Open Access Article
Open Access ArticleEngineering the electronic structure of Ni–Co bimetallic sites toward efficient electrochemical biomass upgrading and CO2 reduction
Qing-Lian Yana,
Xun-Bin Lina,
Ji-dan Liu a,
Ting Ouyang*a and
Zhao-Qing Liu
a,
Ting Ouyang*a and
Zhao-Qing Liu *ab
*ab
aSchool of Chemistry and Chemical Engineering, Institute of Clean Energy and Materials, Key Laboratory for Clean Energy and Materials, Huangpu Hydrogen Innovation Center, Guangzhou University, Guangzhou 510006, P. R. China. E-mail: ouyt@gzhu.edu.cn; lzqgzu@gzhu.edu.cn
bSchool of Chemistry, South China Normal University, Guangzhou 510006, P. R. China
First published on 16th January 2026
Abstract
Electrochemical coupling of the 5-hydroxymethylfurfural oxidation reaction (HMFOR) and CO2 reduction reaction (CO2RR) offers a promising route to produce high-value chemicals while lowering the energy input. A critical bottleneck in the CO2-HMF coupled system is the poor ability of the anode catalyst to adsorb and desorb HMF and OH−, resulting in prohibitively high energy consumption. We construct an anode catalyst NiCo2O4 by regulating the tetrahedral site to increase the ratio of Co3+/Co2+, which achieves a faradaic efficiency (FE) for 2,5-furandicarboxylic acid (FDCA) of 99.1% at 1.5 V vs. RHE. The CO2-HMF coupled system with NiCo2O4 as an anode and Au as a cathode at a low cell voltage of 1.7 V affords a total energy conversion efficiency of 43.3%; the FEFDCA of the anode is 91.9%, and the FE of the cathode is 94.7% (66.1% for CO and 28.6% for H2). In-situ surface-enhanced Raman spectroscopy further elucidates the dynamic evolution of the surface state and intermediates of the integrated system: the NiCo2O4 anode promotes HMF-to-FDCA conversion via potential-dependent formation of Ni3+ and Co3+ intermediates for OH− capture. Meanwhile, the key intermediate *CO for CO2-to-CO conversion is detected at the cathode, and the simultaneous progress of the anodic and cathodic reactions significantly reduces the energy consumption of the coupled system. This work provides important theoretical support and a technical approach for the design and amplification of CO2-HMF coupled systems.
Introduction
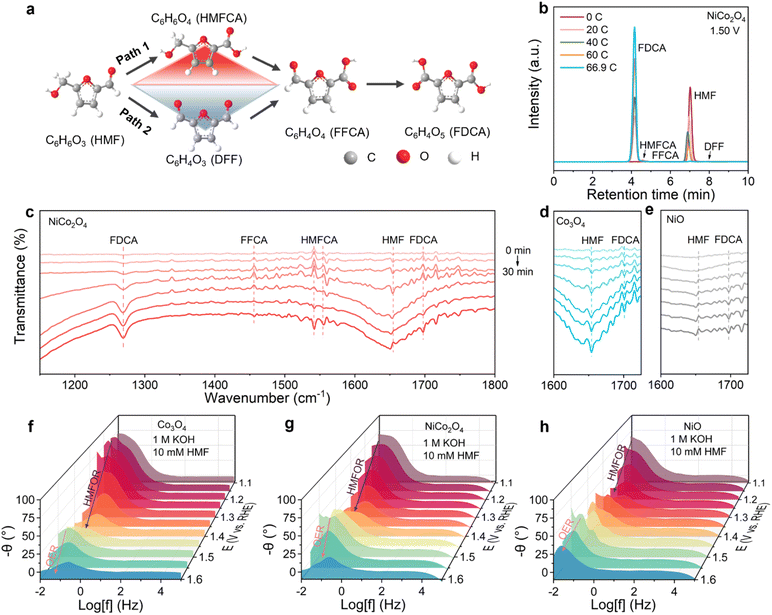
Electrochemical conversion technology provides a green solution for key issues in the field of energy and environment. Among them, water electrolysis hydrogen production (HER system) occupies a core position in the large-scale preparation of hydrogen energy due to the cheap and easy availability of raw materials.1 Nevertheless, relying on a single method for hydrogen production is insufficient to address the complex challenges of carbon emission reduction and the high-value integration of resources. Using the electrocatalytic CO2 reduction reaction (CO2RR) to obtain value-added chemicals represents a critical pathway toward global carbon neutrality.2–7 However, the practical development of CO2RR electrolyzers is hindered by the high overpotential of the anodic oxygen evolution reaction (OER), which increases energy consumption and generates low-value byproduct O2.8–10 A promising strategy to overcome these limitations involves replacing the OER with the electrooxidation of biomass-derived small molecules. These oxidation reactions not only operate at lower overpotentials but also yield high-value products such as organic acids or fuels.11–13 By integrating biomass oxidation with the CO2RR in a single electrolyzer, it could achieve dual benefits: enhanced energy efficiency and simultaneous production of valuable chemicals at both electrodes, thereby advancing sustainable electrochemical synthesis.14–16An electrolyzer system with the anode producing liquid products and the cathode generating gas products is convenient for product detection, separation and collection.17 A promising candidate for the anodic reaction is 5-hydroxymethylfurfural, an important and impressive biomass derivative.18 Compared to the OER (4OH− + O2 → 2H2O + 4e−, 1.23 V), the HMFOR (HMF + 6OH− → FDCA + 4H2O + 6e−, 0.3 V) shows a much lower theoretical potential and produces high-value chemicals.19–21 HMF can be selectively oxidized or reduced to produce polymer monomers, chemicals and liquid fuels because its structure contains active hydroxyl groups and aldehyde groups. Notably, FDCA is one of the 12 sugar-based platform compounds listed by the U.S. Department of Energy and is obtained through the oxidation of HMF’s two oxygen-containing groups into carboxyl groups (Table S1).18,22–24 Accordingly, coupling the cathodic CO2RR with the anodic HMFOR is expected to simultaneously produce high value-added chemicals in an electrolytic cell. The half-cell and overall responses involved are shown below.
| Anodic reaction: C6H6O3 + 2H2O → C6H4O5 + 6H+ + 6e− |
| Cathodic reaction: 3CO2 + 3H2O + 6e− → 3CO + 6OH− |
| The total reaction: C6H6O3 + 3CO2 → C6H4O5 + 3CO + H2O |
Notably, the development of the CO2-HMF coupled system has attracted considerable attention.25–27 However, the integrated system of the HMFOR and CO2RR remains underexplored, with unresolved scientific challenges in understanding the reaction kinetics at both electrodes. Research on this system still faces a key challenge: the current research on the CO2-HMF coupled system mostly focuses on the independent development and performance optimization of anode and cathode catalysts. However, there is a lack of direct and in situ experimental evidence for the dynamic evolution of active sites at both ends of the catalyst, the formation and consumption of key reaction intermediates, and the dynamic synergy mechanism between them under actual working conditions. Co3O4 as a HMFOR catalyst has garnered significant attention for its abundant octahedral Co (Co3+ Oh) active sites.28,29 Co3+ Oh sites with their high oxidation state, optimized electronic structure, and stable lattice environment, serve as critical active centers for enhancing HMFOR activity and selectivity.30–32 However, three key limitations hinder their practical application: (1) in the octahedral crystal field, the fully occupied t2g6 of low-spin Co3+ Oh sites results in excessively strong adsorption of HMF molecules, while the activation capability for OH− remains insufficient. This imbalance impedes the efficient deprotonation of intermediates (such as 5-hydroxymethyl-2-furancarboxylic acid (HMFCA) and 5-formyl-2-furancarboxylic acid (FFCA)); (2) the HMFOR deprotonation process under alkaline conditions relies heavily on the dynamic supply of OH− on the surface of the catalyst. However, the weak adsorption and activation of OH− by Co3O4 lead to sluggish reaction kinetics; (3) Co3+ Oh sites in the spinel structure tend to trigger the OER side reaction and significantly reduce the HMFOR selectivity.
To address the imbalance between HMF adsorption strength and OH− supply efficiency, doping Co3O4 with transition metals (Fe, Zn, Ni…) has become an effective strategy to regulate the electronic and catalytic properties of Co3+ Oh sites.33–35 In this work, a NiCo2O4 catalyst was synthesized via Ni incorporation into Co3O4. Systematic electrochemical measurements, in situ spectroscopic studies, and theoretical calculations reveal that the Ni sites significantly enhance the concurrent adsorption and activation of HMF and OH−, thereby accelerating the dehydrogenation and electron-transfer kinetics during the rate-determining FFCA → FDCA step. Consequently, the NiCo2O4 anode achieves an exceptional FEFDCA of 99.1% at 1.5 V vs. RHE, along with a low potential of 1.22 V vs. RHE to deliver a current density of 10 mA cm−2. A double-chamber membrane electrolytic cell, based on the CO2-HMF coupled system, was constructed. It features a gas diffusion electrode and an ion-selective membrane for the efficient separation of FDCA and CO. In the integrated coupled system, when the cell voltage of the NiCo2O4 catalyst is 1.7 V, the FEFDCA of the anode is 91.9%, the cathode FE is 94.7% (66.1% for CO and 28.6% for H2), and the total energy conversion efficiency is 43.3%, which proves that the combination of biomass pricing and the CO2 conversion reaction is very important to reduce the cost of electricity and improve the economic efficiency and the great prospect of producing high value-added chemicals. Furthermore, in-situ surface-enhanced Raman spectroscopy (SERS) indicates that the NiCo2O4 anode promotes HMF-to-FDCA conversion via potential-dependent formation of Ni3+ and Co3+ intermediates for OH− capture. Meanwhile, the evolution of key intermediates in the CO2 to *CO pathway is monitored at the cathode and directly reveals the dynamic evolution process of the surface state of the catalyst under operating conditions, confirming the high degree of kinetic synergy between the two ends of the reaction, thereby significantly reducing the overall energy consumption of the system. This work elucidates the synergistic mechanism of the two types of reaction kinetics in situ under coupling conditions for the first time, which provides a key experimental basis for optimizing the bifunctional electrocatalytic system and further exploring the coupling reaction mechanism. Through the above research, we aim to provide theoretical support and a technical path for the design and large-scale application of biomass-carbon dioxide co-electrolysis systems and promote the electrosynthesis technology towards the goal of high efficiency.
Results and discussion
Catalyst synthesis and characterization
Based on the above discussion, NiCo2O4 supported on CC was synthesized by the hydrothermal-calcination method (Fig. S1). NiO and Co3O4 were synthesized by the same method as that for the comparison sample. The crystal structure was characterized by X-ray powder diffraction (XRD). The diffraction peaks of NiO, Co3O4 and NiCo2O4 are consistent with the PDF standard cards of NiO (PDF # 44-1159), Co3O4 (PDF # 43-1003) and NiCo2O4 (PDF # 20-0781), respectively (Fig. S2), indicating that the materials were successfully synthesized. The morphology of the obtained electrocatalysts is observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image of NiO shows that its structure is a linear cluster, and Co3O4 shows a nanosheet array structure (Fig. S3b and c). NiCo2O4 exhibits a nanoneedle array structure (Fig. S3a). HRTEM images enlarge the blue framed area of a single nanosheet and show clear lattice fringes with a spacing of 0.234 nm, which corresponds to the (111) crystal plane of NiCo2O4 (Fig. S4). The selected area electron diffraction (SAED) patterns correspond to (111), (220), (311), (222) and (400) crystal planes (Fig. S5), which is consistent with the results of XRD and HRTEM. The corresponding element mapping image displays that the Ni, Co and O elements on the surface of the catalyst are evenly distributed (Fig. S6). The above analysis indicates the successful synthesis of NiCo2O4. Furthermore, it can be seen from TEM that the (222) crystal plane is the main unit cell of NiCo2O4. The analysis of the unit cell configuration of the (111) crystal plane of Co3O4 and NiCo2O4 in Fig. 1a preliminarily speculates that the octahedral interstitial Co3+ with strong adsorption to HMF, after the Ni2+ preferentially replaces the tetrahedral interstitial Co2+. After that, the unit cell locally forms an ‘octahedral interstitial Co3+-tetrahedral interstitial Ni2+’ adjacent coordination structure. Because the oxidation of Co3+ is much stronger than that of Ni2+, in order to maintain the charge balance and reduce the lattice energy, Ni2+ will transfer the outer electrons to Co3+ through the O2− bond, and finally Co3+ is reduced to Co2+ (still occupying the octahedral gap). Ni2+ may be oxidized to Ni3+ (maintaining the tetrahedral/octahedral gap), and the electrons realize the directional flow between the adjacent ions in the tetrahedral-octahedral gap. The increase in Co3+ electron cloud density further strengthens the adsorption of HMF, and the introduced high-energy d orbital of Ni2+ contributes to the desorption of the product. In order to investigate the effect of Ni introduction on the oxidation state of Co, X-ray photoelectron spectroscopy (XPS) measurements were performed on NiO, Co3O4, and NiCo2O4 samples (Fig. S7). The Ni 2p spectra of NiO and NiCo2O4 (Fig. S8a) show two spin–orbit binaries (Ni 2p3/2 and Ni 2p1/2), belonging to Ni3+ (855.5 eV and 873.7 eV) and Ni2+ (853.7 eV and 871.7 eV) peaks. The Co 2p spectra of Co3O4 and NiCo2O4 (Fig. S8b) show two spin–orbit binary peaks (Co 2p3/2 and Co 2p1/2), belonging to Co3+ (779.2 eV and 794.3 eV) and Co2+ (780.6 eV and 795.9 eV) peaks. As shown in Fig. 1b, the ratio of Ni2+/Ni3+ (0.48/1) in NiCo2O4 is higher than that in NiO (0.43/1). At the same time, the ratio of Co2+/Co3+ in NiCo2O4 (2.27) increases compared with Co3O4 (1.43). The above results show that after introducing Ni into Co3O4, electrons can be transferred from Ni to Co and promote the reduction of Co3+ to Co2+, and Co3+ reversely induces the equilibrium results of Ni2+ oxidation and Ni-induced Co3+ reduction. Next, the spinel cell composition of Co3O4 and NiCo2O4 and the corresponding d-orbital electron filling are understood, which is conducive to the interpretation of the experimental results. The effect of Ni introduction on the molecular configuration of Co3O4 was further explored. The d-orbital electron filling orbital distribution maps of the Ni and Co tetrahedron and octahedron were Co3+ Oh (t2g6), Ni3+ Oh (t2g6 eg1), Co2+ Td (e4 t23), and Ni2+ Td (e4 t24) (Fig. 1c and d). It is worth noting that the energy level of the splitting d orbital in the octahedral field is t2g (dxy, dyz, dxz) < eg (dx2−y2, dz2), while the energy level of the splitting d-orbital in the tetrahedral field is opposite, e (dx2−y2, dz2) < t2 (dxy, dyz, dxz). Compared with the octahedral coordination, the tetrahedral coordination has three high-energy t2 (dxy, dyz, dxz) orbitals. More high-energy d-orbitals near the Fermi level provide more opportunities to interact with reactants and intermediates, thus providing a better ability to adjust the catalytic performance.36,37HMFOR performance evaluation
The HMFOR of NiO, Co3O4 and NiCo2O4 was evaluated in a standard three-electrode system. The screening experiments of different concentrations of HMF show that the activity increased with the increase in the HMF concentration (0−40 mM), indicating that the reaction behavior is concentration-dependent (Fig. S9). In order to further study the electro-oxidation behavior and reaction mechanism, 10 mM HMF was selected. Fig. 1e shows the linear sweep voltammetry (LSV) curves of NiO, Co3O4 and NiCo2O4 in the OER and HMFOR. For the OER (dotted line), the curves of Co3O4 and NiCo2O4 both have a peak at 1.49 V vs. RHE, which corresponds to the oxidation of Co2+ to form high valence Co3+ species at potential. The peak at 1.44 V vs. RHE in the curve of NiO corresponds to the high valence Ni3+ species formed by the oxidation of Ni2+ at the potential.38 When 10 mM HMF is introduced into the electrolyte, the NiCo2O4 electrode reaches 10 mA cm−2 at 1.35 V vs. RHE, which is lower than that of NiO (1.39 V) and Co3O4 (1.6 V), indicating that the HMFOR performance of NiCo2O4 is better. When the feed ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co is 1
Co is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, compared with the morphology and LSV response of NiCo2O4 (Fig. S10 and S11), it further indicates that the introduction of Ni greatly improves the HMFOR performance of Co3O4. Under the same conditions, the Tafel slope of NiCo2O4 (255 mV dec−1) is smaller than that of Co3O4 (398 mV dec−1) and NiO (400 mV dec−1, Fig. S12), which also verifies that the HMFOR performance of NiCo2O4 is better than that of Co3O4 and NiO from the kinetic point of view. NiCo2O4 was evaluated by measuring the electrochemical double layer capacitance (Cdl), where a larger Cdl means a larger specific surface area and, consequently, more exposed active sites under set conditions. NiCo2O4 shows a higher Cdl value (3.78 mF cm−2) than NiO (2.65 mF cm−2) and Co3O4 (1.88 mF cm−2, Fig. S13). The larger ECSA value indicates that NiCo2O4 has more specific surface area of reaction, which is beneficial for the improvement of HMFOR activity.
1, compared with the morphology and LSV response of NiCo2O4 (Fig. S10 and S11), it further indicates that the introduction of Ni greatly improves the HMFOR performance of Co3O4. Under the same conditions, the Tafel slope of NiCo2O4 (255 mV dec−1) is smaller than that of Co3O4 (398 mV dec−1) and NiO (400 mV dec−1, Fig. S12), which also verifies that the HMFOR performance of NiCo2O4 is better than that of Co3O4 and NiO from the kinetic point of view. NiCo2O4 was evaluated by measuring the electrochemical double layer capacitance (Cdl), where a larger Cdl means a larger specific surface area and, consequently, more exposed active sites under set conditions. NiCo2O4 shows a higher Cdl value (3.78 mF cm−2) than NiO (2.65 mF cm−2) and Co3O4 (1.88 mF cm−2, Fig. S13). The larger ECSA value indicates that NiCo2O4 has more specific surface area of reaction, which is beneficial for the improvement of HMFOR activity.
The FEFDCA of NiCo2O4 was tested at 1.45–1.60 V vs. RHE (Fig. 1f). With the increase in potential, the FEFDCA decreases gradually due to the emergence of the competitive OER. It is worth noting that the highest FEFDCA is 99.1% at 1.5 V vs. RHE. The substances present and composition of the electrolyte at this potential were determined by HPLC-MS. The results show that there is only one substance present (FDCA), indicating that HMF is completely converted into FDCA, and the electrooxidation process does not stay in the intermediate product step, further indicating the reliability of the data (Fig. S14). A complete comparison of the current density, HMF conversion, FDCA and FE yields and selectivity at 1.5 V vs. RHE is revealed in the radar plot (Fig. 1g), indicating that NiCo2O4 in NiO, Co3O4 and NiCo2O4 has significant HMFOR activity. The current density on the NiCo2O4 electrode reaches 26.2 mA cm−2 at 1.5 V vs. RHE, which is much higher than the same voltage on the Co3O4 electrode (7.05 mA cm−2) and the NiO electrode (18 mA cm−2). This result indicates that NiCo2O4 significantly boosts HMFOR efficiency and facilitates electron transfer. The conversion of HMF on NiO, Co3O4 and NiCo2O4 electrodes is 100%, but it is worth noting that the FEFDCA is 99.1% and the yield is 82.44%, which are higher than those of the NiO electrode (82%/71.24%) and Co3O4 electrode (43.09%/45.58%). According to the FE of Co3O4 which is less than the yield, it is preliminarily speculated that it is more conducive to the formation of intermediate products, namely hydroxyl oxidation. The FE of NiO is lower than its yield, which is more conducive to the formation of FDCA, namely aldehyde oxidation. From the perspective of product collection, the introduction of Ni changes the configuration of Co3O4, which greatly improves the HMFOR efficiency of NiCo2O4.
Corroboration of the reaction pathway
At present, there are two main oxidation pathways of the HMFOR in alkaline electrolyte: 2,5-diformylfuran (DFF) and HMFCA (Fig. 2a).16,39 In the DFF-directed pathway, the hydroxyl groups of HMF are preferentially oxidized to form DFF, and DFF is further oxidized to FFCA by aldehyde oxidation. In the HMFCA-oriented pathway, HMF is preferentially oxidized by the aldehyde group to form HMFCA and then oxidized by the hydroxyl group to form FFCA. Finally, FDCA is obtained by deep oxidation of FFCA. The NiCo2O4-catalyzed HMFOR was used to elucidate the reaction pathways involved at 1.5 V vs. RHE, and traces of HMF consumption and product (HMFCA, FFCA, DFF and FDCA) accumulation were tracked by HPLC (Fig. 2b and S15). In 1 M KOH containing 7.7 mM HMF (15 mL), a decrease in HMF and an increase in FDCA are observed with the accumulation of charge, indicating that HMF could be converted into FDCA by NiCo2O4 (Fig. S16 and S17). The color change of the reaction solution during the specific experiment also confirmed the complete conversion process (Fig. S18). In addition to HMF and FDCA, the intermediate products HMFCA and FFCA can be clearly detected, confirming that the conversion of HMF to FDCA follows the HMFCA-oriented pathway (HMF → HMFCA → FFCA → FDCA). In order to further determine the conversion pathway of the HMFOR catalyzed by NiCo2O4, potential- and time-dependent in situ FTIR spectroscopy was used.40–42 As shown in Fig. 2c–e, NiCo2O4 has a band at 1654 cm−1 (HMF), indicating that HMF is consumed first and then adsorbed, while the downward band of Co3O4 has the largest change, and NiO has no obvious change, indicating that Co3O4 has the largest adsorption of HMF and NiO has no adsorption. At the same time, fork-like bands at 1523 and 1541 cm−1 are observed, indicating the formation of intermediate HMFCA (Fig. S19). With time, a new peak appeared at 1700 cm−1, which belongs to the carboxylate group, which is related to the formation of FDCA. NiCo2O4 has the most obvious change in the descending section, indicating that the formation of FDCA is the fastest. These results indicate that the introduction of Ni with strong desorption of weakly adsorbed reactants on Co3O4, which has strongly adsorbed reactants and difficult to desorb products, can result in the HMFOR with efficient multi-electron transfer and dual-site synergistic catalysis of strong adsorption of Co and strong desorption of Ni. In particular, the absence of the DFF signal once again confirms the dominant position of the HMFCA pathway in the NiCo2O4-catalyzed HMFOR, which is consistent with the results of HPLC analysis. As mentioned, the absence of DFF features is likely to be caused by spontaneous conversion in strongly alkaline solutions.43,44 In addition, NiCo2O4 shows excellent catalytic stability and can be recycled four times continuously (Fig. S20a and b), and the conversion rate, yield and FE are all greater than 95%, which can be attributed to its good structural integrity. After the HMFOR test, the stability of the structure and composition was evaluated by a number of characterization studies. The SEM image shows that the overall outline of the needle-like array structure of NiCo2O4 is well maintained (Fig. S21), and the results show that NiCo2O4 has a stable microstructure. The XRD pattern shows that NiCo2O4 retained the original characteristic diffraction peak, and no new peak appeared (Fig. S22), indicating that the crystal structure of the catalyst did not change significantly after the HMFOR. The surface state of the elements was studied by XPS analysis. The subtle changes in the high-resolution XPS spectra of Co and Ni indicate a subtle change in the surface chemical state of NiCo2O4 (Fig. S23). All these analyses demonstrate the excellent robustness of NiCo2O4 in the HMFOR.In-situ electrochemical impedance spectroscopy (EIS) tests were performed on NiO, Co3O4 and NiCo2O4 in 1 M KOH or 1 M KOH + 10 mM HMF under a potential gradient of 1.1–1.6 V vs. RHE. The HMFOR and OER processes of NiO, Co3O4 and NiCo2O4 show similar trends in the high-frequency and medium-frequency regions (10–105 Hz) (Fig. 2f–h and S24a–c). During the OER process, the Bode plots of NiO, Co3O4 and NiCo2O4 show characteristic peaks in the low-frequency region (0.1–10 Hz). When the potential increases to 1.5 V vs. RHE, an inflection point appears in the low frequency region, which may be related to the adsorption of hydroxyl groups on the surface and surface oxidation, because the initial potential of the OER is in this potential range. The relevant equivalent circuit and Nyquist diagram and the fitted Rct values are shown and summarized (Fig. S25, S26 and Tables S2–S7). By comparison, the introduction of Ni into Co3O4 effectively reduces the transfer resistance and results in a faster reaction rate.
In-depth understanding of HMFOR performance
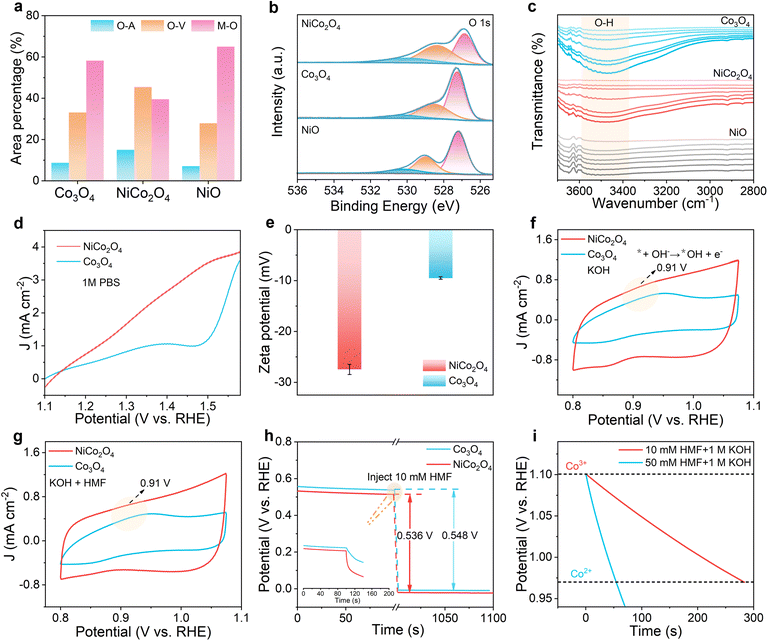
The oxidation of HMF in alkaline solution requires the participation of OH− species.45,46 The adsorption and conversion of OH− is an essential step in the HMFOR, similar to the first step of electrocatalytic water oxidation in an alkaline environment. Therefore, it is necessary to clarify the transformation behavior of OH− by exploring the OER performance of the material. The Tafel slope of NiCo2O4 (373 mV dec−1) is larger than that of Co3O4 (207 mV dec−1) and NiO (320 mV dec−1) (Fig. S27), indicating that the introduction of Ni inhibits the OER kinetics of NiCo2O4, that is, inhibits the conversion of OH− on the surface of NiCo2O4. The O 1s XPS spectra of different samples can be deconvoluted into three parts (Fig. 3a and b), which are surface adsorbed water molecules (530.2 eV), oxygen vacancies (528.3 eV) and lattice oxygen atoms combined with metal ions (526.8 eV). Compared with the high-resolution spectra of NiO and Co3O4, the proportion of lattice oxygen (526.8 eV) in NiCo2O4 is significantly reduced, and the proportion of absorbed water (530.2 eV) is significantly increased, which confirms the existence of abundant oxygen vacancies in NiCo2O4. The ratio of lattice oxygen to adsorbed oxygen in NiCo2O4, Co3O4 and NiO samples is 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6.7
1, 6.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 9.2
1 and 9.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The results show that the proportion of adsorbed oxygen species in NiCo2O4 is significantly higher than that in Co3O4 and NiO, indicating that NiCo2O4 may have better adsorption capacity for oxygen-containing species OH−, which is more conducive to the HMFOR. In the in-situ FTIR spectra (Fig. 3c), in the range of 3100−3600 cm−1, NiO shows little change in the intensity of the O–H vibration peak and NiCo2O4 shows higher O–H vibration peak intensity than Co3O4, indicating that more OH− species are formed or accumulated on the surface of NiCo2O4 during the reaction, while there is no OH− accumulation on the surface of NiO. At the same time, it is detected that when the voltage was turned off at the end, the O–H characteristic signals of Co3O4, NiCo2O4 and NiO did not decrease or disappear (Fig. S28). The peak intensity, peak shape and peak position remain stable, which is completely consistent with the characteristics in the process of electrification. This indicates that this process does not involve the physical adsorption of free OH in the electrolyte, but involves the process of chemical combination of active sites on the catalyst surface with OH to form M-OH. The generated M-OH is part of the chemical structure of the catalyst surface and has a stable chemical bonding state. Next, NiCo2O4 and Co3O4 were further explored, and the adsorption activity of OH− was also determined in an OH− deficient environment (1 M PBS). The results show that the OER performance of NiCo2O4 is much higher than that of Co3O4 (Fig. 3d), which also proves that the introduction of Ni is particularly beneficial for the capture of OH−. In Fig. 3e, NiCo2O4 (−27.5 mV) shows a more negative zeta potential value than Co3O4 (−9.5 mV) in the KOH environment, representing more OH− in the inner Helmholtz layer, which also supports the above conclusion. In addition, in the cyclic voltammetry (CV) curve (Fig. 3f, g and S29), an obvious oxidation peak can be observed in the potential range of 0.8–1.05 V vs. RHE, indicating the adsorption of *OH. At the same time, the linear fitting results show that the process is an adsorption control process (Fig. S30 and S31).
1, respectively. The results show that the proportion of adsorbed oxygen species in NiCo2O4 is significantly higher than that in Co3O4 and NiO, indicating that NiCo2O4 may have better adsorption capacity for oxygen-containing species OH−, which is more conducive to the HMFOR. In the in-situ FTIR spectra (Fig. 3c), in the range of 3100−3600 cm−1, NiO shows little change in the intensity of the O–H vibration peak and NiCo2O4 shows higher O–H vibration peak intensity than Co3O4, indicating that more OH− species are formed or accumulated on the surface of NiCo2O4 during the reaction, while there is no OH− accumulation on the surface of NiO. At the same time, it is detected that when the voltage was turned off at the end, the O–H characteristic signals of Co3O4, NiCo2O4 and NiO did not decrease or disappear (Fig. S28). The peak intensity, peak shape and peak position remain stable, which is completely consistent with the characteristics in the process of electrification. This indicates that this process does not involve the physical adsorption of free OH in the electrolyte, but involves the process of chemical combination of active sites on the catalyst surface with OH to form M-OH. The generated M-OH is part of the chemical structure of the catalyst surface and has a stable chemical bonding state. Next, NiCo2O4 and Co3O4 were further explored, and the adsorption activity of OH− was also determined in an OH− deficient environment (1 M PBS). The results show that the OER performance of NiCo2O4 is much higher than that of Co3O4 (Fig. 3d), which also proves that the introduction of Ni is particularly beneficial for the capture of OH−. In Fig. 3e, NiCo2O4 (−27.5 mV) shows a more negative zeta potential value than Co3O4 (−9.5 mV) in the KOH environment, representing more OH− in the inner Helmholtz layer, which also supports the above conclusion. In addition, in the cyclic voltammetry (CV) curve (Fig. 3f, g and S29), an obvious oxidation peak can be observed in the potential range of 0.8–1.05 V vs. RHE, indicating the adsorption of *OH. At the same time, the linear fitting results show that the process is an adsorption control process (Fig. S30 and S31).
Open circuit potential (OCP) was measured to detect changes in the internal organic adsorbent content of the Helmholtz layer. When 10 mM HMF is injected, although the OCP change of NiCo2O4 (0.536 V) is close to that of Co3O4 (0.548 V), the OCP potential of Co3O4 of NiCo2O4 is significantly smaller, indicating that more HMF molecules are inherently adsorbed in the inner Helmholtz layer. As shown in Fig. 3h, the OCP of NiCo2O4 decreases faster after the addition of HMF, which also means that the introduction of Ni is beneficial for the dynamic adsorption of HMF. The evidence of whether Co3+ is the active site of the HMFOR is further investigated. Therefore, we conducted a Co3+ elimination time test to explore the function of Co3+ species in the HMFOR. As shown in Fig. 3i, NiCo2O4 is first oxidized to form Co3+ species and accumulate at an oxidation potential of 1.1 V vs. RHE, resulting in the enrichment of Co3+, which is completed in 1 M KOH + 10 mM HMF. When accumulated to a certain extent, the potential is converted to OCP, and the potential gradually decreases, indicating the attenuation of Co3+. When an additional amount of HMF (50 mM) is introduced into the backward system in the first 10 s, the decay rate of Co3+ increases sharply, and the decay time is shortened from about 284 s to 55 s, indicating that Co3+ is the active site of the HMFOR.
Density functional theory calculations
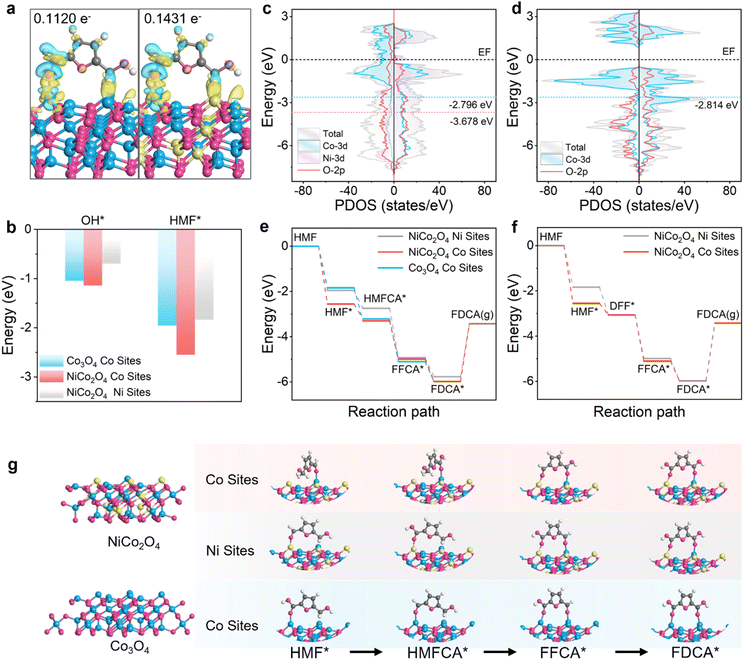
In order to further reveal the introduction of Ni, that NiCo2O4 has a significant improvement in the selectivity and performance of HMF oxidation to FDCA and the internal promotion mechanism for the integration of NiCo2O4 in the HMFOR, we performed DFT calculations.47 According to the optimized stable structures of Co3O4 and NiCo2O4, the (111) crystal plane of the catalyst sample is selected for calculation. After adsorption of HMF (Fig. 4a), NiCo2O4 (0.143 e−) transferred more electrons to HMF than Co3O4 (0.112 e−), indicating a stronger interaction with HMF. Considering that the appropriate adsorption behavior of OH− and HMF is critical to the HMFOR performance, the bonding strength of OH* and HMF* at different sites on the sample was evaluated (Fig. 4b, S32 and Tables S8, S9). For the adsorption of OH*, the Co sites of NiCo2O4 (−1.14 eV) show a smaller bonding strength than the Ni sites of NiCo2O4 (−0.70 eV) and the Co sites of Co3O4 (−1.05 eV). However, for the adsorption of HMF, the Co sites of NiCo2O4 (−2.55 eV) exhibit more negative adsorption energy than the Ni sites of NiCo2O4 (−1.84 eV) and the Co sites of Co3O4 (−1.96 eV), indicating that the introduction of Ni enhances the adsorption behavior of HMF and OH* on the Co sites of NiCo2O4, while the Ni sites of NiCo2O4 exhibit worse HMF and OH* adsorption behavior than the Co sites of Co3O4, which just balances the competitive adsorption of HMF molecules and OH− species, a reaction conducive to subsequent steps. In addition, the total density of states (TDOS) of NiCo2O4 near the Fermi level (EF) is significantly higher than that of Co3O4 due to the introduction of Ni (Fig. 4c and d), indicating that the conductivity of NiCo2O4 is enhanced, which is conducive to achieving faster interfacial electron transfer in the electrocatalytic process, thus effectively promoting the elementary steps involved in multi-electron transfer in the HMFOR, especially the rate-determining step. The smoother electron supply directly reduces the electron transfer energy barrier, thereby improving the overall reaction kinetics. The partial density of states (PDOS) analysis shows that both Co-3d and Ni-3d orbitals have a major contribution to TDOS, and there are strong peaks and signals near EF, indicating that Ni as an active site promotes electron transfer and has a lower energy barrier. After the introduction of Ni, the d-band center (−2.796 eV) of Co in NiCo2O4 is slightly shifted upward compared with Co3O4 (−2.814 eV), indicating that its interaction with adsorbed oxidation intermediates is strengthened, which is consistent with the strengthening of OH* adsorption behaviour. Then, the reaction state of the HMFOR process on the sample was studied (Fig. 4e–g, S33 and Tables S10–S14), which shows the specific adsorption configuration with the lowest energy path and the corresponding intermediate. Since the HMFOR is an exothermic reaction from HMF → HMF* → HMFCA* → FFCA* FDCA* and HMF → HMF* → DFF* → FFCA* → FDCA*, and the last step FDCA* → FDCA is an endothermic reaction, the HMF adsorption and FDCA desorption steps are determined as the reaction rate-determining steps (RDSs). For HMF adsorption, the Co sites of NiCo2O4 adsorb HMF to form HMF* with the lowest energy. Combined with the comparison of adsorption configurations, it can be seen that the Co sites of NiCo2O4 adsorb from the side of HMF. The Ni sites of NiCo2O4 and the Co sites of Co3O4 are adsorbed from the front of HMF, and the steric hindrance is large, indicating that the Co sites of NiCo2O4 are more conducive to the formation of HMF* to accelerate the reaction, which is consistent with the previous in situ FTIR analysis. Compared with the HMFCA path and the DFF path, the Co site of HMF* → HMFCA* is more exothermic than the Co site of HMF* → DFF*, which is more conducive to the generation of HMFCA*, further confirming that the main path is the HMFCA path. Since FDCA* is endothermically desorbed to obtain FDCA, the Ni sites of NiCo2O4 are superior to the Co sites of NiCo2O4 and the Co sites of Co3O4. Combined with the adsorption configuration, it can be seen that the conversion of FDCA* to FDCA requires desorption from adjacent metal atoms, and the Ni adsorption capacity is poor, indicating that the Ni sites of NiCo2O4 are more favorable for FDCA* to form FDCA. The results show that the introduction of Ni and the synergistic effect on NiCo2O4 are beneficial to accelerate the reaction conversion rate in the RDS and HMFOR processes, thereby promoting the improvement of product selectivity and yield.Electrocatalytic performance of the CO2-HMF coupled system reaction
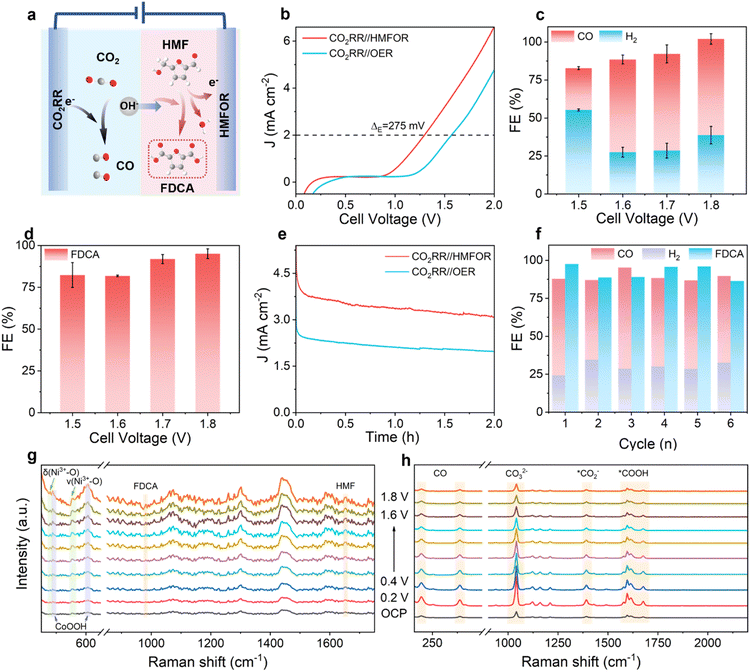
Based on NiCo2O4, a paired electrolysis system was developed with the prepared NiCo2O4 as the HMFOR anode and Au nanoparticles as the CO2RR cathode (Fig. 5a and S34).8,14,25 For comparison, the CO2RR system was also compared with the traditional anode OER. The LSV curve shown in Fig. 5b shows that at the same current density, the voltage required for the CO2-HMF coupled system is lower than that of the OER-CO2RR system (ΔE = 275 mV), indicating that the use of biomass small molecule oxidation instead of the OER can reduce the energy consumption required for the electrolysis process. The cathode and anode products of the coupled system were analyzed at 1.5–1.8 V (Fig. 5c and d). The FEFDCA at the anode is higher than 86%, and the highest value reached 91.9% at 1.7 V. The selectivity of the cathode to CO also reached 69.8% at 1.7 V, the FE for CO was 66.1%, and the FE of H2 was 28.6%. These results are among the top records for both the FE of CO2 and HMF (Table S15). Energy efficiency (EE) is the percentage of the energy retained in the target product to the total energy input into the electrolysis system.48 It can be used to measure the effectiveness of the reaction process that converts the input electric energy into valuable chemical products and stores it in the form of chemical energy. We calculate EE for further evaluation, and it can be seen that the total energy conversion efficiency is stable at about 43% at 1.5–1.8 V (Fig. S35). At 1.7 V, the total energy conversion efficiency is 43.3% (22.2% for FDCA, 15.9% for CO, and 5.2% for H2), which has a good effect on achieving high energy conversion efficiency at low potential (Table S16). High EE is essential to reduce electricity costs and improve the economic benefits of the CO2RR. In the potentiostatic electrolysis process (Fig. 5e), the change in cell current with time showed a significant difference between HMFOR-CO2RR and OER-CO2RR pairing systems in a H-type cell. The average current required for the CO2-HMF coupled system is 4 mA cm−2, and the average current required for the OER-CO2RR system is 2 mA cm−2. In the early stage of electrolysis of the CO2-HMF coupled system, the current density changes rapidly. However, in the later stage, due to the consumption of HMF, the OER dominates and the current change converges with the OER. In this case, the kinetic rate of the anodic reaction becomes slow with the decrease of the reactant concentration. For the OER-CO2RR system, the continuous decrease in current is due to the consumption of OH− by the OER. In addition, the system has excellent catalytic stability (Fig. 5f and S36), and the total FE exceeds 190% after 6 cycles of the stability test, and the FEFDCA is stable between 86.4% and 97.6%. At the same time, the total FE of the cathode is stable at more than 86%, including CO (57.1–66.6%) and H2 (24.2–32.6%). The stability of its structure and composition was evaluated by a series of characterization studies. In the SEM images it can be seen that the needle-like array structure of NiCo2O4 and the overall contour of Au nanoneedles are well maintained (Fig. S37). The XRD pattern shows that the anode cathode catalyst still shows the original characteristic diffraction peak, and no new peaks appear, indicating that the crystal structure of the catalyst does not change significantly after the CO2-HMF coupled system reaction (Fig. S38).In situ Raman spectroscopy of the coupled system
In order to detect and track the dynamic evolution of the intermediate products at both ends of the CO2-HMF coupled system in real time, in situ Raman spectroscopy (SERS) can not only monitor the changes of the intermediates at both ends of the CO2-HMF coupled system, but also analyze the changes of the surface state of the catalysts at both ends. For the HMFOR (Fig. 5g), there are two obvious peaks at 479 and 555 cm−1, which are attributed to the eg bending (δ) and A1g stretching (ν) modes of Ni3+−O, respectively. With the increase of applied potential, abundant NiOOH species are formed and accumulated on the electrode surface.20,49 This phenomenon also implies that the generated Ni3+−O species is an active intermediate; two obvious peaks at 487 and 606 cm−1 are attributed to CoOOH, which accumulates with the increase of applied potential as NiOOH species, while the signal peak of CoO2 is almost undetectable, indicating that Co3+ is the active site of the HMFOR.31 At the same time, with the increase of the potential, the HMF (1653 cm−1) peak gradually increases, indicating that the adsorption of HMF is continuously enhanced, which is consistent with the previous characterization results. The peak of FDCA (982 cm−1) also gradually increases, and the generation of FDCA is positively correlated with the applied potential, which is consistent with Fig. 5d.50,51 This shows that the two-site synergy of NiCo2O4 is applied to high-efficiency HMFOR, which further confirms the reliability of the theoretical calculation results. For the CO2RR (Fig. 5h), the peaks at 195 and 388 cm−1 correspond to the restricted rotation and Au–CO stretching of adsorbed CO, respectively.52 As the potential increases, the CO coverage decreases and Au accelerates CO desorption. No *CO peak at 2100 cm−1 verifies this conjecture. At the same time, *CO2− (1394 cm−1) and *COOH (1563–1686 cm−1) are necessary intermediates for the formation of *CO.53,54 As the potential increases, the peak intensity decreases, which is related to the decrease of CO selectivity, which is consistent with the Fig. 5c results. Overall, in the CO2RR-HMFOR coupled system, the NiCo2O4 anode promotes HMF-to-FDCA conversion via potential-dependent formation of Ni3+ and Co3+ intermediates for OH− capture. Meanwhile, the key intermediates of the CO2-to-CO conversion are observed at the cathode. The simultaneous progress of these anodic and cathodic reactions significantly reduces the overall energy consumption of the process.Conclusions
In summary, we present a cobalt-based spinel anode catalyst with a Ni–Co dual-site synergistic effect, which achieves efficient and stable electrochemical HMFOR under alkaline conditions, delivering a high FEFDCA of 99.1% at 1.5 V vs. RHE. Comprehensive characterization confirms that the introduction of Ni significantly enhances the adsorption and conversion capability of the catalyst towards HMF and OH− species. This enhancement is particularly crucial in the key FFCA → FDCA conversion step, effectively facilitating dehydrogenation and electron transfer processes. In the coupled system, at a cell voltage of 1.7 V, the anode maintains a high FEFDCA of 91.9%, while the cathode FE is 94.7% (CO is 66.1% and H2 is 28.6%), and the total energy conversion efficiency is 43.3%. SERS and DFT are used to reveal the dynamic catalytic mechanisms in the CO2-HMF coupled system. The NiCo2O4 anode promotes HMF-to-FDCA conversion via potential-dependent formation of Ni3+ and Co3+ intermediates for OH− capture. Meanwhile, the key intermediates of the CO2-to-CO conversion are observed at the cathode, offering a real-time experimental basis for optimizing such integrated electrocatalytic systems for concurrent CO2 and biomass conversion. This work provides compelling experimental evidence for the co-production of high-value chemicals via the upgrading of biomass-derived platform molecule HMF and CO2 conversion. More importantly, the adopted electronic structure regulation strategy and the established in situ dynamic monitoring method provide a universal research paradigm and technical path for the design and optimization of other coupled electrosynthesis systems (such as glycerol, ethanol, benzyl alcohol, etc.) involving organic molecule oxidation and CO2 reduction.Author contributions
Z. L. and T. O. conceived and supervised the research and performed funding acquisition and writing – review & editing. Q. Y. conducted the experiments, analyzed the data, and wrote the manuscript. X. L. assisted with the material synthesis and characterization. J. L. supported supervising and reviewing the manuscript. All authors contributed to the interpretation of the results.Conflicts of interest
There are no conflicts to declare.Data availability
All the data supporting this article have been included in the main text and the supplementary information (SI). Supplementary information: detailed experimental methods (catalyst synthesis, characterization techniques, electrochemical testing, and product analysis), supplementary figures (materials characterization, electrochemical performance data, in situ spectroscopy results, and computation l models), and supplementary tables (EIS fitting parameternsive DFT calculation data) that support the findings discussed in the main text. Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc10014.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22379033, U24A20541 and 22278094), National Key Research and Development Program Project (No. 2025YFF0516504), Guangdong Basic and Applied Basic Research Foundation (No. 2025B1515020046), Guangzhou Science and Technology Program (No. 2025A03J0011), Basic and Applied Basic Research Program of Guangzhou (No. 2024A03J0236), and Open Project of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry (2026-09). We would like to thank the Analysis and Test Center of Guangzhou University for their technical support.Notes and references
- S. W. Boettcher, Chem. Rev., 2024, 124, 13095–13098 Search PubMed.
- L. Lin, P. Su, Y. Han, Y. Xu, Q. Ni, X. Zhang, P. Xiong, Z. Sun, G. Sun and X. Chen, eScience, 2025, 5, 100264 Search PubMed.
- Z. Deng, B. Zhu, S. J. Davis, P. Ciais, D. Guan, P. Gong and Z. Liu, Nat. Rev. Earth Environ., 2025, 6, 231–233 CrossRef.
- T. Liu, H. Luo, T. Ouyang and Z. Liu, Adv. Funct. Mater., 2025, 35, 2415367 CrossRef CAS.
- R. Zhao, Z. Zhu, T. Ouyang and Z. Liu, Angew. Chem., Int. Ed., 2024, 63, e202313597 CrossRef CAS PubMed.
- T. Xiang, T. Liu, T. Ouyang, S. Zhao and Z. Liu, Interdiscip. Mater., 2024, 3, 380–388 CAS.
- R. Zhao, H. Luo, T. Ouyang and Z.-Q. Liu, ACS Nano, 2024, 18, 35749–35757 CrossRef CAS PubMed.
- F. Ye, S. Zhang, Q. Cheng, Y. Long, D. Liu, R. Paul, Y. Fang, Y. Su, L. Qu, L. Dai and C. Hu, Nat. Commun., 2023, 14, 2040 CrossRef CAS PubMed.
- H. Wu, L. Huang, J. Timoshenko, K. Qi, W. Wang, J. Liu, Y. Zhang, S. Yang, E. Petit, V. Flaud, J. Li, C. Salameh, P. Miele, L. Lajaunie, B. Roldán Cuenya, D. Rao and D. Voiry, Nat. Energy, 2024, 9, 422–433 Search PubMed.
- X. Li, Q. Chen, W. Sun, C. He and Z. Wen, Angew. Chem., Int. Ed., 2024, 63, e202412410 Search PubMed.
- S.-Q. Liu, M.-R. Gao, S. Wu, R. Feng, Y. Wang, L. Cui, Y. Guo, X.-Z. Fu and J.-L. Luo, Energy Environ. Sci., 2023, 16, 5305–5314 RSC.
- S. Liu, B. Tian, X. Xu, X. Wang, P. Ran, Y. Sun, J. Wu, A. Qiu, F. Wang, L. Tang, J. Ma and M. Ding, ACS Catal., 2024, 14, 9476–9486 CrossRef CAS.
- X. Jiang, K. Zhao, H. Feng, L. Ke, X. Wang, Y. Liu, L. Li, P. Sun, Z. Chen, Y. Sun, Z. Wang, L. Yu and N. Yan, J. Am. Chem. Soc., 2025, 147, 13471–13482 CrossRef CAS PubMed.
- W. Guo, X. Cao, D. Tan, B. Wulan, J. Ma and J. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202401333 CrossRef CAS PubMed.
- C. Lu, P. Shi, S. Huang, C. Yang, J. Zhu, J. Zhang, C. Ke, Y. Su, X. Zhuang and T. Wang, Angew. Chem., Int. Ed., 2025, 64, e202423263 CrossRef CAS PubMed.
- C. Lu, S. Yang, P. Shi, S. Huang, C. Cai, J. Zhu, X. Zhuang and T. Wang, Angew. Chem., Int. Ed., 2025, 64, e202502846 Search PubMed.
- L. Chen, C. Yu, X. Song, J. Dong, J. Mu and J. Qiu, Nat. Commun., 2024, 15, 8072 Search PubMed.
- D. Chen, W. Li, J. Liu and L. Sun, Energy Environ. Sci., 2025, 18, 3120–3128 RSC.
- L. Zeng, Y. Chen, M. Sun, Q. Huang, K. Sun, J. Ma, J. Li, H. Tan, M. Li, Y. Pan, Y. Liu, M. Luo, B. Huang and S. Guo, J. Am. Chem. Soc., 2023, 145, 17577–17587 CrossRef CAS PubMed.
- J. Woo, J. Choi, J. Choi, M. Lee, E. Kim, S. Yun, S. Yoo, E. Lee, U. Lee, D. H. Won, J. H. Park, Y. J. Hwang, J. S. Yoo and D. K. Lee, Adv. Funct. Mater., 2025, 35, 2413951 Search PubMed.
- Y. Lu, T. Liu, C. Dong, C. Yang, L. Zhou, Y. Huang, Y. Li, B. Zhou, Y. Zou and S. Wang, Adv. Mater., 2022, 34, 2107185 CrossRef CAS PubMed.
- M. Zubair, P. M. Usov, H. Ohtsu, J. A. Yuwono, C. S. Gerke, G. D. Y. Foley, H. Hackbarth, R. F. Webster, Y. Yang, W. H. Lie, Z. Ma, L. Thomsen, M. Kawano and N. M. Bedford, Adv. Energy Mater., 2024, 14, 2400676 CrossRef CAS.
- L. Zhang, P. Jin, Z. Wu, B. Zhou, J. Jiang, A. Deng, Q. Li, T. Hussain, Y. Zhang, H. Liu and S. Wang, Energy Environ. Mater., 2024, 7, e12725 CrossRef CAS.
- X. Jiang, X. Ma, Y. Liu, L. Zhao, Y. Zhang, B.-Q. Li and Q. Zhang, Appl. Catal., B, 2024, 347, 123785 CrossRef CAS.
- X. Liu, Y. Zhang, Y. Li, B. Yan and X. Zhao, Appl. Catal., B, 2025, 368, 125146 CrossRef CAS.
- J. Bi, Q. Zhu, W. Guo, P. Li, S. Jia, J. Liu, J. Ma, J. Zhang, Z. Liu and B. Han, ACS Sustain. Chem. Eng., 2022, 10, 8043–8050 Search PubMed.
- X. Cao, B. Wulan, Y. Wang, J. Ma, S. Hou and J. Zhang, Sci. Bull., 2023, 68, 1008–1016 CrossRef CAS PubMed.
- S. Liu, B. Zhang, Y. Cao, H. Wang, Y. Zhang, S. Zhang, Y. Li, H. Gong, S. Liu, Z. Yang and J. Sun, ACS Energy Lett., 2023, 8, 159–168 CrossRef CAS.
- J. Ma, X. Wang, J. Song, Y. Tang, T. Sun, L. Liu, J. Wang, J. Wang and M. Yang, Angew. Chem., Int. Ed., 2024, 63, e202319153 Search PubMed.
- G. Zhao, G. Hai, P. Zhou, Z. Liu, Y. Zhang, B. Peng, W. Xia, X. Huang and G. Wang, Adv. Funct. Mater., 2023, 33, 2213170 CrossRef CAS.
- X. Deng, G. Xu, Y. Zhang, L. Wang, J. Zhang, J. Li, X. Fu and J. Luo, Angew. Chem., Int. Ed., 2021, 60, 20535–20542 CrossRef CAS PubMed.
- H. G. Xu, X. R. Ning, J. Y. Zhao, H. Y. Lin, H. Q. Fu, S. Wang, Y. Guo, H. Wu, M. Zhu, H. Y. Yuan, P. F. Liu and H. G. Yang, Chem, 2024, 10, 2147–2169 Search PubMed.
- Y. Lu, C. Dong, Y. Huang, Y. Zou, Z. Liu, Y. Liu, Y. Li, N. He, J. Shi and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 19215–19221 Search PubMed.
- Q. Hu, S. Qi, Q. Huo, Y. Zhao, J. Sun, X. Chen, M. Lv, W. Zhou, C. Feng, X. Chai, H. Yang and C. He, J. Am. Chem. Soc., 2024, 146, 2967–2976 Search PubMed.
- X. Wang, T. Ouyang, L. Wang, J. Zhong, T. Ma and Z. Liu, Angew. Chem., Int. Ed., 2019, 58, 13291–13296 Search PubMed.
- Y. Liu, X. Ren, J. Wang, H. Wang, Z. Yin, Y. Wang, W. Huang, X. Hu, Z. J. Xu and Y. Deng, J. Am. Chem. Soc., 2025, 147, 20318–20328 CrossRef CAS PubMed.
- Y. Zhang, Q. Wu, J. Z. Y. Seow, Y. Jia, X. Ren and Z. J. Xu, Chem. Soc. Rev., 2024, 53, 8123–8136 RSC.
- P. Xu, Z. Bao, Y. Zhao, L. Zheng, Z. Lv, X. Shi, H. Wang, X. Fang and H. Zheng, Adv. Energy Mater., 2024, 14, 2303557 Search PubMed.
- C. Xu, E. Paone, D. Rodríguez-Padrón, R. Luque and F. Mauriello, Chem. Soc. Rev., 2020, 49, 4273–4306 Search PubMed.
- M. Qin, J. Chen, M. Qi, H. Wang, S. Mao, L. Xi and Y. Wang, ACS Catal., 2024, 14, 8414–8426 Search PubMed.
- J. Zhang, Y. Shen, Z. Wu, X. Zhang, J. Kang, Y. Wu, S. Zhang, S. Chen, G. Wang, H. Zhang, H. Yin and H. Zhao, Angew. Chem., Int. Ed., 2025, 64, e202423109 CrossRef CAS PubMed.
- C. Wang, Z. Zhao, W. Cao, Y. Peng, M. Song and Y. Wu, Adv. Funct. Mater., 2025, 2502618 CrossRef CAS.
- P. Zhou, X. Liu, Z. Chen, C. Tang, X. Zhao, J. Zheng, R. Ge and H. Duan, Adv. Funct. Mater., 2025, 2502081 CrossRef CAS.
- A. Prajapati, N. Govindarajan, W. Sun, J. Huang, H. Bemana, J. T. Feaster, S. A. Akhade, N. Kornienko and C. Hahn, ACS Catal., 2024, 14, 10122–10131 Search PubMed.
- P. Zhou, X. Lv, S. Tao, J. Wu, H. Wang, X. Wei, T. Wang, B. Zhou, Y. Lu, T. Frauenheim, X. Fu, S. Wang and Y. Zou, Adv. Mater., 2022, 34, 2204089 Search PubMed.
- K. Wang, M. Wu, Y. Zhang, B. Jiang, Y. Su, S. Yang, X. Lu and H. Li, Adv. Funct. Mater., 2025, 35, 2424435 CrossRef CAS.
- Y. Ma, Y. Xiao, C. Lei, X. Zhang, W. Sheng, S. Liu, P. Chen, P. Zhou, H. Duan, P. Li and M. Gong, Adv. Funct. Mater., 2025, e12292 Search PubMed.
- W. Lai, Y. Qiao, J. Zhang, Z. Lin and H. Huang, Energy Environ. Sci., 2022, 15, 3603–3629 Search PubMed.
- H. Zhang, Q. Yang, S. Luo, Z. Liu, J. Huang, Y. Zheng, C. Hu, J. Zhang, X. Bao, P. Yuan and X. Yao, ACS Catal., 2024, 14, 9565–9574 CrossRef CAS.
- G. Fu, X. Kang, Y. Zhang, Y. Guo, Z. Li, J. Liu, L. Wang, J. Zhang, X.-Z. Fu and J.-L. Luo, Nat. Commun., 2023, 14, 8395 CrossRef CAS PubMed.
- T. Huang, J. Han, Z. Li, Y. Hong, X. Gu, Y. Wu, Y. Zhang and S. Liu, Angew. Chem., Int. Ed., 2025, 64, e202500269 CrossRef CAS PubMed.
- D. Zhang, X. Liu, Y. Zhao, H. Zhang, A. V. Rudnev and J.-F. Li, Chem. Sci., 2025, 16, 4916–4936 RSC.
- M. Zheng, P. Wang, X. Zhi, K. Yang, Y. Jiao, J. Duan, Y. Zheng and S.-Z. Qiao, J. Am. Chem. Soc., 2022, 144, 14936–14944 CrossRef CAS PubMed.
- M. Wang, M. Fang, Y. Liu, C. Chen, Y. Zhang, S. Jia, H. Wu, M. He and B. Han, J. Am. Chem. Soc., 2025, 147, 16450–16458 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |