 2011 Search PubMed.
- Paper-Based Optical Chemosensors, ed. L. Feng, Elsevier, 2025 Search PubMed.
- Paper Microfluidics, ed. S. Bhattacharya, S. Kumar and A. K. Agarwal, Springer-Verlag, 2019 Search PubMed.
- Absorbent Technology, ed. P. Chatterjee and B. Gupta, Elsevier, 2025 Search PubMed.
- U. K. Krieger, F. Siegrist, C. Marcolli, E. U. Emanuelsson, F. M. Gøbel, M. Bilde, A. Marsh, J. P. Reid, A. J. Huisman, I. Riipinen, N. Hyttinen, N. Myllys, T. Kurtén, T. Bannan, C. J. Percival and D. Topping, Atmos. Meas. Tech., 2018, 11, 49–63 CrossRef.
- S. P. Verevkin, D. H. Zaitsau, V. N. Emel'yanenko and A. A. Zhabina, Fluid Phase Equilib., 2015, 397, 87–94 CrossRef CAS.
- S. Basu, U. Shivhare and A. Mujumdar, Drying Technol., 2006, 24, 917–930 CrossRef.
- J. J. v Laar, Z. Phys. Chem., 1910, 72U, 723–751 CrossRef.
- J. J. Van Laar, Z. anorg. allgem. Chem., 1928, 171, 42–60 CrossRef CAS.
- H. Nakagawa and T. Oyama, Frontiers Chem., 2019, 7, 731 CrossRef CAS.
- A. Eliassi, H. Modarress and G. A. Mansoori, J. Chem. Eng. Data, 1999, 44, 52–55 Search PubMed.
- K. W. Lang and M. P. Steinberg, J. Food Sci., 1980, 45, 1228–1230 Search PubMed.
- M. Chen, B. Coasne, R. Guyer, D. Derome and J. Carmeliet, Nat. Commun., 2018, 9, 3507 CrossRef.
- A. Berens and H. Hopfenberg, Polymer, 1978, 19, 489–496 Search PubMed.
- A. C. O'Sullivan, Cellulose, 1997, 4, 173–207 Search PubMed.
- K. Hofstetter, B. Hinterstoisser and L. Salmén, Cellulose, 2006, 13, 131–145 Search PubMed.
- A. Mihranyan, A. P. Llagostera, R. Karmhag, M. Stromme and R. Ek, Int. J. Pharm., 2004, 269, 433–442 Search PubMed.
- M. Ioelovich and A. Leykin, BioResources, 2011, 6, 178–195 CAS.
- M. E. Parker, J. E. Bronlund and A. J. Mawson, Packag. Technol. Sci., 2006, 19, 193–209 Search PubMed.
- A. H. Bedane, H. Xiao and M. Ei, Adsorption, 2014, 20, 863–874 Search PubMed.
- P. A. C. Gane and C. J. Ridgway, Nord. Pulp Paper Res. J, 2009, 24, 298–308 CrossRef.
- Y. Hu and Q. Yu, Geoenergy Sci. Eng., 2024, 236, 212740 Search PubMed.
- T. Uesaka, Handbook of physical testing of paper, CRC Press, 2001, pp. 135–192 Search PubMed.
- S. Hou, J. Wang, F. Yin, C. Qi and J. Mu, Wood Sci. Technol., 2022, 56, 1087–1102 CrossRef CAS.
- J. G. Kapsalis, Water Activity Influences on Food Quality, ed. L. B. Rockland, G. F. Stewart, 1981, pp. 143–177 Search PubMed.
- C. Weinmüller, C. Langel, F. Fornasiero, C. Radke and J. Prausnitz, J. Biomed. Mat. Res. A, 2006, 77A, 230–241 CrossRef.
- M. Chen, B. Coasne, D. Derome and J. Carmeliet, Cellulose, 2020, 27, 6945–6960 Search PubMed.
- L. Salmén and P. A. Larsson, Carbohydr. Polym., 2018, 182, 15–20 Search PubMed.
- Y. Zou, B. Maillet, P. Coussot and L. Brochard, Phys. Rev. Res., 2025, 7, 013205 CrossRef CAS.
- R. M. Sala and I. A. Tomka, Angew. Makromol. Chem., 1992, 199, 45–63 CrossRef CAS.
- L. Weng, C. Chen, J. Zuo and W. Li, J. Phys. Chem. A, 2011, 115, 4729–4737 Search PubMed.
- J. A. Baird, R. Olayo-Valles, C. Rinaldi and L. S. Taylor, J. Pharm. Sci., 2010, 99, 154–168 CrossRef CAS.
- P. Myllärinen, R. Partanen, J. Seppälä and P. Forssell, Carbohydr. Polym., 2002, 50, 355–361 CrossRef.
- J. I. Enrione, S. E. Hill and J. R. Mitchell, J. Agric. Food Chem., 2007, 55, 2956–2963 CrossRef CAS.
- P. H. Hermans and D. Vermaas, J. Polym. Sci., 1946, 1, 149–155 CrossRef CAS.
- B. Simončič and V. Rozman, Colloids Surf., A, 2007, 292, 236–245 CrossRef.
- A. Lundberg, J. Ortegren and O. Norberg, J. Imaging Sci. Technol., 2011, 55, 506051–506058 CrossRef.
- Y. Yu and F. von Gottberg, Proc. IS&T's NIP18: Int. Conf. Digital Printing Technol., 2002, 383–387 Search PubMed.
- A. J. Stamm and H. Tarkow, J. Phys. Colloid Chem., 1950, 54, 745–753 Search PubMed.
- B. Andreasson, J. Forsström and L. Wågberg, Cellulose, 2005, 12, 253–265 CrossRef CAS.
- R.-M. P. Karlsson, P. T. Larsson, T. Pettersson and L. Wågberg, Langmuir, 2020, 36, 12261–12271 CrossRef CAS.
- T. Akyazi, L. Basabe-Desmonts and F. Benito-Lopez, Anal. Chim. Acta, 2018, 1001, 1–17 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |






