Research advances and future perspectives in Fischer–Tropsch synthesis for sustainable aviation fuel
Ang
Li
*abc,
Junhui
Zheng
b,
Ziqi
Wang
b and
Zongwei
Zhang
*bc
aSchool of Sciences, Civil Aviation University of China, Jinbei Road 2898, Dongli District, Tianjin 300300, P. R. China. E-mail: a_li@cauc.edu.cn
bAeronautical Engineering Institute, Civil Aviation University of China, Jinbei Road 2898, Dongli District, Tianjin 300300, P. R. China
cFuture Aviation Fuel Engineering and Technology Laboratory, Civil Aviation University of China, Jinbei Road 2898, Dongli District, Tianjin 300300, P. R. China
First published on 27th November 2025
Abstract
Amid growing global concerns over climate change, the aviation industry is reinforcing its commitment to sustainable development. Current studies confirm that Sustainable Aviation Fuel (SAF) has become a central strategic measure for reducing carbon emissions intensity and mitigating environmental impacts throughout the fuel's life cycle. Among various production pathways, Fischer–Tropsch synthesis (FTS) is widely considered one of the most promising routes for near-term industrial-scale SAF deployment, owing to its high technological maturity and well-established scalability. This article provides a systematic review of the relationship between the composition and content of jet fuel components and their physicochemical properties. It further interprets the technical requirements specified in the ASTM International standard ASTM D7566, Standard Specification for Aviation Turbine Fuels Containing Synthesized Hydrocarbons, with particular emphasis on FTS-derived synthetic jet fuels. Based on current research progress, the paper concludes with a summary and outlook on future technological directions for sustainable aviation fuels.
1. Introduction
With the rapid development of the civil aviation industry, the energy consumption costs and environmental pollution associated with aviation kerosene have attracted widespread attention. According to the International Air Transport Association (IATA), global aviation fuel consumption is projected to reach approximately 107 billion gallons in 2025, with total fuel expenditure amounting to about $248 billion, accounting for 26.4% of operating costs. On the emissions front, civil aviation released 0.8 Gt of CO2 in 2023, representing approximately 2.0% of worldwide anthropogenic emissions. This figure is expected to rebound to the pre-pandemic level of 1.06 Gt by 2025.1 As electrification advances in other transportation sectors, aviation's proportion of total emissions is projected to increase significantly in the coming decades, presenting a major challenge to the achievement of global net-zero emissions targets by 2050. In response, the 41st Assembly of the International Civil Aviation Organization (ICAO) in 2022 reached a consensus among its 193 member states to adopt a long-term aspirational goal (LTAG) of achieving net-zero carbon emissions in international aviation by 2050. Additionally, in October 2023, the European Union enacted the Regulation on Ensuring Sustainable and Fair Aviation, which mandates that airlines use sustainable aviation fuel (SAF) starting from 2025. The regulation requires progressively increasing SAF blending ratios: at least 2% by 2025, 6% by 2030, 20% by 2035, and 70% by 2050.2The impact of Sustainable Aviation Fuel (SAF) on non-CO2 emissions in the aviation industry is primarily reflected in reducing contrails and soot emissions, while simultaneously delivering the co-benefit of improving air quality, making it one of the key pathways to mitigate the non-CO2 climate effects of the aviation sector.3 Specifically, SAF—especially categories such as Fischer–Tropsch Synthetic Paraffinic Kerosene (FT-SPK)—contains no or low levels of aromatics. This avoids the massive generation of soot particles resulting from the incomplete combustion of aromatics in conventional jet fuel. Soot serves as the core “carrier” for contrail formation: water vapor in the atmosphere condenses on the surface of soot particles, and ice crystals nucleate and grow around these particles, ultimately forming contrail cirrus with a warming effect (positive radiative forcing). Therefore, SAF can directly reduce the number of emitted soot particles, inhibiting contrail formation at the source. This not only lowers the probability of contrail cirrus generation but also shortens the lifetime of existing contrails and reduces their optical depth, thereby mitigating the positive radiative forcing (warming effect) they induce. Existing studies have confirmed that a roughly 50% reduction in the initial number of ice crystals (achieved via SAF) can lead to a corresponding 20% decrease in radiative forcing. Moreover, the reduction in soot emissions from SAF improves near-surface air quality, resulting in a synergistic effect of climate mitigation and environmental improvement. Importantly, this process requires no adjustments to flight routes or modifications to operational procedures, and unlike some nitrogen oxide (NOx) emission reduction technologies, it does not increase fuel consumption—thus avoiding the “CO2 penalty” (i.e., increasing CO2 emissions while reducing non-CO2 emissions) and demonstrating high technical feasibility and environmental benefits. From a scientific research perspective, the academic community has clearly established that non-CO2 impacts (including contrails, NOx, etc.) are a crucial component of the aviation industry's climate effects. In 2018, the net Effective Radiative Forcing (ERF) from non-CO2 impacts in the aviation sector accounted for 66% of the total net radiative forcing, and its contribution to the uncertainty of overall climate impacts was even 8 times that of CO2. The inhibitory effect of SAF on soot and contrails directly targets contrail cirrus—a key factor among non-CO2 impacts (characterized by large radiative forcing magnitude and high uncertainty, and one of the core sources of non-CO2 effects)—which further confirms SAF's core value in mitigating the non-CO2 climate effects of the aviation industry.
SAF is currently sourced from sustainable biomass, municipal solid waste, or used cooking oil, or alternatively include unavoidable and biogenic CO2 in combination with renewable hydrogen as sources. The EU distinguishes SAFs as drop-in aviation fuels that can be: advanced biofuels or biofuels produced from the feedstock in line with sustainability criteria, recycled carbon fuels or synthetic fuels. Within the mandate and for ease of differentiation, they are further refined into: sustainable aviation fuels, meaning fuels of a biological origin, which can include fuels like HEFA (hydro processed esters and fatty acid fuels), advanced biofuels and “sustainable” biofuels, or, as a sub-category of SAF: synthetic aviation fuels, meaning fuels of a non-biological origin, which can also be referred to as “e-fuels”, “e-kerosene”, “synthetic fuels” or “power-to-liquids” (PtL).2 It is considered an ideal drop-in replacement for conventional jet fuel,4–6 offering substantially reduced lifecycle greenhouse gas (GHG) emissions, comparable or higher energy density, and strong compatibility with existing engine designs and infrastructure.6 Current aviation-fuel certifications (e.g., ASTM D7566) mandate that SAF must be blended with conventional kerosene, with a maximum blending ratio of 50% for most formulations. Notably, 100% neat SAF is not yet certified for general use in commercial jet engines, a constraint that is critical to framing the near-term application of SAF. According to the IATA, SAF is projected to deliver more than 60% of the emission reductions required to curb aviation emissions and achieve net-zero carbon goals by 2050. In contrast, other mitigation measures, such as carbon offsets, operational efficiencies, hydrogen, and electric aircraft, will play a much smaller role in decarbonizing this sector. As a result, SAF is widely recognized by the global aviation sector as a critical pathway enabling the transition to low-carbon or even carbon-negative aviation.7 Thus, its large-scale adoption is deemed a cornerstone strategy for decarbonizing the industry.8 Existing studies have demonstrated the significant emission reduction potential of SAF. Through a systematic lifecycle assessment (LCA) of algae-derived SAF, Fortier et al. revealed that this pathway can reduce CO2 emissions by 55.4–76.0% compared to conventional jet fuel.9 Similarly, Han et al. reported in a comprehensive LCA study on multiple biomass-based SAF production routes that such biofuels can achieve CO2 emission reductions ranging from 41% to 89%.7 To date, researchers have developed multiple technological pathways for converting biomass into SAF, primarily including oil-to-jet (OTJ), gas-to-jet (GTJ), alcohol-to-jet (ATJ), and sugar-to-jet (STJ).8–11
Fischer–Tropsch Synthesis (FTS) serves as a core pathway for transforming syngas and other gaseous feedstocks into hydrocarbon intermediates, which can be upgraded to aviation fuel through subsequent refining. This positions FTS as a highly promising near-to-mid-term solution with immense industrial-scale potential.12,13 This article provides a systematic review of current synthetic aviation fuel (SAF) production technologies via the FTS pathway that are compliant with A1 and A4 of the ASTM D7566 standard for aviation turbine fuel.14 It examines the relationship between the composition and content of jet fuels and their physicochemical properties, with a specific focus on technology pathways related to FTS. By integrating industrial scalability perspectives with fundamental laboratory research, the article summarizes catalyst design strategies, reaction mechanisms, and performance optimization methods for aviation fuel synthesis (Fig. 1). Additionally, it identifies future research directions grounded in existing challenges. The overarching goal of this review is to offer theoretical and technical guidance for future studies on FTS-derived SAF, thereby facilitating the scaling of SAF production and supporting the transition toward carbon-neutral aviation and sustainable development.
2. Aviation kerosene
Aviation turbine fuel specifications play a critical role in ensuring fuel performance, flight operational safety, and compatibility with existing aviation infrastructure. The ASTM D1655 standard, established by the American Society for Testing and Materials (ASTM), specifies the technical requirements for conventional petroleum-derived aviation turbine fuels. These fuels are further categorized into civil (JET A, JET A-1, JET B) and military, (JP-4, JP-8, etc.), applications.15 Among them, JET A-1 has become one of the most widely used civil aviation turbine fuels worldwide due to its excellent versatility. As an ideal alternative to JET A-1 aviation fuel, the development of SAF that complies with its technical standards represents the most viable pathway. This necessitates that SAF simultaneously meets strict performance, operability, and drop in requirements equivalent to those of JET A-1 (Fig. 2). While global technical specifications for aviation fuels are gradually converging, nationally retained standard systems continue to hold significant importance. The DEF STAN 91-091 issued by the UK Ministry of Defence represents one such critical standard, providing essential technical basis particularly for the certification of fully synthetic and semi-synthetic jet fuels produced by South Africa's Sasol. This standard functions in a complementary manner with the ASTM framework, collectively forming a crucial safeguard for aviation fuel quality and safety.2.1. Molecular composition
From a molecular composition perspective, aviation kerosene typically consists of hydrocarbons with carbon chain lengths ranging from C8 to C16 with an average molecular weight typically around 152–166 g mol−1, primarily including n-alkanes, iso-alkanes (collectively referred to as chain alkanes), cycloalkanes, and aromatic hydrocarbons.16 The molecular structures and concentrations of these components collectively dictate the key physicochemical properties and operational performance of the fuel in various aspects. Specifically, chain alkanes significantly contribute to both the gravimetric (MJ kg−1) and volumetric (MJ L−1) energy density of aviation kerosene. A high gravimetric energy density helps reduce aircraft weight associated with fuel load, thereby improving flight efficiency. A high volumetric energy density allows for a smaller fuel tank design while maintaining the same range capability. However, the content of n-alkanes must be carefully balanced: excessive levels raise the freeze point, increasing the risk of fuel-line blockage at high-altitude, low-temperature conditions, whereas insufficient levels depress the flash point below safety thresholds, heightening fire hazards during flight operations. Compared with n-alkanes, iso-alkanes offer the crucial advantage of a low freezing point, significantly improving the fluidity of aviation fuel under low-temperature conditions and preventing blockages in fuel system lines.17 Additionally, iso-alkanes exhibit superior thermal stability, resisting thermal decomposition into carbon deposits under high-temperature engine operating conditions, thereby reducing engine fouling and extending service life.Cycloalkanes constitute essential components of aviation kerosene, owing to their exceptionally low freeze point and relatively high density.18,19 These characteristics significantly improve the low-temperature performance of the fuel while enhancing the fuel's volumetric energy density. Overall, the proportional blending of alkanes (including both linear and cyclic structures) serves as a crucial tuning parameter for optimizing the balance between combustion efficiency, freeze resistance, and thermal stability in aviation fuels.
Polycyclic aromatics can lead to soot formation and higher maintenance costs due to their tendency for incomplete combustion. However, aromatics are indispensable in aviation fuel systems because they maintain the structural integrity of seals by causing moderate swelling in elastomeric materials.20 The mechanism lies in their ability to induce moderate swelling in elastomeric sealing materials (e.g., nitrile rubber O-rings) commonly used in engine fuel systems. This swelling prevents the seals from shrinking and hardening upon prolonged exposure to the fuel, thereby avoiding fuel leaks and ensuring flight safety.
2.2. Carbon numbers
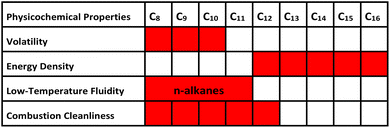
From the molecular perspective of carbon chain length, intermolecular forces play a critical role in governing the properties of hydrocarbon molecules. Their carbon numbers are closely linked to physicochemical characteristics, thereby influencing their practical application performance.21 Lower carbon-chain lengths correspond to reduced molecular mass, which gives rise to lower boiling points, increased volatility, and enhanced low-temperature fluidity. However, these advantages are also accompanied by a reduction in volumetric energy density. In contrast, higher carbon numbers are characterized by greater molecular mass, contributing to significantly higher boiling points and improved energy density. Nevertheless, these benefits come at the expense of impaired low-temperature fluidity and a higher propensity for incomplete combustion during combustion, thereby increasing the risk of carbon deposit formation. This relationship exerts multifaceted impacts on the performance of aviation kerosene, which can be broadly categorized into the following four aspects (Table 1).2.3. Distillation range and volatility
The distillation range of jet fuel is characterized by a strict final boiling point limit of 300 °C (with the lower end indirectly defined by the flash point specification), and this specification is intrinsically linked to maintaining an optimal balance between light and heavy fractions.22 As low-carbon components in jet fuel, light fractions (C8–C10) is to ensure stable engine operation under critical conditions, such as during startup, low-speed high-altitude flight, and idling. These fractions facilitate the rapid formation of a uniform and combustible mixture, thereby ensuring reliable ignition and stable combustion. An excessive proportion of these high-carbon components can raise the final boiling point of the fuel beyond standard specifications. This not only reduces atomization efficiency at low temperatures but also increases the burden on the combustion system, indirectly impairing combustion efficiency.2.4. Energy density
Energy density exhibits a direct, positive correlation with the carbon number of the hydrocarbon molecules in jet fuel.23 Owing to their lower carbon and hydrogen atom counts, light fractions (C8–C10) deliver comparatively limited energy per unit volume. If their proportion in jet fuel is excessive, the aircraft must carry a larger fuel load to meet a given range requirement, increasing overall weight and potentially undermining efficiency manifested as higher fuel burn and reduced payload capacity. With more carbon and hydrogen atoms per molecule, heavier fractions release substantially more thermal energy during combustion, resulting in a significantly higher volumetric energy density compared to lighter fractions. Appropriately increasing the proportion of high-carbon components such as C12–C16 can extend aircraft range for a given fuel volume, making it one of the crucial strategies for enhancing the energy performance of jet fuel.2.5. Low-temperature fluidity
When an aircraft flies at an altitude of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 meters, the ambient temperature often drops below −50 °C. Therefore, aviation fuel must remain liquid at this temperature and maintain good fluidity to prevent solidification from causing blockages in the fuel lines.24 This performance characteristic is closely related to the carbon number, which is primarily reflected in both the structure and the carbon number of alkanes. Low-carbon n-alkanes (C8–C11) have relatively low freezing points. In particular, n-octane (C8, −56 °C) and n-nonane (C9, −51 °C) can remain liquid even at −50 °C. High-carbon n-alkanes (C12–C16) possess a pronounced propensity to crystallize. Even trace amounts can precipitate as wax crystals under cold conditions; these crystals agglomerate and progressively obstruct fuel filters and supply lines, starving the engine of fuel and potentially precipitating an in-flight shutdown. Consequently, industrial practice mandates the isomerization of C12–C16n-alkanes into their branched counterparts. The irregular molecular architecture of iso-alkanes disrupts ordered crystal packing, markedly depressing the freeze point and safeguarding fuel flow at high altitudes.
000 meters, the ambient temperature often drops below −50 °C. Therefore, aviation fuel must remain liquid at this temperature and maintain good fluidity to prevent solidification from causing blockages in the fuel lines.24 This performance characteristic is closely related to the carbon number, which is primarily reflected in both the structure and the carbon number of alkanes. Low-carbon n-alkanes (C8–C11) have relatively low freezing points. In particular, n-octane (C8, −56 °C) and n-nonane (C9, −51 °C) can remain liquid even at −50 °C. High-carbon n-alkanes (C12–C16) possess a pronounced propensity to crystallize. Even trace amounts can precipitate as wax crystals under cold conditions; these crystals agglomerate and progressively obstruct fuel filters and supply lines, starving the engine of fuel and potentially precipitating an in-flight shutdown. Consequently, industrial practice mandates the isomerization of C12–C16n-alkanes into their branched counterparts. The irregular molecular architecture of iso-alkanes disrupts ordered crystal packing, markedly depressing the freeze point and safeguarding fuel flow at high altitudes.
2.6. Combustion cleanliness
The cleanliness of jet fuel combustion directly governs engine efficiency and service life, and carbon number is the decisive determinant of both combustion completeness and carbon deposit formation.25,26 Hydrocarbon molecules within C8–C12 carbon range achieve an optimal balance between vaporization characteristics and combustion reactivity. They readily vaporize completely in the combustion chamber, mix thoroughly with air, and undergo nearly complete oxidation (producing primarily CO2 and H2O). As a result, soot and carbon deposit are minimized, reducing contamination of critical components such as the combustion chamber and fuel nozzles. This contributes to sustained high-efficiency engine operation over extended periods. As carbon number increases (C13–C16), hydrocarbon molecules become significantly more difficult to vaporize. They are less likely to vaporize rapidly and completely in the combustion chamber, particularly when minor issues arise in the fuel atomization system, such as insufficient injection pressure or nozzle wear. Incompletely vaporized fuel droplets are prone to thermal cracking under high temperatures, forming carbon deposit or soot. These deposits accumulate on combustion chamber walls, valves, and injector nozzles, which can impair heat dissipation, disrupt fuel spray patterns, reduce engine power, increase fuel consumption, and ultimately shorten the service life of engine.Indeed, the overall performance of aviation kerosene is not determined by a single carbon number fraction, but rather through the precise regulation of the content and molecular structures of hydrocarbons across different carbon number ranges, such as light fractions (C8–C10) and medium-to-heavy fractions (C12–C16). This strategic balancing enables the simultaneous optimization of four properties: volatility, energy density, low-temperature fluidity, and combustion cleanliness, ultimately ensuring the safe and efficient operation of aero-engines under diverse and demanding conditions. It is crucial to emphasize that the jet fuel specifications themselves preclude the inclusion of any component or concentration that would result in non-compliant properties. While trends in molecular properties can indicate formulation challenges, the definitive assessment is always compliance with the established specification limits.
ASTM D7566, entitled Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbon Fuels, establishes the technical criteria and approval framework for the safe blending of SAF components with conventional petroleum-derived turbine fuel that conforms to ASTM D1655 (Table 2). This standard outlines the refining pathways for sustainable aviation fuel (SAF) production and specifically addresses the following two technological routes: A1. Fischer–Tropsch Hydroprocessed Synthesized Paraffinic Kerosine and A4. Synthesized Fuel with Aromatics Derived by Alkylation of Light Aromatics from Non-Petroleum Sources.
| Origin of aviation fuel | Typical representative | |||
|---|---|---|---|---|
| n-Alkanes | Iso-alkanes | Cycloalkanes | Aromatics | |
| Conventional petroleum derivatives | ✓ | ✓ | ✓ | ✓ |
| A1. Fischer–Tropsch hydroprocessed synthesized paraffinic kerosine (FT-SPK) | ✓ | ✓ | ✓ | ✗ |
| A2. Synthesized paraffinic kerosine from hydroprocessed esters and fatty acids | ✓ | ✓ | ✓ | ✗ |
| A3. Synthesized iso-paraffins from hydroprocessed fermented sugars | ✓ | ✓ | ✓ | ✗ |
| A4. Synthesized kerosine with aromatics derived by alkylation of light aromatics from nonpetroleum sources (FT-SPK/A) | ✓ | ✓ | ✓ | ✓ |
| A5. Alcohol-to-jet synthetic paraffinic kerosene (ATJ-SPK) | ✓ | ✓ | ✓ | ✗ |
| A6. Synthesized kerosene from hydrothermal conversion of fatty acid esters and fatty acids | ✓ | ✓ | ✓ | ✓ |
| A7. Synthesized paraffinic kerosine fromhydroprocessed hydrocarbons, esters and fatty acids | ✓ | ✓ | ✓ | ✗ |
| A8. Alcohol-to-jet synthetic paraffinic kerosene with aromatics (ATJ-SKA) | ✓ | ✓ | ✓ | ✓ |
Differences in the composition of the products from the two pathways, particularly in aromatic content, lead to significant distinctions in their physicochemical properties and compatibility with aviation applications: The density of FT-SPK fuel is typically lower than that required by the ASTM D1655 standard for conventional jet fuel. This reduced density may compromise the volumetric energy density of the fuel; direct use in large quantities could potentially affect aircraft range and requires careful evaluation. Due to the near absence of aromatic hydrocarbons (which in conventional petroleum-derived jet fuel help maintain the swelling of elastomeric seals), the low aromatic content in FT-SPK fuel can lead to seal shrinkage; prolonged exposure may subsequently degrade sealing performance and increase the risk of fuel leakage. Through alkylation reactions that introduce aromatic and other heavy components, FT-SPK/A fuel readily meets the density requirements of ASTM D1655. It matches the energy density level of conventional jet fuel without further adjustment, thereby avoiding range limitations associated with low density. The aromatics present in FT-SPK/A fuel simulate the swelling effect that conventional jet fuel has on elastomeric seals, effectively mitigating the shrinkage risk associated with FT-SPK fuel. Moreover, its compositional properties offer better compatibility with metallic engine components, rubber fuel lines, and other materials, reducing equipment adaptation issues caused by fuel composition differences. Currently, A1 FT-SPK dominates SAF industrial deployment. Globally, leading energy and bioenergy companies have adopted this route for large-scale production. Leading companies including Sasol, TotalEnergies, Shell, and JM/bp, and Qatar Fuel have also adopted it for SAF production, collectively forming the backbone of the current SAF market.
3. Fischer–Tropsch synthesis
Fischer–Tropsch Synthesis (FTS) is fundamentally a chemical process that converts syngas (a mixture primarily composed of CO and H2) into hydrocarbons. Syngas can be derived from diverse sources: conventional routes such as natural-gas reforming or coal gasification, as well as renewable feedstocks including biomass.27 This diversity in raw materials, coupled with its potential sustainability, makes FTS widely regarded as one of the most promising technological pathways for producing SAF.The reaction mechanisms of FTS have been highly controversial since the early studies by Fischer and Tropsch.28–30 Given that an in-depth exploration of the mechanisms is beyond the scope of this review, only an overview of the two main mechanisms is provided here:
3.1. Carbide mechanism
As the earliest proposed and most widely accepted pathway, the carbide mechanism holds that after the direct dissociation of CO and H2, surface carbon atoms are hydrogenated to form CHx species, which then polymerize. Chain termination is achieved through the abstraction or addition of hydrogen atoms.31,323.2. CO insertion mechanism
The CO insertion mechanism suggests that adsorbed CO reacts with surface hydrogen to generate aldehyde intermediates.33,34 Chain growth is realized via CO insertion, and chain termination occurs through hydrogenation. Studies have shown that the energy barrier of this pathway on cobalt terrace sites is lower than that of direct dissociation, so there is a possibility of the coexistence of multiple mechanisms.In addition to reaction pathways, the rate-determining step is also controversial. CO dissociation,35 carbon hydrogenation,36 oxygen removal,37 and chain termination38 have all been identified as key steps. This controversy arises from the complex influences of parameters such as temperature and hydrogen concentration. For example, CO dissociation is the dominant rate-controlling step at low temperatures, while hydrogenation plays a more significant role at high temperatures.30 Furthermore, the phase structure of cobalt under reaction conditions is crucial for catalytic activity and selectivity.
The mechanisms underlying FTS generally comprise three elementary steps: chain initiation, chain growth, and chain termination. These steps are well described by the Anderson–Schulz–Flory (ASF) model, a classical statistical framework that governs the product distribution in chain-growth polymerization under ideal conditions. The model is characterized by the chain growth probability factor (α-value), defined as the ratio of the chain propagation rate to the sum of the propagation and termination rates. By modulating the α-value, the selectivity of conventional FTS catalysts toward liquid fuels can be predicted (Fig. 3a). Since the core components of jet fuel are hydrocarbons in the C8–C16 range (including n-alkanes, iso-alkanes, cyclic hydrocarbons, and aromatics), the maximum theoretical selectivity for C8–C16 hydrocarbons in FTS products is limited to only 41% due to the constraints of the thermodynamics of the ASF distribution. This represents a bottleneck that restricts the direct production of jet fuel components via FTS.
 | ||
| Fig. 3 (a) Product selectivity of FTS under the ASF distribution law, (b) process conditions for the industrial FTS. | ||
The catalytic active sites for the FTS reaction primarily consist of transition metals such as nickel (Ni), cobalt (Co), iron (Fe), and ruthenium (Ru). Among these, Co-based and Fe-based catalysts are widely used in industrial applications due to their superior overall performance. Most researchers agree that the core active phase of iron-based catalysts is iron carbide (FexCy), while the active component of cobalt-based catalysts is metallic cobalt (Co0). However, some studies have reported that cobalt oxide, cobalt carbide, carbon-deposited cobalt species, and cobalt–support interfaces may also serve as active phases for this reaction.29 It should be noted, though, that these cobalt species must meet specific conditions to exhibit Fischer–Tropsch synthesis activity. Based on the reaction temperature, industrial FTS processes can be categorized into three types: Low-Temperature Fischer–Tropsch (LTFT), Medium-Temperature Fischer–Tropsch (MTFT), and High-Temperature Fischer–Tropsch (HTFT). The suitable temperature ranges for different catalysts are as follows: Co-based catalysts are exclusively used in LTFT processes, with an operating temperature range of 190–240 °C. Fe-based catalysts exhibit a broader operating temperature range and can be applied across all three process types: LTFT (approximately 210–240 °C), MTFT (approximately 270–290 °C), and HTFT (approximately 330–350 °C) (Fig. 3b).
Currently, the dominant industrial routes for producing SAF via FTS are Co-LTFT and Fe-HTFT. In terms of Co-LTFT, the products are predominantly n-alkanes with a high α-value (indicating good selectivity for long-chain hydrocarbons). These alkanes can be directly converted into hydrocarbons within the C8–C16 jet fuel range through subsequent hydrocracking processes, effectively ensuring a high yield of jet fuel. This makes Co-LTFT the preferred route for large-scale SAF production at present. Conversely, the product composition of Fe-HTFT is relatively complex, containing not only n-alkanes but also significant amounts of iso-alkanes, olefins, aromatics, and oxygenates (such as alcohols, aldehydes, and acids). Although these by-products have high potential value for chemical utilization, they require complex downstream separation and upgrading processes, such as hydrotreating and isomerization, to be converted into qualified jet fuel components. This leads to significantly increased process costs and technical complexity.39
4. Future trends in Fischer–Tropsch synthesis to aviation kerosene
As the aviation industry expands its demand for SAF production capacity and imposes higher requirements on low-carbon and cost-effective processes under the dual-carbon goals, the limitations of the two-step process have become increasingly evident. On the one hand, the separation, transportation, and secondary reactions of the intermediate product (the FTS-derived wax) require additional energy consumption and equipment investment, resulting in overall higher process energy consumption. On the other hand, conventional FTS processes largely depend on high-purity syngas (CO/H2) and do not adequately consider the resource utilization of CO2 in the feed gas. This not only increases the cost of upstream syngas purification but also leads to carbon resources waste and higher greenhouse gas emissions. Therefore, this section will provide a systematic outlook on the future development trends of FTS for producing SAF, with a focus on Direct Biomass Syngas-to-SAF and CO2-Modified Fischer–Tropsch Synthesis.4.1. Direct biomass syngas-to-SAF
Biomass as a widely available and sustainable resource can be converted into high-calorific-value syngas through thermochemical gasification in the presence of gasifying agents (typically pure oxygen, steam, or carbon dioxide).40 The resulting syngas, primarily composed of H2, CO, CH4, and CO2, requires pretreatment to remove particulates, ash, tar, trace metals, and acid gases before being fed to FTS, where it is transformed into a mixture of liquid fuels and waxes. To further enhance the selectivity toward liquid fuels, the FTS products must undergo additional refining steps such as hydrocracking. Currently, the two-step process of coupling FT with hydrocracking for producing SAF is relatively mature and consequently falls outside the scope of this review. Alternatively, a one-step system integrating FTS and hydrocracking offers a pathway for the direct conversion of CO to hydrocarbons.41,42 meeting the distillation requirements of SAF. This integrated approach significantly simplifies the process, offering potential advantages in both efficiency and cost-effectiveness. Therefore, the development of highly selective FTS technologies capable of directly synthesizing specific liquid fuel fraction, avoiding subsequent refining steps, represents a critical research direction for optimizing current industrial processes.43The core challenge in converting biomass-derived syngas into aviation fuel lies in the precise design of bifunctional catalysts.44 Such catalysts must simultaneously possess active sites for FTS, to facilitate long-chain hydrocarbon formation, and sites capable of catalyzing isomerization reactions, thereby achieving a synergistic “chain growth-structure regulation” mechanism.45 From a product distribution perspective, Co-based catalysts predominantly yield linear alkanes (n-paraffins) in FTS reactions, accompanied by a high chain growth probability factor (α-value). This product profile is particularly conducive to subsequent isomerization processes. As a result, Co-based catalysts have emerged as a central focus in one-step aviation fuel synthesis research.46
In term of Co-based catalysts, the active FTS sites are metallic cobalt (Co0) nanoparticles whose size distribution critically dictates intrinsic activity, structural stability, and product selectivity.47 Moreover, the acidic sites of the zeolite support serve as active centers for hydrocracking reactions in bifunctional catalysts. The acidic properties of the support (such as acid strength and density) directly determine hydrocracking efficiency and the resulting carbon chain distribution of products.48,49 Current research trends highlight that catalyst design featuring mesoporous structures and the multi-functionalization of zeolites (acid site-metal site synergy) has become central to FTS. For zeolite supports, the pore structure significantly influences FTS product distribution by modulating confinement effects and diffusion behavior:50,51 excessively small pores favor the formation of C1–C4 short-chain hydrocarbons, while overly large pores promote non-selective cracking of long-chain hydrocarbons, making it challenging to precisely target the aviation fuel range (C8–C16). Furthermore, the acidic properties of zeolite supports, including acid strength, acid density, and acid type, are also critical factors affecting FTS product distribution, as they collectively determine both the carbon chain length and the degree of structural isomerization.52
Cai et al. prepared a series of cobalt-based catalysts (with 15 wt% Co loading) supported on aluminum-doped SBA-15 (average pore size: 4–5 nm) with varying Al/Si ratios, and applied them to FTS for aviation fuel.53 The study revealed that although the mesoporous SBA-15 support exhibits typical characteristics of mesoporous materials, it also imposes certain restrictions on the formation of long-chain hydrocarbons. Meanwhile, as the number of Brønsted acid sites increased (with higher Al content), the selectivity toward aviation fuel-range hydrocarbons (C8–C18) showed a trend of first increasing and then decreasing. At an Al/Si ratio of 0.01, the cobalt-based catalyst achieved the highest selectivity toward C8–C18 aviation fuel fractions (52.4%), along with a high ratio of iso- to n-alkanes (i/n-alkanes) of 20.2, significantly outperforming the pure SBA-15-supported catalyst without Al doping (Fig. 4a). Recently, SeongWoo Jeong et al.54 further investigated the regulatory mechanism of the physicochemical properties of zeolites on the selectivity of hydrocarbon products in FTS. Experimental results demonstrated that, compared to conventional microporous FT-H-ZSM-5-40 zeolite, the mesopore-modified FT-meso-H-ZSM-5-40 zeolite reduced the formation of heavy hydrocarbon components by 60% due to the introduced mesoporous structure (Fig. 4b). In particular, when the Si/Al ratio of H-ZSM-5 zeolite was ≤40, its isomerization and cracking functionalities were fully utilized, significantly enhancing the selectivity toward C5–C20 liquid fuels.
The type and loading of promoters also significantly influence the selectivity toward aviation kerosene-range hydrocarbons. Li et al. designed and synthesized a Co nanoparticle-supported mesoporous Y zeolite catalyst (Co/Ymeso) and systematically investigated the effects of different alkali and rare earth metal promoters (Na, Ce, La, Li, K) on the catalytic activity and product selectivity in FTS (Fig. 4c).55 The study revealed that through precise modulation of the pore structure and acidic properties of the mesoporous Y zeolite, the prepared Co/Ymeso-La catalyst achieved a selectivity of 72% toward the aviation kerosene fraction, demonstrating excellent catalytic performance. Bai et al. reported a series of Co/TUD-1 catalysts in which different promoter (Al, Zr, La, Ce) were incorporated into the TUD-1 framework to systematically pore structure and acidic properties.56 The introduction of different metals altered both the density and strength of Brønsted and Lewis acid sites on the catalyst surface. Among them, Co/Al-TUD-1, possessing an optimal pore structure and abundant Brønsted acid sites, efficiently promoted the C–C coupling of chain growth while simultaneously enabling hydrocracking/isomerization of long-chain hydrocarbons to the targeted jet-fuel range, affording an aviation-fuel selectivity of 51.3% (Fig. 4d). Yang et al. prepared a series of Co/Y-b-x catalysts.57 Experimental results demonstrated that with an appropriate Y incorporation ratio (e.g., in the Co/Y-b-3 catalyst), the selectivity toward aviation kerosene-range hydrocarbons reached 41.2%. This improvement was attributed to the role of Y in significantly enhancing the dispersion and reducibility of the Co3O4 precursor, thereby reducing the average crystallite size of metallic Co and optimizing the distribution of active sites. However, it should be noted that excessive Y3+ addition can lead to hydrolysis upon adsorption on the H-β zeolite surface, resulting in an abnormal increase in the number of Brønsted acid sites, which adversely affects the catalytic performance.
Therefore, achieving high selectivity toward aviation kerosene requires the selection of a zeolite support with a pore size matched to the target carbon chain length (C8–C16) and appropriate acidic properties, including acid strength, density, and type. By leveraging spatial confinement effects and modulating diffusion behavior through the carrier's pore structure, it is essential to facilitate the efficient generation of target hydrocarbons (C8–C16) while simultaneously suppressing excessive cracking (which produces short-chain hydrocarbons) and the formation of undesired long-chain hydrocarbons (>C16). This strategy ultimately enhances both the yield and quality of aviation fuel (e.g., by increasing the iso-alkane ratio and improving the uniformity of the fractional distribution).
Although the one-step synthesis of aviation kerosene from biomass-derived syngas offers notable advantages—such as process simplification, cost reduction, and significant potential for carbon emission reduction—this technology still faces the critical challenge of low jet fuel yield. Addressing this issue urgently requires breakthroughs in the development of novel catalysts (e.g.), highly active bifunctional catalysts and promoters with atom-level precision and innovations in synthesis processes, such as the optimization of reaction conditions and improvements in reactor design.
4.2. Tandem catalysis—CO2-modified Fischer–Tropsch synthesis
The original impetus for developing SAF was to curtail the CO2 emissions associated with conventional petroleum-derived jet fuel. The effective utilization of carbon dioxide is also a critical research direction for SAF. Conventional FTS processes largely depend on high-purity syngas (CO/H2) and do not adequately consider the utilization of CO2 in the feed gas as a resource. CO2-Modified Fischer–Tropsch Synthesis (CO2-MFTS) is a process that integrates the reverse water–gas shift (RWGS) reaction with the FTS in a single reactor for hydrocarbon synthesis.58–60 Since the RWGS reaction is endothermic and the FTS is exothermic, the process not only utilizes CO2 effectively but also reduces overall energy consumption.61 The tandem reaction process62 comprises two steps: the partial reduction of CO2 to CO via the RWGS reaction, followed by the conversion of the resulting CO into hydrocarbons through the FTS reaction.63,64 The reaction mechanism primarily involves the following steps (Fig. 5): (1) adsorption of CO and H2 onto active sites; (2) hydrogenation of adsorbed CO2 and H species to form HCOO* or *HOCO intermediates; (3) reduction of these intermediates to CO; and (4) chain growth via FTS. One proposed pathway suggests that CO is first converted into HCO*, which is subsequently reduced to an alkyl group to initiate chain growth. In contrast, an alternative mechanism based on carburization proposes that CO dissociates into C* and O*, followed by hydrogenation to form *CH2 and H2O(g). The surface *CH2 species then undergo coupling to facilitate carbon chain growth. This tandem catalytic process relies two distinct active sites on the catalyst, one for RWGS and the other for FTS, to directly convert CO2 and H2 into hydrocarbons. Compared to Co-based catalysts with low RWGS activity, Fe-based catalysts have emerged as a research hotspot in CO2-MFTS due to their intrinsic activity for both RWGS and FTS. Therefore, this section primarily focuses on reviewing iron-based catalysts. Existing research findings will be summarized from two perspectives: catalyst design and reaction condition control.The support plays a decisive role in CO2 hydrogenation. Beyond merely dispersing the active phase, it directs the reaction pathway via metal–support interactions. Commonly used supports in CO2-MFTS include classical materials such as SiO2,71 ZrO2 (ref. 72) and Al2O3,73 as well as emerging carbon-based materials like MOF-derived carbons74 and carbon nanofibers.75 In recent years, crystalline microporous and mesoporous molecular sieves have established themselves as highly versatile catalytic platforms for CO2-based Fischer–Tropsch synthesis (CO2-FTS). These materials leverage their intrinsic Brønsted and Lewis acid sites to drive essential elementary reactions—including C–C bond scission and skeletal isomerization.76 Concurrently, their well-defined channel structures facilitate the efficient dispersion and stabilization of active metal phases via spatial confinement, shape selectivity, and electronic synergy.77 As a result, the integrated catalytic system exhibits enhanced activity, improved product selectivity, and exceptional hydrothermal stability. For example, zeolites with large internal channels favor C5+ hydrocarbon formation. Eun Cheol Ra et al. reports the composite of Na/ZnFe2O4 with molecular sieves of different topological structures (including ZSM-5, ZSM-11, and SSZ-13) leads to significant differences in the distribution of hydrocarbon products (Fig. 7).78 When combined with ZSM-5 (pore size: 0.55–0.60 nm, zigzag channel structure), the catalyst produces hydrocarbons rich in aromatics and iso-alkanes, making it suitable for gasoline blending. In contrast, composites with ZSM-11 (straight channels, similar pore size to ZSM-5) show significantly reduced aromatic content, and the product composition better meets the specifications of jet fuel, thus being more suitable for its production. When SSZ-13 (pore size only 3.8 Å, classified as a small-pore molecular sieve) is used, the catalytic products are predominantly C2–C4 light olefins. Besides topology, the nature, density and strength of acid sites in the zeolite are equally important for selectivity. Jiang et al. engineered a CoFe/HZSM-5(40) catalyst, in which the synergy between tailored zeolite acidity and a proximity effect enhanced the C5+ selectivity to 73.4%.79 More recently, Amoo introduced a C–Na–Fe ternary system integrated with integrated with ZSM-22 and ZSM-5 for the conversion of CO2 to liquid fuels for CO2-to-liquid fuels conversion. Under CO2 hydrogenation conditions, the HZSM-22 composite oligomerizes light olefins into C5+ hydrocarbons that are dominated by iso-alkenes and iso-paraffins, whereas the ZSM-5 analogue enriches aromatics.80 Wei et al. developed a Na-Fe3O4/HZSM-5 multifunctional catalyst in which Fe3O4 drives the RWGS step, Fe5C2 generates light olefins, and Brønsted acid sites in the zeolite mediate their subsequent oligomerization, aromatization and isomerization. This catalyst exhibits 78% selectivity toward C5–C11 hydrocarbons with low CH4 and CO formation, yielding a product fraction rich in iso-paraffins and aromatic compounds.81
Alkali metals (especially K and Na) enhance surface basicity, promoting CO2 conversion, olefin formation and chain growth.82 Potassium is commonly introduced as K2CO3 through impregnation. Krausser et al. systematically compared the catalytic performance of three potassium promoters (K2CO3, KCl and K2Si2O5) in CO2-FTS and employed in situ XPS to probe their interactions with an Fe-based catalyst. The study revealed that, under reaction conditions, K2CO3 exhibits a markedly superior interfacial mobility relative to the other potassium salts, enabling more efficient enrichment on the catalyst surface.63 Adrian Ramirez and colleagues utilized the unique CO2 capture capability of K2CO3 to fabricate a K2CO3-promoted iron-based catalyst (Fe2O3@K2CO3). In this system, K2CO3 activates CO2 to form CO through intermediates such as KHCO3 and KOOCH. The released CO subsequently undergoes Fischer–Tropsch synthesis (FTS) on iron carbide sites to produce olefins. Notably, this catalyst demonstrates high selectivity comparable to that of commercial Fischer–Tropsch catalysts.83 Chen et al. demonstrated that potassium promoters can reduce the size of iron nanoparticles, enhance CO2 adsorption, and facilitate the formation of iron carbide active phases, thereby promoting the generation of C5+ hydrocarbons through C–C coupling.74 Zhu et al. employed in situ X-ray diffraction (XRD), in situ Fourier transform infrared spectroscopy (FTIR), and temperature-programmed techniques to systematically investigate the regulatory mechanism of potassium promoters on iron-based catalysts during CO2 hydrogenation. The results revealed that the incorporation of K significantly enhances the CO2 adsorption capacity of the catalyst. It not only promotes the reaction kinetics between CO2 and dissociated hydrogen but also accelerates the carburization process of iron species, specifically facilitating the transformation from Fe(0) to Fe3C, and further to Fe5C2. Moreover, through a series of competitive adsorption and surface reactions, the K promoter helps establish a microenvironment that effectively suppresses oxidative attack on Fe5C2 by H2O and CO2, thereby preserving catalytic activity. By optimizing both the K loading and the CO pretreatment parameters, the developed iron-based catalyst achieved highly efficient conversion of CO2 into C2–C4 olefins and C5+ long-chain hydrocarbons.84 Dai et al. synthesized a series of K-promoted Fe/CNT catalysts via co-impregnation and systematically evaluated their performance for CO2 hydrogenation in a slurry-phase reactor. The experimental results indicated that as the K/Fe molar ratio increased from 0 to 0.3, both the CO2 conversion and the selectivity toward C5+ hydrocarbons showed a synchronous improvement. However, further increasing the K/Fe molar ratio beyond 0.3 resulted in only marginal enhancements in catalytic performance. The catalyst with a K/Fe molar ratio of 0.3 exhibited the optimal performance, achieving a CO2 conversion of 23.7% and a C5+ hydrocarbon selectivity of 56%. Multi-scale characterization established the following structure–activity relationships: an appropriate K loading enlarges the specific surface area and strengthens CO2 chemisorption, thereby enhancing activity; however, excessive K loading leads to a reduction in specific surface area, decreased reduction degree of Fe-based catalyst, and diminished graphitization of the CNT support. These factors collectively suppress CO2 chemisorption and inhibit the formation of C5+ hydrocarbons.85
Focusing on sodium promoter, Yang et al. conducted a comprehensive investigation of the time-on-stream behavior of Fe-based catalysts during CO2 hydrogenation.86 By combining in situ characterization, ex situ techniques, and precise catalytic performance evaluations, the role of Na in maintaining catalytic stability and regulating the composition of the catalyst was demonstrated under dynamic reaction conditions. The experimental results indicate that the Na promoter effectively protects the active iron carbide phase from oxidative erosion by H2O/CO2 and prevents excessive reduction by H2, thereby ensuring structural and performance stability of the iron-based catalyst even under deliberately dynamic reaction environments. Liang et al. prepared a series of xNa/Fe-based catalysts by modulating the loading of Na promoter, which exhibited outstanding catalytic performance in the hydrogenation of CO2 to olefins.87 The study revealed a positive correlation between the amount of Na added and the formation of the active Fe5C2 phase, as well as the catalytic performance, specifically reflected in CO2 conversion and olefin selectivity. As the Na content increased, both CO2 conversion and olefin selectivity showed significant improvement initially, then gradually plateaued. Notably, the introduction of Na markedly promoted the formation and structural stability of the Fe5C2 phase under CO2 hydrogenation conditions. Based on these findings, the authors proposed a novel catalyst design strategy that enables simultaneous achievement of high C5+ yield and long-term operational stability in direct CO2 hydrogenation. Complementarily, Yoon et al. showed that an excessive Na loading (20 wt%) can further stabilize Fe-based catalysts. During 2000 h of continuous operation, the catalyst maintained a stable C5+ yield of 22% with a deactivation rate of merely 0.005% h−1.88
In the field of CO2-MFTS, transition metal promoters (e.g., Cu, Zn, Co, Mn) have garnered substantial attention.89–91 These promoters are often used in combination with alkali metals as co-promoters to cooperatively modulate the performance of Fe-based catalysts. Yao et al. demonstrated that an Fe-based catalyst without any promoter, while exhibiting high CO2 hydrogenation activity, yields an undesirable methane selectivity of 32.2% with only trace amounts of targeted liquid hydrocarbons. In contrast, catalysts (named as Fe–Zn–K, Fe–Cu–K and Fe–Mn–K) co-promoted with K and either Zn, Cu, or Mn demonstrated excellent CO2 conversion and high selectivity toward aviation kerosene fractions (C8–C16). Although the overall performances of these three systems are closely matched, the Fe–Mn–K catalyst still achieved slightly higher aviation kerosene selectivity (47.8%) compared to the Fe–Cu–K (40.8%) and Fe–Zn–K (45.1%) catalysts.92 Guo et al. employed co-precipitation to synthesize a series of NaFeMe catalysts (Me = Al, Co, Cu, Mn, Ni, Zn) for CO2 hydrogenation to liquid C5+ fuels. Among them, the NaFeZn catalyst exhibited the best performance at 320 °C, 2 MPa and 4000 mL gcat−1 h−1, delivering a CO2 conversion of 28.1% and a C5+ selectivity of 21.3%.93 H2-TPR and XPS tests confirmed strong Fe–Zn interaction and electron transfer favorable for CO2 reduction. Hwang et al. further elucidated the synergistic role of co-incorporating Cu and K into Fe-based catalysts. The results demonstrated that the Fe–Cu–K catalyst achieved a C5+ yield of 18.1%, which is 1.4 times and 7.8 times higher than those of the Fe–K (12.8%) and Fe–Cu (2.3%) catalysts. Through characterization techniques, including XRD, HR-TEM, H2-TPR, XPS and XAS, it was confirmed that in the presence of K, Cu can be successfully incorporated into the bulk crystal lattice of Fe. More importantly, the K promoter effectively maintains the active state of the Fe–Cu alloy phase during the reaction, preventing its deactivation due to structural reconstruction or oxidation.94
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is generally accepted as the optimal target.95 Fiato et al. conducted a systematic study on the effect of the H2/CO2 ratio, and their results indicated that although a higher H2/CO2 ratio can enhance the equilibrium conversion of CO2 to CO, it also leads to shorter hydrocarbon chain lengths, manifested as a significant increase in CH4 selectivity.96
1 is generally accepted as the optimal target.95 Fiato et al. conducted a systematic study on the effect of the H2/CO2 ratio, and their results indicated that although a higher H2/CO2 ratio can enhance the equilibrium conversion of CO2 to CO, it also leads to shorter hydrocarbon chain lengths, manifested as a significant increase in CH4 selectivity.96
The reaction temperature also exerts a pronounced influence on the product distribution in CO2-MFTS. This process integrates the endothermic reaction (RWGS) and the exothermic reaction (FTS) within a single reactor to achieve one-step hydrocarbon synthesis. Although increasing the temperature enhances CO2 conversion, excessively high temperatures tend to favor hydrogenation reactions over C–C coupling. In current studies, the reaction temperature is typically maintained within the range of 230–260 °C.97 Excessively low temperatures result in insufficient CO generation, hindering carbon chain initiation in FTS, while overly high temperatures shift the product distribution toward shorter-chain hydrocarbons.
Thermodynamically, higher reaction pressures favor carbon chain growth. However, high-pressure processes are typically associated with increased capital and operational costs. Consequently, most studies reported in the literature employ reaction pressures in the range of 1–3 MPa.98 Due to constraints imposed by reaction kinetics, thermodynamics, and the requirements of the tandem catalytic process, catalysts used in CO2-MFTS must possess both FexOγ phases active for the RWGS reaction and FexCγ phases active for the FTS. Notably, the active carbide phases a are seldom synthesized directly via chemical methods. Instead, they are typically formed gradually during catalyst pretreatment or under in situ reaction conditions through a three-step pathway: precursor reduction, carburization of reduced iron, and phase refinement. Under CO or syngas atmospheres, these reduced iron species can be further converted into various iron carbide polymorphs.99 Davis and co-workers further demonstrated that α-Fe and various carbides can coexist and interconvert dynamically, with the prevailing phase assemblage continuously responding to changes in the reaction environment.100 Samir Bensaid et al. conducted the first systematic study on the influence of pretreatment conditions on the performance of Na/Fe3O4 catalysts for CO2-MFTS. The experimental results demonstrated that certain pretreatment (particularly carburization at 400 °C) significantly promotes the formation of έ-Fe2.2C (the highly active iron-carbide phase), thereby enhancing the selectivity toward C5+ hydrocarbons. After this pretreatment, the C5+ yield rose from 14% to 18%, while the product slate achieved an optimal balance between light and heavy oil fractions. In conclusion, precise control over both catalyst pretreatment and reaction conditions is a crucial prerequisite for achieving high efficiency in CO2-MFTS.101
5. Outlook
FTS is the cornerstone technology for converting carbonaceous feedstocks such as coal, natural gas, or biomass into clean liquid fuels. The hydrocarbons with carbon chain lengths of C8–C16 produced by FTS can serve as serve as drop-in components for Jet A-1 and other certified aviation kerosene, providing a viable pathway to displace fossil-based fuels and curtail aviation-related CO2 emissions. However, the industrial application of FTS-derived jet fuel still faces two major technical bottlenecks: firstly, low CO conversion leads to insufficient feedstock utilization and high energy consumption; secondly, the selective control of C8–C16 hydrocarbon formation remains difficult. Due to the ASF distribution governing FTS, the carbon chain lengths of products follow a continuous distribution, often resulting in excessive formation of light hydrocarbons (C1–C4, such as methane and ethane) or long-chain waxes (C20+). These by-products necessitate additional separation and purification steps, significantly reducing the yield and economic viability of the jet fuel fraction. In response to the aforementioned challenges, recent research has adopted a combined strategy of catalyst system innovation and precise control of reaction conditions, leading to a notable increase in the selectivity toward jet fuel fractions and paving the way for practical application.5.1. Optimization direction of the catalyst system
The catalyst governs the reaction pathway and product distribution in FTS. Current research efforts are predominantly focused on the rational design of composite catalytic systems comprising active metal species and tailored supports. Among these, cobalt-based catalysts have emerged as the preferred active components for jet fuel-oriented synthesis due to their high CO hydrogenation activity, low methane selectivity, and strong ability to generate long-chain hydrocarbons. Meanwhile, zeolite molecular sieves with well-defined pore structures (0.5–0.6 nm, matching the kinetic diameter of C8–C16 hydrocarbons) and tunable acid sites are coupled with cobalt catalysts to construct metal–acid bifunctional composite systems. This integration enables precise control over the carbon chain length of the products. In addition, reaction temperature, pressure, and the H2/CO ratio dictate both catalyst activity and product distribution; their values must therefore be optimized in strict concert with the chosen catalyst architecture.5.2. Re-upgrading of the conventional FTS
Conventional FTS processes largely depend on high-purity syngas (CO/H2) and do not adequately consider the utilization of CO2 in the feed gas as a resource. This not only increases the cost of upstream syngas purification but also leads to carbon resource waste and higher greenhouse gas emissions. The CO2-MFTS route for producing aviation kerosene demonstrates significant advantages over conventional CO-FTS in terms of carbon emission reduction, feedstock flexibility, target product selectivity, process simplification, and sustainability, positioning it as a cutting-edge technological pathway for advancing green aviation fuel industrialization. Furthermore, CO2-FTS technology can be integrated with conventional FTS by converting CO2-rich tail gas into hydrocarbons through hydrogenation, enabling complete recycling of carbon. Although considerable progress has been made in the production of aviation kerosene via CO2-FTS, several scientific and technical challenges remain unresolved. These include the difficulty in the efficient activation of C–O bonds in CO2, the precise control over the selectivity of jet fuel-range hydrocarbons, the fulfillment of strict compositional requirements for aviation kerosene, and the suppression of competing side reactions such as methanation and short-chain hydrocarbon formation.FTS-derived SAF, now established as a cornerstone for the replacement of conventional petroleum derivatives, has evolved from the stage of “laboratory performance breakthroughs” to that of “industrial-scale technology maturation.” Future research and development efforts will focus on advancing one-step synthesis processes, achieved through the synergistic integration of reactors and catalytic systems design. This strategy enables the direct and complete production of certified jet fuel from syngas within a single reactor unit, significantly streamlining the traditional multi-stage production pathway and enhancing process efficiency.
Author contributions
In the development of this paper, the contributions of the authors were clearly delineated. Ang Li, Junhui Zheng, and Ziqi Wang collectively contributed to the conceptualization and original draft preparation, establishing the foundational research framework and core content. Ang Li and Zongwei Zhang were responsible for reviewing and revising the manuscript, enhancing its accuracy, logical coherence, and fluency to ensure the quality of the final work.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results have been included, and no new data have been generated or analyzed as part of this review.Acknowledgements
The authors gratefully acknowledge the support of Natural Science Foundation of Tianjin (Grant No. 23JCQNJC00200).Notes and references
- American Society of Testing Materials (ASTM), Global Outlook for Air Transport Protectionism on the Rise, 2025 Search PubMed.
- European Commission, ReFuelEU Aviation, 2023 Search PubMed.
- C. Voigt, J. Kleine, D. Sauer, R. H. Moore, T. Bräuer, P. Le Clercq, S. Kaufmann, M. Scheibe, T. Jurkat-Witschas, M. Aigner, U. Bauder, Y. Boose, S. Borrmann, E. Crosbie, G. S. Diskin, J. DiGangi, V. Hahn, C. Heckl, F. Huber, J. B. Nowak, M. Rapp, B. Rauch, C. Robinson, T. Schripp, M. Shook, E. Winstead, L. Ziemba, H. Schlager and B. E. Anderson, Commun. Earth Environ., 2021, 2, 114 CrossRef.
- J. Yang, Y. Chang, P. Ye, Z. Zhang, C. Zhang and B. Teng, Bioresour. Technol., 2025, 434, 132768 CrossRef CAS.
- A. E. Mansy, S. Daniel, C. K. Fonzeu Monguen, H. Wang, A. I. Osman and Z.-Y. Tian, Environ. Chem. Lett., 2025, 23, 419–461 CrossRef CAS.
- I. Zahid, M. H. Nazir, K. Chiang, F. Christo and M. Ameen, Curr. Opin. Green Sustainable Chem., 2024, 49, 100959 CrossRef CAS.
- J. Han, A. Elgowainy, H. Cai and M. Q. Wang, Bioresour. Technol., 2013, 150, 447–456 CrossRef CAS PubMed.
- H. Wei, W. Liu, X. Chen, Q. Yang, J. Li and H. Chen, Fuel, 2019, 254, 115599 CrossRef CAS.
- M.-O. P. Fortier, G. W. Roberts, S. M. Stagg-Williams and B. S. M. Sturm, Appl. Energy, 2014, 122, 73–2 CrossRef CAS.
- J. A. Okolie, D. Awotoye, M. E. Tabat, P. U. Okoye, E. I. Epelle, C. C. Ogbaga, F. Güleç and B. Oboirien, iScience, 2023, 26, 106944 CrossRef CAS.
- S. Bube, N. Bullerdiek, S. Voß and M. Kaltschmitt, Fuel, 2024, 366, 131269 CrossRef CAS.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- C. T. Chong and J. H. Ng, Nat. Commun., 2023, 14, 8156 CrossRef CAS PubMed.
- Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons, ASTM Standard D7566-24, ASTM International, 2024 Search PubMed.
- B. H. H. Goh, C. T. Chong, H. C. Ong, T. Seljak, T. Katrašnik, V. Józsa, J.-H. Ng, B. Tian, S. Karmarkar and V. Ashokkumar, Energy Convers. Manage., 2022, 251, 114974 CrossRef CAS.
- J. D. Guthrie, C. Rowell, S. E. Nowling, Y. J. Lee, R. J. Chen, C. B. Meier, G. Kilaz, M. E. Peretich and H. I. Kenttämaa, Energy Fuels, 2025, 39, 6319–6331 CrossRef CAS.
- Y. X. Jing, Q. E. Xia, X. H. Liu and Y. Q. Wang, ChemSusChem, 2017, 10, 4817–4823 CrossRef CAS PubMed.
- G. H. Lai, F. Wang, J. Jin and W. J. Tao, Fuel, 2026, 405, 136731 CrossRef.
- X. L. Chen, K. A. Orton, C. Mukarakate, K. Gaston, G. M. Fioroni, R. L. McCormick, M. B. Griffin and K. Iisa, Sustainable Energy Fuels, 2024, 8, 5504–5513 RSC.
- H. Jin, W. Yuan, W. Li, J. Yang, Z. Zhou, L. Zhao, Y. Li and F. Qi, Prog. Energy Combust. Sci., 2023, 96, 101076 CrossRef.
- Z. H. Song, X. S. Yan, Z. Y. Liu and X. Y. Yang, Appl. Sci., 2025, 15, 8098 CrossRef CAS.
- R. C. Boehm, Z. B. Yang, D. C. Bell, C. Faulhaber, E. Mayhew, U. Bauder, G. Eckel and J. S. Heyne, Energy Fuels, 2024, 38, 17128–17145 CrossRef CAS.
- L. Pan, Q. Deng, E. Xiutianfeng, G. K. Nie, X. W. Zhang and J. J. Zou, Prog. Chem., 2015, 27, 1531–1541 CAS.
- E. S. K. Why, H. C. Ong, H. V. Lee, W. H. Chen, N. Asikin-Mijan and M. Varman, Energy Convers. Manage., 2021, 243, 114311 CrossRef CAS.
- C. Ruan, L. Yu and X. C. Lu, Prog. Aerosp. Sci., 2025, 153, 101054 CrossRef.
- Z. Xu, W. T. Shi, M. H. Wang, S. H. Zhong, Y. Zhou, J. Z. Pei, L. T. Shao, K. Pan and Y. Song, Sustainable Energy Technol. Assess., 2025, 75, 104210 CrossRef.
- L. Yang, X. Ge, C. Wan, F. Yu and Y. Li, Renewable Sustainable Energy Rev., 2014, 40, 1133–1152 CrossRef CAS.
- T. H. Pham, Y. Qi, J. Yang, X. Duan, G. Qian, X. Zhou, D. Chen and W. Yuan, ACS Catal., 2015, 5, 2203–2208 CrossRef CAS.
- I. C. ten Have and B. M. Weckhuysen, Chem Catal., 2021, 1, 339–363 CAS.
- B. Zijlstra, R. J. P. Broos, W. Chen, G. L. Bezemer, I. A. W. Filot and E. J. M. Hensen, ACS Catal., 2020, 10, 9376–9400 CrossRef CAS.
- R. A. van Santen, M. M. Ghouri, S. Shetty and E. M. H. Hensen, Catal. Sci. Technol., 2011, 1, 891–911 RSC.
- W. Zhou, K. Cheng, J. Kang, C. Zhou, V. Subramanian, Q. Zhang and Y. Wang, Chem. Soc. Rev., 2019, 48, 3193–3228 RSC.
- B. Böller, K. M. Durner and J. Wintterlin, Nat. Catal., 2019, 2, 1027–1034 CrossRef.
- R. A. van Santen, I. M. Ciobîcă, E. van Steen and M. M. Ghouri, in Advances in Catalysis, ed. B. C. Gates and H. Knözinger, Academic Press, 2011, vol. 54, pp. 127–187 Search PubMed.
- S. Lyu, L. Wang, J. Zhang, C. Liu, J. Sun, B. Peng, Y. Wang, K. G. Rappé, Y. Zhang, J. Li and L. Nie, ACS Catal., 2018, 8, 7787–7798 CrossRef.
- R. Pestman, W. Chen and E. Hensen, ACS Catal., 2019, 9, 4189–4195 CrossRef CAS.
- W. Chen, R. Pestman, B. Zijlstra, I. A. W. Filot and E. J. M. Hensen, ACS Catal., 2017, 7, 8050–8060 CrossRef CAS PubMed.
- B. Zijlstra, R. J. P. Broos, W. Chen, H. Oosterbeek, I. A. W. Filot and E. J. M. Hensen, ACS Catal., 2019, 9, 7365–7372 CrossRef CAS.
- D. Moodley, T. Botha, R. Crous, J. Potgieter, J. Visagie, R. Walmsley and C. Dwyer, in Catalysis for a Sustainable Environment, 2024, ch. 6, pp. 73–116 Search PubMed.
- S. M. You, Y. S. Ok, D. C. W. Tsang, E. E. Kwon and C. H. Wang, Crit. Rev. Environ. Sci. Technol., 2018, 48, 1165–1213 CrossRef CAS.
- S. Sartipi, K. Parashar, M. J. Valero-Romero, V. P. Santos, B. van der Linden, M. Makkee, F. Kapteijn and J. Gascon, J. Catal., 2013, 305, 179–190 CrossRef CAS.
- X. H. Li, K. Asami, M. F. Luo, K. Michiki, N. Tsubaki and K. Fujimoto, Catal. Today, 2003, 84, 59–65 CrossRef CAS.
- L. C. Caballero, J. P. L. Perez and M. M. Nigra, J. Mater. Chem. A, 2025, 13, 25974–25990 RSC.
- H. Wang, Y. Pei, M. Qiao and B. Zong, in Production of Biofuels and Chemicals with Bifunctional Catalysts, ed. Z. Fang, R. L. Smith and H. Li, 2017, pp. 137–158, DOI:10.1007/978-981-10-5137-1_4.
- T. S. Zhao, J. Chang, Y. Yoneyama and N. Tsubaki, Ind. Eng. Chem. Res., 2005, 44, 769–775 CrossRef CAS.
- Y. C. Zhang, R. J. Hu, H. Q. Su and Y. Su, Energy Technol., 2024, 12, 2301280 CrossRef CAS.
- J.-Y. Park, Y.-J. Lee, P. R. Karandikar, K.-W. Jun, K.-S. Ha and H.-G. Park, Appl. Catal., A, 2012, 411–412, 15–23 CrossRef CAS.
- D. Deviana, G. Bae Rhim, Y.-e. Kim, H. Song Lee, G. Woo Lee, M. Hye Youn, K. Young Kim, K. Young Koo, J. Park and D. Hyun Chun, Chem. Eng. J., 2023, 455, 140646 CrossRef CAS.
- X. Li, Y. Chen, S. Liu, N. Zhao, X. Jiang, M. Su and Z. Li, Chem. Eng. J., 2021, 416, 129180 CrossRef CAS.
- G. H. Yang, J. J. He, Y. Yoneyama, Y. S. Tan, Y. Z. Han and N. Tsubaki, Appl. Catal., A, 2007, 329, 99–105 CrossRef CAS.
- Q. H. Lin, Q. D. Zhang, G. H. Yang, Q. J. Chen, J. Li, Q. H. Wei, Y. S. Tan, H. L. Wan and N. Tsubaki, J. Catal., 2016, 344, 378–388 CrossRef CAS.
- X. H. Li, M. F. Luo and K. Asami, Catal. Today, 2004, 89, 439–446 CrossRef CAS.
- Y. Cai, X. Xu, H. Wang, L. Wang, L. Chen, R. Li, J. Ding, H. Wan and G. Guan, Ind. Eng. Chem. Res., 2018, 57, 3844–3854 CrossRef CAS.
- S. Jeong, J. J. Lim, J. Yoo, Y. S. Yun, S. J. Park, J. W. Lee, K. Kim, H. S. Chae, Y. J. Lee and S. J. Han, Fuel, 2026, 404, 136159 CrossRef CAS.
- J. Li, Y. He, L. Tan, P. Zhang, X. Peng, A. Oruganti, G. Yang, H. Abe, Y. Wang and N. Tsubaki, Nat. Catal., 2018, 1, 787–793 CrossRef CAS.
- J. Bai, W. Chen, F. B. Kabwe, X. Shang, G. Lin, Q. Jiang and C. Wang, Ind. Eng. Chem. Res., 2025, 64, 10414–10424 CrossRef CAS.
- M. Yang, L. Zhu, Y. Zhuo, J. Liang and S. Wang, Sustainable Energy Fuels, 2020, 4, 3528–3536 RSC.
- E. Corrao, F. Salomone, E. Giglio, M. Castellino, S. M. Ronchetti, M. Armandi, R. Pirone and S. Bensaid, Chem. Eng. Res. Des., 2023, 197, 449–465 CrossRef CAS.
- S. J. Han, J. Chen, H.-G. Park, K.-W. Jun and S. K. Kim, Chem. Eng. J., 2025, 508, 161006 CrossRef CAS.
- F. Jiang, B. Liu, S. Geng, Y. Xu and X. Liu, Catal. Sci. Technol., 2018, 8, 4097–4107 RSC.
- W. Meng, B. C. A. d. Jong, H. v. d. Bovenkamp, G.-J. Boer, G. Leendert Bezemer, A. Iulian Dugulan and J. Xie, Chem. Eng. J., 2024, 489, 151166 CrossRef CAS.
- E. V. Ramos-Fernandez, J. L. Santos, D. K. Alsaadi, A. Bavykina, J. M. R. Gallo and J. Gascon, Chem. Sci., 2025, 16, 530–551 RSC.
- L. Krausser, V. A. Kondratenko, H. Lund, S. Bartling, E. A. Fedorova, Q. Yang and E. V. Kondratenko, ACS Catal., 2025, 15, 10627–10638 CrossRef CAS.
- J. Zhu, S. Shaikhutdinov and B. R. Cuenya, Chem. Sci., 2025, 16, 1071–1092 RSC.
- M. Claeys, E. van Steen, T. Botha, R. Crous, A. Ferreira, A. Harilal, D. J. Moodley, P. Moodley, E. du Plessis and J. L. Visagie, ACS Catal., 2021, 11, 13866–13879 CrossRef CAS.
- D. Liuzzi, E. Fernandez, S. Perez, E. Ipiñazar, A. Arteche, J. L. G. Fierro, J. L. Viviente, D. A. P. Tanaka and S. Rojas, Rev. Chem. Eng., 2022, 38, 55–76 CrossRef CAS.
- F. C. Emiel de Smit, A. M. Beale, O. V. Safonova, W. van Beek, P. Sautet and B. M. Weckhuysen, J. Am. Chem. Soc., 2010, 132, 14928–14941 CrossRef PubMed.
- L. Krausser, Q. Yang and E. V. Kondratenko, ChemCatChem, 2024, 16, e202301716 CrossRef CAS.
- T. Li, H. Zhao, L. Guo, G. Liu, J. Wu, T. Xing, T. Li, Q. Liu, J. Sui, Y. Han, J. Liang, Y. He and N. Tsubaki, ACS Catal., 2025, 15, 1112–1122 CrossRef CAS.
- D. Wang, Z. Xie, M. D. Porosoff and J. G. Chen, Chem, 2021, 7, 2277–2311 CAS.
- X. Y. Liang, F. Yan, H. J. Liu, F. Xie, Y. X. Lu, L. Y. Huang, X. H. Shen and Z. T. Zhang, J. Environ. Chem. Eng., 2025, 13, 117429 CrossRef CAS.
- S. S. Bibi, H. Jo, J. R. Sugiarto, S. Ahmed, M. Irshad, W. Yoon and J. Kim, J. Energy Chem., 2025, 108, 769–784 CrossRef CAS.
- D. Uykun Mangaloğlu, P. Güzel, S. Şenkan and H. Atakül, Fuel, 2024, 371, 131935 CrossRef.
- X. Chen, R. Gao, Q. Wang, K. Hu, F. Wang, C. Deng, L. Xu, C. Zhang, K.-W. Jun, S. Ki Kim, T. Zhao, H. Wan and G. Guan, Fuel, 2024, 364, 131061 CrossRef CAS.
- F. Karaçoban, Y. Bonne, T. van Haasterecht and J. H. Bitter, J. CO2 Util., 2025, 97, 103122 CrossRef.
- H. Kirsch, N. Lochmahr, C. Staudt, P. Pfeifer and R. Dittmeyer, Chem. Eng. J., 2020, 393, 124553 CrossRef CAS.
- H. D. Kim, H.-t. Song, A. Fazeli, A. Alizadeh Eslami, Y. S. Noh, N. Ghaffari Saeidabad, K.-Y. Lee and D. J. Moon, Catal. Today, 2022, 388–389, 410–416 CrossRef CAS.
- E. C. Ra, S. Jang, D. G. Oh, J. H. Lee, H. E. Kim, E. H. Kim, K. Y. Kim, M. H. Lee, K. H. Kim, J. H. Kwak and J. S. Lee, Chem. Eng. J., 2023, 470, 144335 CrossRef CAS.
- Q. Jiang, K. Jin, L. Wei, P. Ren, H. Wang, T. Zhu, Y. Cui, Q. Zhang, X. Wen and C. Wang, ACS Catal., 2025, 15, 13233–13242 CrossRef CAS.
- C. C. Amoo, J. I. Orege, Q. Ge and J. Sun, Appl. Catal., B, 2024, 340, 123193 CrossRef CAS.
- K. Wang, N. Liu, J. Wei, Y. Yu, J. Zhang, J. I. Orege, L. Song, Q. Ge and J. Sun, Chem. Commun., 2023, 59, 13767–13770 RSC.
- W. Ngantsoue-Hoc, Y. Q. Zhang, R. J. O'Brien, M. S. Luo and B. H. Davis, Appl. Catal., A, 2002, 236, 77–89 CrossRef CAS.
- A. Ramirez, S. Ould-Chikh, L. Gevers, A. D. Chowdhury, E. Abou-Hamad, A. Aguilar-Tapia, J. L. Hazemann, N. Wehbe, A. J. Al Abdulghani, S. M. Kozlov, L. Cavallo and J. Gascon, ChemCatChem, 2019, 11, 2879–2886 CrossRef CAS.
- J. Zhu, M. Mu, Y. Liu, M. Zhang, G. Zhang, Z. Cheng, B. Hang Yin, A. C. K. Yip, C. Song and X. Guo, Chem. Eng. Sci., 2023, 282, 119228 CrossRef CAS.
- L. Y. Dai, Y. Chen, R. J. Liu, X. Li, N. Ullah and Z. H. Li, Appl. Organomet. Chem., 2021, 35, e6253 CrossRef CAS.
- Y. Wang, S. Kazumi, W. Z. Gao, X. H. Gao, H. J. Li, X. Y. Guo, Y. Yoneyama, G. H. Yang and N. Tsubaki, Appl. Catal., B, 2020, 269, 118792 CrossRef CAS.
- B. L. Liang, H. M. Duan, T. Sun, J. G. Ma, X. Liu, J. H. Xu, X. Su, Y. Q. Huang and T. Zhang, ACS Sustainable Chem. Eng., 2019, 7, 925–932 CrossRef CAS.
- W. Yoon, H. Jo, S. Ahmed, M. K. Khan, M. Irshad, J. Lee, S. S. Bibi and J. Kim, Chem. Eng. J., 2024, 495, 153617 CrossRef CAS.
- L. Zhang, J. Zhao, T. Li, W. Gao, H. Li, L. Wu, W. Xia, W. Wu, C. Wang, F. Wang, S. Yasuda, X. Guo, Y. He, G. Yang, G. Liu, Z. Jin, J. Zeng and N. Tsubaki, Nano Lett., 2025, 25, 4904–4912 CrossRef CAS PubMed.
- G. Song, M. Li, L. Xu, X. Yang, M. A. Nawaz, H. Yuan, Z. Zhang, X. Xu and D. Liu, Ind. Eng. Chem. Res., 2022, 61, 6820–6830 CrossRef CAS.
- C. Wen, X. Xu, X. Song, L. Lu, X. Zhuang, K. Jin, Q. Jiang, X. Zhang, L. Chen, C. Wang and L. Ma, Energy Fuels, 2023, 37, 518–528 CrossRef CAS.
- B. Yao, T. Xiao, O. A. Makgae, X. Jie, S. Gonzalez-Cortes, S. Guan, A. I. Kirkland, J. R. Dilworth, H. A. Al-Megren, S. M. Alshihri, P. J. Dobson, G. P. Owen, J. M. Thomas and P. P. Edwards, Nat. Commun., 2020, 11, 1–4 CrossRef PubMed.
- L. Guo, H. Y. Yang, J. Qiu, T. Zhang, Y. H. Xu, Z. Y. Duan, Y. L. Yang, X. M. Dai, L. N. Liu and C. Y. Zhang, J. Environ. Chem. Eng., 2025, 13, 117796 CrossRef CAS.
- S.-M. Hwang, S. J. Han, J. E. Min, H.-G. Park, K.-W. Jun and S. K. Kim, J. CO2 Util., 2019, 34, 522–532 CrossRef CAS.
- L. Torrente-Murciano, R. S. L. Chapman, A. Narvaez-Dinamarca, D. Mattia and M. D. Jones, Phys. Chem. Chem. Phys., 2016, 18, 15496–15500 RSC.
- R. A. Fiato, E. Iglesia, G. W. Rice and S. L. Soled, in Advances in Chemical Conversions for Mitigating Carbon Dioxide, ed. T. Inui, M. Anpo, K. Izui, S. Yanagida and T. Yamaguchi, 1998, vol. 114, pp. 339–344 Search PubMed.
- M. A. Lwazzani, A. A. García Blanco, M. Biset-Peiró, E. Martín-Morales and J. Guilera, Sustainable Energy Fuels, 2025, 9, 1095–1108 RSC.
- B. Ali, M. T. Arslan, I. Hussain, S. H. Talib, A. S. Farooqi, A. M. Alhassan, J. N. Musa, B. O. Yusuf, K. R. Alhooshani and S. A. Ganiyu, Int. J. Hydrogen Energy, 2025, 141, 615–649 CrossRef.
- M. Ding, Y. Yang, B. Wu, Y. Li, T. Wang and L. Ma, Appl. Energy, 2015, 160, 982–989 CrossRef CAS.
- S. Li, R. J. O'Brien, G. D. Meitzner, H. Hamdeh, B. H. Davis and E. Iglesia, Appl. Catal., A, 2001, 219, 215–222 CrossRef CAS.
- A. Tauro, F. Salomone, F. Celoria, M. Armandi, L. Nodari, L. Romagnoletti, E. Felli, R. Pirone and S. Bensaid, Chem. Eng. J., 2025, 515, 163154 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |