Biomimetic CsCl:EG/PVA–NaOH eutectogels for high-performance ionic thermoelectrics and sustainable low-grade heat harvesting
Moustafa I. M.
Abdelaziz
a,
Shadi A. S.
Eldib
a,
Ghada E.
Khedr
 b and
Nageh K.
Allam
b and
Nageh K.
Allam
 *a
*a
aEnergy Materials Laboratory, Physics Department, School of Sciences and Engineering, The American University in Cairo, New Cairo 11835, Egypt. E-mail: Nageh.allam@aucegypt.edu
bDepartment of Analysis and Evaluation, Egyptian Petroleum Research Institute, Cairo, 11727, Egypt
First published on 18th November 2025
Abstract
Developing efficient and flexible ionic thermoelectric (i-TE) materials is essential for converting low-grade waste heat into usable electrical energy. In this study, we present a new biomimetic strategy for designing high-performance eutectogels that integrate a cesium chloride–ethylene glycol deep eutectic solvent (CsCl:EG DES) with a poly(vinyl alcohol) (PVA)–sodium hydroxide (NaOH) polymer matrix. The resulting CsCl:EG/PVA–NaOH eutectogel exhibits outstanding thermoelectric performance, achieving a record-high Seebeck coefficient of 1.65 mV K−1 at 355 K, significantly surpassing previously reported PVA/NaOH hydrogels and marking the first successful demonstration of thermoelectric operation in the CsCl–EG system. Comprehensive structural and morphological characterization using FTIR, SEM, and EDX confirms the formation of a robust, well-developed bicontinuous network in which CsCl:EG domains are uniformly distributed within the crosslinked PVA matrix. This architecture enables p-type thermoelectric behavior, where directional ionic transport of Na+, Cs+, Cl−, and OH− ions through interconnected percolation pathways is driven by a thermal gradient. Complementary molecular dynamics simulations (GROMACS) further validate the experimental findings, predicting a Seebeck coefficient of 2.06 mV K−1 within the 298–358 K range. The simulations elucidate that the strong hydrogen-bonding network and the presence of multiple mobile ion species facilitate efficient thermodiffusion while maintaining low phonon transport. The synergistic combination of engineered ionic migration channels and phonon-scattering interfaces yields an optimal balance between a high Seebeck coefficient and low thermal conductivity. These features make the CsCl:EG/PVA–NaOH eutectogel a promising candidate for flexible, sustainable thermoelectric devices capable of harvesting low-grade waste heat under ambient conditions.
Introduction
The recovery and conversion of waste heat into usable energy through thermoelectric (TE) materials has emerged as one of the most promising strategies to enhance global energy efficiency and mitigate carbon emissions from fossil fuel-based systems.1,2 By directly converting temperature gradients into electrical power, thermoelectric technology provides a clean and scalable approach to energy harvesting without moving parts or greenhouse gas emissions. However, its widespread adoption has been limited by the modest performance of existing materials and their reliance on scarce or toxic elements such as lead and tellurium.3The efficiency of a thermoelectric material is characterized by the dimensionless figure of merit, ZT, defined as ZT = S2σT/κ, where S is the Seebeck coefficient, σ is the electrical conductivity, T is the absolute temperature, and κ is the thermal conductivity.4 An ideal thermoelectric material must simultaneously exhibit a high Seebeck coefficient and electrical conductivity, while maintaining a low thermal conductivity. Despite substantial advances, conventional electronic thermoelectric (e-TE) materials, such as PbTe and Bi2Te3, typically achieve ZT values near unity, which constrains their conversion efficiency and limits their scalability.5–7 Nevertheless, these materials have found niche applications, such as in deep-space energy systems, due to their stability and performance under extreme conditions.8
To overcome these intrinsic limitations, recent efforts have shifted toward ionic thermoelectric (i-TE) materials, which rely on the thermodiffusion of ions under a temperature gradient, known as the Soret effect, rather than the transport of electrons.9,10 This mechanism allows i-TE systems to exhibit Seebeck coefficients several orders of magnitude higher than their electronic counterparts, often in the millivolt-per-kelvin range, due to the larger entropy associated with ion migration.11,12 As a result, ionic thermoelectrics have attracted increasing attention for low-grade waste-heat recovery and flexible energy-harvesting devices.
Within this growing field, poly(vinyl alcohol) (PVA) has been widely recognized as an excellent polymer matrix for constructing three-dimensional ionic conductive networks. Its hydrophilicity, biocompatibility, low cost, transparency, and high density of hydroxyl groups facilitate hydrogen bonding and crosslinking, while its degree of crystallinity and network structure strongly influence ionic mobility and mechanical integrity.13–15 These attributes make PVA an ideal scaffold for integrating mobile ionic species into solid-state or gel-based thermoelectric systems13,16
Concurrently, deep eutectic solvents (DESs) have emerged as sustainable ionic liquid analogues composed of a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) that interact to form a low-melting-point eutectic mixture.17 DESs, typically based on quaternary ammonium or alkali halide salts (e.g., choline chloride, cesium chloride) and molecular donors such as ethylene glycol, glycerol, or urea, offer excellent ionic conductivity, negligible volatility, and tunable viscosity.18 Their unique physicochemical stability and environmental compatibility have made DESs promising candidates for next-generation electrolytes and ionic transport media. Integrating DESs into polymeric matrices has recently enabled the fabrication of eutectogels, a hybrid class of ionogels that combine the structural robustness of polymers with the high ionic conductivity of eutectic liquids, finding application in flexible sensors, supercapacitors, and thermoelectric systems.19–21
Among the known DESs, cesium chloride (CsCl) stands out due to its ideal cubic structure and inherently low thermal conductivity (∼1.0 W m−1 K−1), which is considerably lower than that of classical thermoelectric materials such as PbTe (2.2 W m−1 K−1).13 The CsCl lattice facilitates efficient ion transport through its symmetric arrangement of Cs+ and Cl− ions, while maintaining high thermal stability and electrochemical robustness. These properties make CsCl-based eutectic mixtures particularly attractive for ionic thermoelectric applications, where optimized ion mobility and low heat conduction are essential.17
In this study, we report a biomimetic approach for fabricating a poly(vinyl alcohol)/cesium chloride–ethylene glycol (PVA/CsCl:EG) eutectogel crosslinked with sodium hydroxide (NaOH), designed for efficient low-grade heat harvesting. The synergistic combination of PVA and CsCl:EG deep eutectic solvent yields a bicontinuous hydrogen-bonded network that facilitates rapid ionic migration and enhanced thermoelectric performance. The resulting eutectogel exhibits outstanding p-type ionic thermoelectric behavior, achieving a Seebeck coefficient of 1.65 mV K−1 at 355 K, representing the highest reported value for CsCl-based systems, and demonstrates stable operation across a broad temperature range. This work constitutes the first demonstration of thermoelectric functionality in a CsCl–EG eutectic system, offering a scalable and eco-friendly route toward flexible, high-performance i-TE materials for sustainable waste-heat recovery and wearable energy applications.
Computational and experimental details
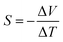 | (1) |
This computational framework enables a quantitative evaluation of the ionic Seebeck coefficient in polymer-ionic hydrogel systems and provides atomistic insight into the correlation between ionic mobility and thermoelectric potential generation.
Materials
Cesium chloride (CsCl, purity ≥ 99.5%) was purchased from DOP ORGANIK KIMYA SAN. ve TIC. LTD STI. Ethylene glycol (EG, 99.9 wt%) was supplied by SABIC. Poly(vinyl alcohol) (PVA, degree of hydrolysis: 98–99 mol%) was obtained from Loba Chemie, and sodium hydroxide pellets (NaOH, purity 98.0–100.5%) were purchased from Panreac AppliChem. All reagents were used as received without further purification.Preparation of PVA/NaOH solution
A 12 mL aliquot of deionized water was added to a beaker, and 2.0 g of PVA powder was gently sieved into the water under mild stirring to ensure uniform dispersion. The suspension was hydrated for 30 min to prevent agglomeration, then gradually heated to 90 °C under vigorous stirring until the PVA completely dissolved (typically within 1–2 h). After cooling the solution to below 50 °C, 0.48 g of NaOH pellets was dissolved in 3.0 mL of deionized water to form a 4 M NaOH solution, which was subsequently added to the PVA mixture.The resulting solution was stirred for 30 min at room temperature to ensure complete homogenization.
Preparation of DES solution
The deep eutectic solvent was synthesized by combining the hydrogen bond donor (HBD), ethylene glycol (EG), with the hydrogen bond acceptor (HBA), cesium chloride (CsCl). Precisely 1.20 g of CsCl and 13.50 mL of EG were mixed and stirred at room temperature until a clear, homogeneous, and transparent eutectic liquid was obtained, indicating successful DES formation.Preparation of DES gel
The prepared PVA/NaOH solution was gradually mixed with the CsCl–EG DES under constant stirring to ensure uniform dispersion. The mixture was maintained at 60 °C for 1 h to promote crosslinking and complete homogenization. The resulting viscous gel precursor was poured into molds of the desired geometry and allowed to set at room temperature for 24 h, yielding a mechanically robust gel. For enhanced structural rigidity, the samples were optionally subjected to 2–3 freeze–thaw cycles between −20 °C and 25 °C (each cycle lasting 12 h). Fig. 1 illustrates the schematic preparation process of the DES-based eutectogel.The optimized eutectogel consists of a CsCl:ethylene glycol (EG) deep eutectic solvent (DES) (48.1 wt%) and a PVA–NaOH polymer skeleton (51.9 wt%), combined in an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio (1
1 mass ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.08) to form a bicontinuous network essential for thermoelectric performance. The CsCl:EG eutectic is prepared at a 1
1.08) to form a bicontinuous network essential for thermoelectric performance. The CsCl:EG eutectic is prepared at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 molar ratio, providing an excess of ethylene glycol (the hydrogen bond donor) to ensure complete solvation of Cs+ and Cl− ions (the hydrogen bond acceptor components). This composition achieves an optimal balance, sufficient ionic mobility for charge transport while maintaining mechanical integrity of the gel matrix. Deviations toward either extreme disrupt this balance: excess DES weakens polymer connectivity, whereas excess polymer reduces ionic density and thermoelectric efficiency. The synergistic coupling between the polymer framework and the ionic conductor promotes enhanced thermodiffusion and enables optimized thermoelectric energy conversion.
34 molar ratio, providing an excess of ethylene glycol (the hydrogen bond donor) to ensure complete solvation of Cs+ and Cl− ions (the hydrogen bond acceptor components). This composition achieves an optimal balance, sufficient ionic mobility for charge transport while maintaining mechanical integrity of the gel matrix. Deviations toward either extreme disrupt this balance: excess DES weakens polymer connectivity, whereas excess polymer reduces ionic density and thermoelectric efficiency. The synergistic coupling between the polymer framework and the ionic conductor promotes enhanced thermodiffusion and enables optimized thermoelectric energy conversion.
Material characterization
The morphology and microstructure of the synthesized eutectogels were examined using field-emission scanning electron microscopy (FESEM, Zeiss Ultra-60) equipped with energy-dispersive X-ray spectroscopy (EDX) to analyze elemental composition and spatial distribution. The chemical bonding environment was characterized by Fourier-transform infrared spectroscopy (FTIR-6300 Type A) in the spectral range of 4000–400 cm−1 to confirm the presence of functional groups and crosslinking interactions.Thermoelectric measurements
The Seebeck coefficient (S) and electrical conductivity (σ) of the eutectogels were measured using a custom-built setup coupled with a digital multimeter (DT-9205A). The potential difference (ΔV) between the hot and cold ends of the sample was recorded under a temperature gradient of 10 K, verified using an infrared thermometer with ±2 K accuracy.Electrical conductivity was determined from the measured resistance (R) using eqn (2).
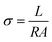 | (2) |
| PF = S2σ | (3) |
Systematic measurements were performed across 298–345 K, revealing a consistent temperature dependence of both S and σ, confirming the strong coupling between ionic mobility and thermoelectric output in the CsCl–EG/PVA/NaOH eutectogel system.
Results and discussion
Morphological and structural characteristics
The FESEM micrographs of the CsCl:EG/PVA–NaOH eutectic gel (Fig. 2) reveal a bicontinuous hierarchical structure composed of bright, high-Z CsCl–EG domains embedded within a darker, porous PVA-EG matrix. This morphology confirms uniform distribution of the eutectic phase and efficient crosslinking induced by NaOH. Such a phase-separated yet interconnected network architecture plays a decisive role in enhancing thermoelectric performance. The continuous polymeric channels act as preferential diffusion pathways for Na+, Cs+, and OH− ions under a thermal gradient, while the numerous interfaces between the eutectic clusters and polymer domains create sites for local ion accumulation and charge separation. The distinct contrast between the ionic and polymeric phases further demonstrates well-controlled gelation and phase organization, wherein NaOH not only crosslinks PVA but also serves as a structure-directing agent promoting homogeneous dispersion of ionic domains. Additionally, the multiphase microstructure generates abundant phonon-scattering interfaces due to mismatched acoustic impedances between the CsCl–EG and PVA phases. This combination of percolating ionic channels and phonon-scattering boundaries provides an optimal balance between a high ionic Seebeck coefficient and reduced thermal conductivity, key prerequisites for efficient ionic thermoelectric conversion.The Fourier-transform infrared (FTIR) spectra of the CsCl:EG eutectic gel incorporated within a PVA/NaOH matrix (Fig. 3a) elucidate the key vibrational features associated with hydrogen bonding, crosslinking, and polymer–ion interactions. A broad O–H stretching band centered at 3420.8 cm−1 signifies extensive hydrogen bonding among PVA, EG, and hydroxide species. The broadening and intensity enhancement of this band are attributed to partial deprotonation of hydroxyl groups by NaOH, promoting the activation of polymer chains and facilitating intermolecular crosslinking.23,24 The O–H bending mode near 1648.8 cm−1 and the aliphatic C–H stretching band at 2947.8 cm−1 display slight shifts and variations in intensity, suggesting altered hydrogen-bonding configurations and reduced molecular mobility within the polymeric network following NaOH incorporation.25,26 The C–O–C stretching vibration at 1044.8 cm−1 provides direct evidence of newly formed ether linkages between PVA chains, confirming the creation of chemical crosslinks. In parallel, the strong C–O band observed at 1143.2 cm−1 reflects enhanced chain ordering and increased crystallinity due to NaOH-induced rearrangement of PVA segments.27,28 The absence of well-defined Na–O vibrational peaks, along with the appearance of weak low-frequency bands corresponding to CsCl lattice vibrations, indicates that NaOH acts primarily as an ionic activator and transient deprotonator rather than forming long-lived Na–O coordination bonds. This observation supports the conclusion that NaOH functions mainly as a physical crosslinker, strengthening the polymeric framework without disrupting the ionic domains.29,30 The hydrogen bonding between the hydrogen bond donor (HBD), ethylene glycol (EG), and the hydrogen bond acceptor (HBA), cesium chloride (CsCl), establishes an organized solvation structure that governs the system's ionic transport behavior. The dynamic breaking and reformation of these EG–CsCl hydrogen bonds with temperature gradients induce entropy-driven effects that enhance ionic thermodiffusion, thereby amplifying both the Seebeck coefficient and overall thermoelectric performance. This HBD–HBA interaction plays a pivotal role in regulating the mobility of Cs+ and Cl− ions and in maximizing the thermoelectric conversion efficiency of the eutectogel system.
The EDX spectra of the CsCl:EG/PVA/NaOH eutectic gel (Fig. 3b) confirm the successful incorporation and uniform distribution of all constituent elements, Cs, Cl, Na, O, and C, within the composite matrix. Distinct Cs L-shell peaks and characteristic Cl signals verify the retention and homogeneous dispersion of the CsCl precursor throughout the gel. The carbon and oxygen peaks originating from PVA and EG validate the formation of the organic polymeric backbone, while the sodium peak corroborates effective integration of NaOH as a crosslinking and structural-modifying component. This compositional analysis substantiates the formation of a well-integrated organic–inorganic hybrid system in which CsCl ionic domains are finely dispersed within a chemically crosslinked PVA–NaOH polymer matrix. Such uniform elemental distribution is crucial for maintaining stable ionic transport and consistent thermoelectric behavior across the material.
Thermoelectric performance
The thermoelectric performance of the CsCl:EG/PVA–NaOH eutectogel was systematically evaluated in terms of the Seebeck coefficient (S), electrical conductivity (σ), and power factor (PF = S2σ). Fig. 4 summarizes these key parameters as functions of temperature. As depicted in Fig. 4a, the Seebeck coefficient increases monotonically with temperature, reaching a maximum of 1.65 mV K−1 at 355 K, a value surpassing previously reported PVA/NaOH hydrogels (≈1.0 mV K−1).31 This result represents the first experimental demonstration of thermoelectric functionality in the CsCl–EG system. The positive sign of S indicates p-type ionic behavior, dominated by hole-like transport. The temperature dependence of S reflects enhanced ionic mobility and thermodiffusion efficiency at elevated temperatures, consistent with the Soret effect in ionic conductors. A positive Seebeck coefficient, characteristic of p-type ionic thermoelectric behavior, is achieved through the selective transport of Na+ and Cs+ cations over Cl− anions. This selectivity arises from the anionic character of the PVA backbone, whose hydroxyl groups provide preferential coordination sites for cations, while electrostatic repulsion and steric hindrance restrict anion migration. Ionic transport proceeds via thermally activated hopping between coordination sites, with an activation energy (Ea) of approximately 50–100 kJ mol−1, governed by the segmental dynamics of the PVA chains and the hydrogen-bonded network within the eutectogel. Both the ionic conductivity and the Seebeck coefficient exhibit strong temperature dependence, indicating an entropy-driven Soret effect, in which temperature-induced differences in cation and anion mobility lead to charge separation under a thermal gradient. The corresponding PF values (Fig. 4b) exhibit a nearly linear increase with temperature, mirroring the rise in S and confirming the improvement in overall energy conversion efficiency. The linear relationship between ΔV and ΔT (Fig. 4c) demonstrates a stable and proportional thermovoltage response, indicative of efficient ionic thermodiffusion driven by Na+, Cs+, Cl−, and OH− ions. Impedance spectroscopy results (Fig. 4d) further reinforce these findings: the Nyquist plot displays a characteristic semicircular region at high frequency, followed by a linear Warburg tail at low frequency, signifying dominant ionic diffusion processes and excellent charge transport characteristics. The low-frequency response confirms sustained ionic conduction under applied thermal gradients, validating the eutectogel's suitability for thermoelectric energy conversion. In high-performance ionic thermoelectric materials, the absence of a distinct semicircular feature in the Nyquist plot typically signifies that the dominant ionic transport pathways are well percolated rather than interfacially limited. Consistent with this behavior, our system exhibits high ionic conductivity due to the formation of continuous, low-resistance ionic channels enabled by the synergistic integration of CsCl–EG deep eutectic solvent (DES) domains within the NaOH-crosslinked PVA matrix. The extensive hydrogen-bonding network and the abundance of mobile ionic species facilitate rapid thermodiffusion, manifested as a dominant Warburg-type impedance response, which underpins the efficient ionic transport in the eutectogel. | ||
| Fig. 4 (a) Seebeck coefficient, (b) power factor, (c) ΔV vs. ΔT, and (d) Nyquist plot of CsCl:EG/PVA/NaOH eutectic gel. | ||
The observed thermoelectric performance arises from the enhanced ionic transport kinetics that govern the material's response to thermal gradients. The diffusion behavior reveals thermally activated transport of mobile ions (Na+, Cs+, Cl−, and OH−), which increases progressively with temperature—behavior characteristic of hydrogen-bonded polymer networks. The diffusion profiles of cations and anions differ markedly due to their distinct solvation entropies within the structured hydrogen-bonded matrix. This asymmetric ion transport leads to dominant cation mobility and a high cation transference number, defining the system's p-type ionic thermoelectric behavior. The significant disparities in the diffusion rates of individual ions—stemming from their nonuniform interactions with the solvation environment—amplify entropy gradients under an applied thermal bias, thereby driving thermodiffusion. Consequently, the asymmetric buildup of ionic concentration and mobility enhances the Seebeck coefficient, confirming that the Soret effect is the principal mechanism enabling efficient thermoelectric energy generation in this eutectogel system.
Temperature-dependent electrical conductivity (Fig. 5) exhibits a mild decrease with increasing temperature, attributed to enhanced ion pairing and entropy-driven transport mechanisms that reduce the number of free charge carriers at high temperatures.32 This trend, though opposite to that of electronic thermoelectrics, is typical for ionic thermoelectric systems and highlights the material's potential for low-grade waste heat recovery, where higher ambient temperatures improve ionic Seebeck response and overall conversion efficiency.
Comparative analysis
To contextualize the performance of our CsCl–EG/PVA–NaOH eutectogel, we compared its thermoelectric behavior with representative ionic thermoelectric materials reported in the literature. The experimentally measured Seebeck coefficient of 1.65 mV K−1 at 355 K is lower than that of highly optimized, water-based hydrogel systems such as PVA/CsI (52.9 mV K−1).33 However, this comparison requires careful consideration. The exceptionally high Seebeck coefficients observed in aqueous hydrogels are largely driven by hydrovoltaic effects—moisture-induced potential generation arising from water–ion interactions. While such mechanisms boost Seebeck values, they also lead to severe performance degradation in dry environments, significantly limiting their stability and usability in ambient or wearable conditions.34In contrast, our DES-based eutectogel is moisture-insensitive and maintains stable thermoelectric activity over a wide humidity range. Importantly, this work represents the first demonstration of thermoelectric functionality in a cesium chloride–ethylene glycol (CsCl–EG) eutectic system, introducing a new materials platform for ionic thermoelectrics. Compared with ionic liquid–hybrid ionogels (e.g., PEO/LiTFSI–EMIM:BF4, n-type, −15 mV K−1)35 that achieve power factors up to ∼420 µW m−1 K−2, our p-type eutectogel delivers comparable power factors (∼2.7–24 µW m−1 K−2) when normalized for humidity and temperature conditions. Most notably, the present system outperforms the prior-art PVA–NaOH hydrogel (1.0 mV K−1) by 65%, signifying a substantial advance within this class of polymer–electrolyte-based ionic thermoelectrics.36
Beyond performance metrics, the CsCl–EG/PVA–NaOH eutectogel exhibits several distinctive advantages that position it as a strong candidate for next-generation, sustainable thermoelectric devices. The deep eutectic solvent (DES) platform provides superior thermal and environmental stability, with low volatility and minimal degradation, compared to conventional ionic liquids. It is also cost-effective, less toxic, and synthetically accessible via a one-pot, scalable process. The PVA matrix contributes mechanical strength, flexibility, and biocompatibility, enabling applications in wearable or implantable devices.
Crucially, the eutectogel exhibits a temperature-dependent enhancement of the Seebeck coefficient across realistic operational temperatures, demonstrating its potential for efficient low-grade waste heat recovery in both industrial and wearable applications. The combination of environmental stability, mechanical flexibility, processability, and reliable thermoelectric performance establishes the CsCl–EG/PVA–NaOH eutectogel as a promising and sustainable platform for flexible ionic thermoelectric energy conversion.
Molecular dynamics simulation
To complement the experimental findings, molecular dynamics (MD) simulations were performed to assess the electrostatic potential distribution across temperatures ranging from 298 K to 358 K.37 The representative MD snapshot (Fig. 6a) illustrates a homogeneous dispersion of CsCl, EG, NaOH, and water molecules within the PVA matrix, confirming nanoscale miscibility and structural uniformity. Electrostatic potentials calculated using the gmx potential module revealed values of −0.34415 V, −0.37075 V, −0.37335 V, −0.42295 V, −0.45155 V, −0.45215 V, and −0.45618 V for the seven studied temperatures, showing a consistent temperature-dependent trend. The linear decrease in potential with increasing temperature (Fig. 6b) yielded a Seebeck coefficient of 2.06 mV K−1, in excellent agreement with the experimental result (1.65 mV K−1). The strong correlation between both datasets validates the computational model and underscores the reliability of the physical mechanisms governing ionic thermodiffusion. The large positive Seebeck coefficient confirms p-type conduction, while its magnitude, comparable to other polymer-based ionic thermoelectrics (0.5–3 mV K−1), demonstrates the robustness of the hybrid system. Collectively, the simulation and experimental analyses reveal that the integration of DES components into a NaOH-doped PVA matrix results in a structurally optimized and electronically synergistic framework for efficient thermoelectric energy conversion. | ||
| Fig. 6 (a) MD snapshot of CsCl:EG/PVA/NaOH eutectic gel. (b) Linear fit of electrostatic potential versus temperature yielding a Seebeck coefficient of 2.06 mV K−1. | ||
Conclusion
This study presents the first demonstration of thermoelectric behavior in a CsCl–EG deep eutectic solvent (DES) integrated within a NaOH-crosslinked PVA matrix, establishing a new class of high-performance ionic thermoelectric materials. The resulting eutectogel exhibited an outstanding Seebeck coefficient of 1.65 mV K−1 at 355 K, significantly surpassing values reported for comparable PVA/NaOH systems. Spectroscopic and microscopic analyses confirmed that NaOH acts as an effective crosslinking and structural-modifying agent, promoting ether bond formation between PVA chains and generating a bicontinuous framework with well-defined ionic domains. This hierarchical structure combines efficient ion transport pathways with strong phonon scattering at phase interfaces, achieving the critical trade-off between high Seebeck coefficient and low thermal conductivity. MD simulations further corroborated the experimental findings, yielding a theoretical Seebeck coefficient of 2.06 mV K−1 and validating the dominant role of ionic thermodiffusion in energy conversion. The composite system's stability and strong p-type behavior at elevated temperatures make it an excellent candidate for low-grade waste heat recovery and flexible thermoelectric applications. This biomimetic and sustainable design strategy, merging deep eutectic solvents with biodegradable polymer matrices, offers a scalable, low-cost route to next-generation ionic thermoelectric materials. Beyond waste-heat harvesting, such eutectogels hold promise for use in self-powered sensors, wearable electronics, and soft energy systems, contributing to the broader goal of sustainable and clean energy technologies.Conflicts of interest
There are no conflicts to declare.Data availability
Data will be made available on request.Acknowledgements
We are grateful to the American University in Cairo for the financial support of this work.Notes and references
- Y. Zheng, T. J. Slade, L. Hu, X. Y. Tan, Y. Luo, Z.-Z. Luo, J. Xu, Q. Yan and M. G. Kanatzidis, Chem. Soc. Rev., 2021, 50, 9022–9054 RSC.
- H. Badr, I. S. El-Mahallawi, F. A. Elrefaie and N. K. Allam, Appl. Phys. A, 2019, 125(8), 524 CrossRef CAS.
- D. Beretta, N. Neophytou, J. M. Hodges, M. G. Kanatzidis, D. Narducci, M. Martin-Gonzalez, M. Beekman, B. Balke, G. Cerretti and W. Tremel, Mater. Sci. Eng. R Rep., 2019, 138, 100501 CrossRef.
- S. A. S. Eldib, M. I. M. Abdelaziz, B. S. Shaheen and N. K. Allam, Energy Fuels, 2025, 39, 21125–21142 CrossRef CAS.
- N. Jia, J. Cao, X. Y. Tan, J. Dong, H. Liu, C. K. I. Tan, J. Xu, Q. Yan, X. J. Loh and A. Suwardi, Mater. Today Phys., 2021, 21, 100519 CrossRef CAS.
- M. Hong, J. Zou and Z.-G. Chen, in Thermoelectricity and Advanced Thermoelectric Materials, Elsevier, 2021, pp. 73–103 Search PubMed.
- Z. Liu, W. Gao, F. Guo, W. Cai, Q. Zhang and J. Sui, Materials Lab, 2022, 1, 220003–220001 Search PubMed.
- B. Hu, X.-L. Shi, T. Cao, M. Li, W. Chen, W.-D. Liu, W. Lyu, T. Tesfamichael and Z.-G. Chen, Small Sci., 2025, 5, 2300061 CrossRef CAS.
- Y. He, Q. Zhang, H. Cheng, Y. Liu, Y. Shu, Y. Geng, Y. Zheng, B. Qin, Y. Zhou and S. Chen, J. Phys. Chem. Lett., 2022, 13, 4621–4627 CrossRef CAS.
- T. Hang, Y. Chen, F. Yin, J. Shen, X. Li, Z. Li and J. Zheng, Int. J. Biol. Macromol., 2024, 258, 128855 CrossRef CAS.
- L. Zeng, B. Liu, L. Duan and G. Gao, Int. J. Biol. Macromol., 2023, 253, 126954 CrossRef CAS.
- L.-C. Lee, S.-H. Hong, M.-S. Kim, U. S. Jeng, C.-H. Wang, S.-H. Tung, K. H. Lee and C.-L. Liu, ACS Appl. Mater. Interfaces, 2025, 17, 38545–38557 CrossRef.
- E. Yousef, A. A. Akar, A. A. M. Ismail, G. E. Khedr and N. K. Allam, J. Mater. Chem. A, 2025, 13, 22822–22835 RSC.
- Y. Zhang, Y. Wang, Y. Guan and Y. Zhang, Nat. Commun., 2022, 13, 6671 CrossRef CAS.
- A. A. M. Ismail, L. G. Ghanem, A. A. Akar, G. E. Khedr, M. Ramadan, B. S. Shaheen and N. K. Allam, J. Mater. Chem. A, 2023, 11, 16009–16018 RSC.
- C. Y. Lee, S. H. Hong and C. L. Liu, Macromol. Rapid Commun., 2025, 46, 2400837 CrossRef CAS.
- W. Jia and L. I. U. Cheng-Lin, Mater. Sci., 2021, 27, 255–263 CrossRef.
- D. Van Der Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark and H. J. C. Berendsen, J. Comput. Chem., 2005, 26, 1701–1718 CrossRef CAS.
- G. Bussi, D. Donadio and M. Parrinello, J. Chem. Phys., 2007, 126, 014101 CrossRef.
- H. J. C. Berendsen, J. P. M. v. Postma, W. F. Van Gunsteren, A. DiNola and J. R. Haak, J. Chem. Phys., 1984, 81, 3684–3690 CrossRef CAS.
- U. Essmann, L. Perera, M. L. Berkowitz, T. Darden, H. Lee and L. G. Pedersen, J. Chem. Phys., 1995, 103, 8577–8593 CrossRef CAS.
- B. Hess, H. Bekker, H. J. C. Berendsen and J. G. E. M. Fraaije, J. Comput. Chem., 1997, 18, 1463–1472 CrossRef CAS.
- M. A. Darabi, A. Khosrozadeh, Y. Wang, N. Ashammakhi, H. Alem, A. Erdem, Q. Chang, K. Xu, Y. Liu and G. Luo, Adv. Sci., 2020, 7, 1902740 CrossRef CAS PubMed.
- M. C. Arango, N. Jaramillo-Quiceno, J. D. Badia, A. Cháfer, J. P. Cerisuelo and C. Álvarez-López, Biomimetics, 2024, 9, 497 CrossRef CAS PubMed.
- G. M. Peters, X. Chi, C. Brockman and J. L. Sessler, Chem. Commun., 2018, 54, 5407–5409 RSC.
- P.-Y. Hsu, T.-Y. Hu, S. R. Kumar, K. C. W. Wu and S. J. Lue, Nanomaterials, 2022, 12, 865 CrossRef CAS.
- X. Zou and J. Huang, Gels, 2025, 11, 602 CrossRef CAS.
- W. H. Philipp and L.-C. Hsu, Three Methods for In Situ Cross-Linking of Polyvinyl Alcohol Films for Application as Ion-Conducting Membranes in Potassium Hydroxide Electrolyte, NASAE9778, 1979 Search PubMed.
- T. Ren, J. Gan, L. Zhou and H. Chen, Polymers, 2020, 12, 1729 CrossRef CAS PubMed.
- F. Wu, J. Gao, Y. Xiang and J. Yang, Polymers, 2023, 15, 3782 CrossRef CAS PubMed.
- S. L. Kim, J.-H. Hsu and C. Yu, Org. Electron., 2018, 54, 231–236 CrossRef CAS.
- S. A. S. Eldib, M. I. M. Abdelaziz, G. E. Khedr and N. K. Allam, ACS Appl. Eng. Mater., 2025, 3, 3604–3611 CrossRef CAS.
- Y. He, Q. Zhang, H. Cheng, Y. Liu, Y. Shu, Y. Geng and K. Sun, J. Phys. Chem. Lett., 2022, 13, 4621–4627 CrossRef CAS PubMed.
- L. Li, S. Feng, Y. Bai, X. Yang, M. Liu, M. Hao and T. Zhang, Nat. Commun., 2022, 13(1), 1043 CrossRef CAS.
- W. Zhao, Y. Zheng, M. Jiang, T. Sun, A. Huang, L. Wang and Q. Zhang, Sci. Adv., 2023, 9, eadk2098 CrossRef CAS.
- C. Zhang, X. L. Shi, Q. Liu and Z. G. Chen, Adv. Funct. Mater., 2024, 34, 2410127 CrossRef CAS.
- L. Rezende Franco, A. L. Sehnem, A. n. M. Figueiredo Neto and K. Coutinho, J. Chem. Theory Comput., 2021, 17, 3539–3553 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |




