Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Atomic-level interface engineering enables efficient and durable acidic hydrogen evolution of osmium at large current densities
Qianyi Lin,
Jun Yu*,
Mansheng Liao,
Weidong Liang,
Yayun Hong,
Huiqi Li,
Zhongxin Song and
Lei Zhang
and
Lei Zhang *
*
College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518071, China. E-mail: yujun@szu.edu.cn; lei.zhang@szu.edu.cn
First published on 10th February 2026
Abstract
Osmium (Os), the least expensive member of the platinum-group metals, has emerged as a promising alternative to Pt-based catalysts for the hydrogen evolution reaction (HER). However, Os-based electrocatalysts still suffer from poor stability under acidic conditions, despite recent efforts to mitigate H* over-adsorption for improved intrinsic activity. Here, we design a porous CeO2 support that enables the atomic dispersion of Os, forming an Os single-atom catalyst (OsSA–CeO2). Unlike traditional flat-film supports, the porous CeO2 architecture prevents Os aggregation and achieves 100% interfacial anchoring of Os atoms. The resulting strong electronic coupling enables tight anchoring of Os and activates the CeO2 matrix with abundant oxygen vacancies, which facilitate H2O dissociation to sustainably supply protons for rapid consumption at large current densities. Also, the generated OH* species are adsorbed by the oxygen vacancies, thus preventing the Os sites from oxidative dissolution. As a result, OsSA–CeO2 exhibits over 500 h of durability at 100 mA cm−2 without performance decay—surpassing all previously reported Os-based HER catalysts. This work provides a general strategy for achieving complete interfacial anchoring of active metal atoms to enhance catalytic stability without sacrificing activity through support activation.
Introduction
The transition to sustainable and non-polluting energy sources has become increasingly urgent amid the global energy, environmental, and geopolitical crises. Among various clean energy technologies, “green hydrogen” has attracted particular attention as a zero-carbon fuel to play a dominant role in the future hydrogen economy.1–4 Electrocatalytic water splitting powered by renewable energy sources (e.g., solar and wind) represents one of the most promising routes for producing high-purity green hydrogen.5–7 As the cost of renewable electricity continues to decline, the price, activity, and durability of electrocatalysts for the hydrogen evolution reaction (HER) are becoming the primary factors determining the overall economic feasibility of this process.6,8–10Platinum (Pt)-based catalysts exhibit the highest HER activity due to their optimal hydrogen adsorption free energy. However, the high cost and scarcity of Pt significantly hinder their large-scale application.6,11–13 Osmium (Os), the least expensive member of the platinum group metals (PGMs), has recently emerged as a potential alternative to Pt-based catalysts.14–17 A fundamental challenge for Os-based HER catalysts is their tendency to over-adsorb hydrogen intermediates (H*), which results in inherently low HER activity.18,19 To address this, several strategies have been developed to tune the electronic structure of Os and modulate its hydrogen adsorption behavior, such as supporting Os nanoparticles on TiO2,20 constructing heterostructures like Os–OsSe2,15,18,19 and introducing anion doping.21 While these approaches have improved catalytic performance, achieving long-term stability, particularly at high current densities (≥100 mA cm−2), remains a major challenge.15,18,19
The instability of Os-based catalysts primarily originates from nanoparticle aggregation via Ostwald ripening and the easily fluctuating valence states of Os under the harsh conditions of the HER.14,22 At large current densities, oxidative dissolution of Os becomes more pronounced due to the rapid consumption of local H+ and the accumulation of newly generated OH− species derived from H2O dissociation.23 Anchoring Os onto reducible metal oxides, such as CeO2, offers a promising pathway to enhance stability. The facile Ce4+/Ce3+ redox cycling and high-order f orbitals of CeO2 facilitate strong electronic coupling with supported metal atoms.24–27 Moreover, the oxyphilic nature of Ce and the ease of oxygen vacancy formation endow CeO2 with a strong affinity for OH− species,23,28–30 which can effectively suppress the adsorption of OH− on Os and thus prevent its oxidative dissolution. To achieve these benefits, each Os atom must directly interact with neighboring Ce atoms i.g. achieving atomic dispersion of Os on CeO2.31–33 However, the fabrication of this precise architectural structure presents a great challenge and it requires the investigation of the thermodynamic stability properties of isolated Os atoms.
In this work, we first investigated the structural stability and hydrogen adsorption behavior of atomically dispersed Os on CeO2 (OsSA–CeO2) using density functional theory (DFT) calculations. The results reveal that Os atoms can be strongly anchored within the CeO2 lattice, while their excessive H* adsorption is effectively mitigated. Guided by these insights, we synthesized OsSA–CeO2 via a two-step electrodeposition method. A CeO2 thin film was first deposited onto a conductive carbon fiber substrate, followed by O2 annealing to create a porous structure that promotes atomic Os dispersion. This architecture introduces abundant oxygen vacancies and induces lattice compression, which shortens the distance between Os atoms and adjacent vacancies. These features facilitate H2O dissociation and the subsequent migration of H* species to neighboring Os sites, providing a continuous hydrogen source for proton consumption at large current densities. Meanwhile, the generated OH* intermediates are preferentially adsorbed by the oxygen vacancies rather than the Os sites, effectively suppressing oxidative dissolution. Consequently, OsSA–CeO2 exhibits a remarkably low overpotential of 97 mV and over 500 h of durability at 100 mA cm−2 with negligible degradation, surpassing nearly all previously reported Os-based HER catalysts.
Results and discussion
DFT calculations for material design
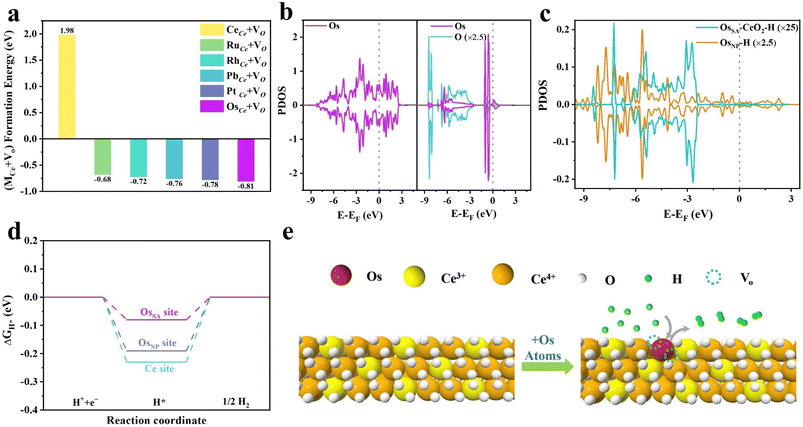
To assess the feasibility of Os incorporation into CeO2, we calculated the formation energies (FEs) of Os substituting Ce (OsCe) and OsCe accompanied by an oxygen vacancy (OsCe + VO). The FE of OsCe on the CeO2 (111) surface is 0.64 eV (Fig. S1), whereas that of OsCe + VO is −0.81 eV (Fig. 1a). The significantly lower FEs of OsCe + VO compared to OsCe indicate that oxygen vacancies form readily in Os-doped CeO2. Compared to other investigated platinum-group metals, OsCe + VO exhibits the lowest FE, demonstrating the high structural stability of atomically dispersed Os in CeO2 (OsSA–CeO2).The strong anchoring of Os atoms originates from pronounced interfacial electronic coupling with the CeO2 lattice, as revealed by DFT calculations. For metallic Os nanoparticles (OsNP), the projected density of states (PDOS) displays delocalized Os 5d orbitals with occupied states at the Fermi level (Fig. 1b, left), showing metallic behavior. Upon Os doping into CeO2, the 5d orbitals become localized, and unoccupied states above the Fermi level nearly vanish (Fig. 1b, right). This reflects charge redistribution and strong hybridization between Os and CeO2. The modified electronic structure of Os in OsSA–CeO2 brings in different hydrogen adsorption behavior (Fig. 1c). The energy range of its hybridization with the H 1s orbital is approximately −6.3 to −2.3 eV for OsSA–CeO2 compared to −8 to −4 eV for OsNP, indicating the weaker interaction between Os and H in OsSA–CeO2. The Gibbs free energy diagram for the HER (Fig. 1d) further supports this result. The calculated ΔGH* values for the Ce site in OsSA–CeO2 and Os site in OsNP are −0.23 eV and −0.19 eV, respectively, implying facile H adsorption. However, this hinders the desorption of H*, thereby limiting the formation of H2. In contrast, the Os site in OsSA–CeO2 exhibits a moderate ΔGH* of −0.08 eV and a shortened H–H coupling distance (1.60 Å vs. 1.97 Å for OsNP, Fig. S2), suggesting optimized hydrogen binding and improved HER kinetics. Overall, atomic Os incorporation into CeO2 yields a stable configuration with balanced H* adsorption energy and robust structural anchoring (Fig. 1e).
Material synthesis and structural characterization
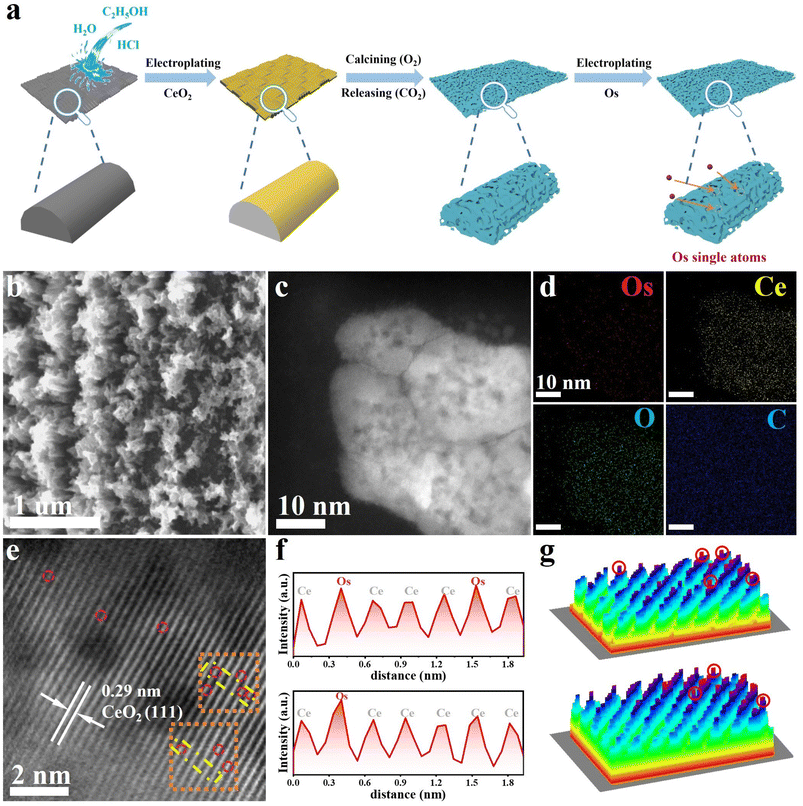
Guided by the theoretical predictions, we synthesized the OsSA–CeO2 sample consisting of Os SAs dispersed in the CeO2 matrix (Fig. 2a). Compared with the dense film structure of CeO2 (Fig. S3), the O2 post-treatment induced CO2 release from the carbon substrate, generating a porous structure of CeO2–O2 (Fig. S4) that was preserved in OsSA–CeO2 (Fig. 2b). The aberration-corrected high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image (Fig. 2c) reveals abundant dark regions within the sheet-like aggregates, corresponding to the voids seen in the scanning electron microscopy (SEM) image (Fig. 2b). Elemental mapping (Fig. 2d and Table S1) and high-resolution STEM imaging (Fig. 2e) confirm the sheet-like CeO2 structure with atomically dispersed Os. The reduced lattice spacing (0.29 nm vs. 0.31 nm for pristine CeO2) indicates lattice compression due to Os incorporation. Fig. 2f shows the 2D atom distribution map of the yellow area framed in Fig. 2e. The atoms with higher intensity are Os single atoms, and those with weaker intensity are Ce atoms. The 3D atom distribution map of the orange area in Fig. 2e also demonstrates the successful preparation of Os single atoms.Reference samples (OsNP/CeO2 and OsNP) were prepared via the same electrodeposition route for Os (Fig. S5–S6). Compared to OsSA–CeO2, the electrodeposited CeO2 film was annealed in Ar instead of O2 in OsNP/CeO2, and the morphology remains flat for CeO2–Ar (Fig. S7). For OsNP, the carbon fiber without CeO2 was directly used as the support for the deposition of Os. In contrast to OsSA–CeO2, Os aggregated into nanoparticles in both OsNP/CeO2 (Fig. S8) and OsNP (Fig. S9). This indicates that the porous structure of the CeO2 matrix is crucial for obtaining atomically dispersed Os atoms due to its much larger exposed surface area (Fig. S10). In addition, the Os loading amount is also necessary for the successful formation of Os single atoms (Fig. S11).
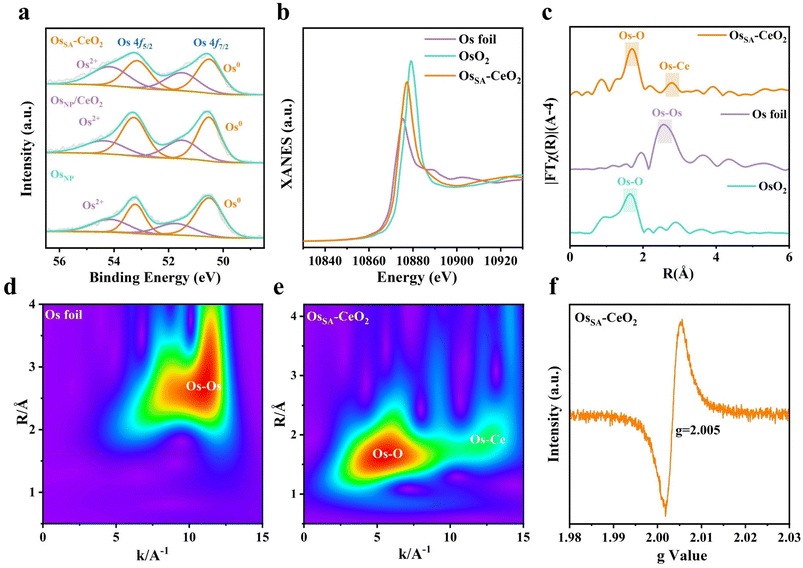
The electronic structure of Os in samples was investigated by X-ray photoelectron spectroscopy (XPS) and X-ray absorption near-edge structure (XANES) spectroscopy. In the Os 4f XPS spectra, the Os2+ fraction in OsNP/CeO2 is approximately 37 at%, which is a bit higher than the 36 at% for OsNP (Fig. 3a). This is probably due to the electronic transfer between OsNP and the CeO2 support.31,32 In contrast, OsSA–CeO2 exhibits a substantially increased Os2+ ratio of 46 at%. As revealed in Os L3 edge XANES spectra, Os in OsSA–CeO2 possesses an oxidation state between those of metallic Os and OsO2 (Fig. 3b). The much higher valence state of Os in OsSA–CeO2 further evidences the successful doping of Os into CeO2 that enables strong electronic interactions between them. Also, the relatively lower valence of Os compared to Ce4+ will induce the generation of oxygen vacancies to keep the electronic balance,34 consistent with the DFT results.
We studied the extended X-ray absorption fine structure (EXAFS) spectra to determine the coordination environments of Os in OsSA–CeO2. The Os L-edge R-space spectrum of OsSA–CeO2 (Fig. 3c) shows a dominant peak at ∼1.72 Å corresponding to the Os–O band, and no peak of Os–Os bond is observed. The small peak at ∼2.83 Å is probably ascribed to the Os–Ce bond,24,35 as illustrated by the Fourier transform (FT) EXAFS fitting (Fig. S12 and Table S2). These verify the atomic dispersion of Os in CeO2, consistent with the STEM results. Wavelet transform (WT)-EXAFS analysis (Fig. 3d and e) further confirms the appearance of Os–O and Os–Ce bands in OsSA–CeO2. The Os–O coordination number (3.2, Table S2) is significantly lower than the Ce–O coordination (7.7) in CeO2, indicating abundant oxygen vacancies, as confirmed by the electron paramagnetic resonance (EPR, Fig. 3f) and O 1s spectrum (Fig. S13) of OsSA–CeO2. The shorter Os–Ce bond (2.83 Å vs. Ce–Ce 3.30 Å) reflects lattice compression, consistent with the STEM results (Fig. 2e). Thus, we can conclude that the porous CeO2 support enables the successful fabrication of atomically dispersed Os SAs, accompanied by the generation of numerous oxygen vacancies within the CeO2 matrix.
Electrochemical performances
HER measurements were conducted in 0.5 M H2SO4 using a three-electrode setup. As shown in Fig. 4a, OsSA–CeO2 exhibits markedly superior HER activity compared to OsNP/CeO2 and OsNP, approaching the performance of commercial 20% Pt/C. The overpotentials of OsSA–CeO2 are only 43 mV and 97 mV at current densities of 10 and 100 mA cm−2, respectively (Fig. 4b). The Tafel slope (47 mV dec−1, Fig. 4c) is significantly lower than that of OsNP/CeO2 (139 mV dec−1) and OsNP (145 mV dec−1), indicating faster kinetics and a favorable HER pathway.36–38 Electrochemical impedance spectroscopy (Fig. 4d) shows that OsSA–CeO2 possesses the lowest charge transfer resistance, as summarized in Table S3. These factors contribute to its exceptionally high mass activity (Fig. 4e) and TOF (Fig. 4f). The mass activity of OsSA–CeO2 is 13.25 A mgOs−1 at 100 mV overpotential (Fig. 4e), 2.4 times higher than that of OsNP/CeO2 and 18.2 times higher than that of OsNP (Table S4). The effect of Os loading on HER performance was further investigated by varying the deposition cycles (Fig. S14). Os-50 CV (OsSA–CeO2) exhibits markedly higher activity than Os-10 CV, while showing slightly lower activity than Os-90 CV. However, excessive Os loading in Os-90 CV leads to the formation of large aggregated nanoparticles (Fig. S11c), which is detrimental to achieving atomically dispersed active sites. Therefore, Os-50 CV, which balances high catalytic activity with atomic dispersion, was selected as the representative sample for further investigation.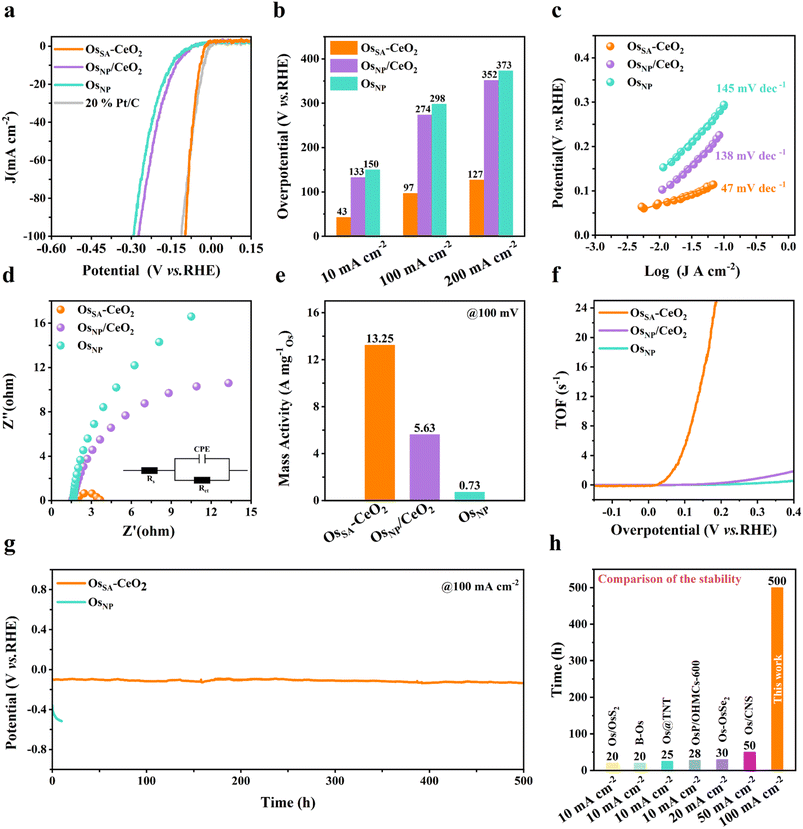 | ||
| Fig. 4 HER performance of OsSA–CeO2 in acidic media. (a) HER polarization curves of various catalysts in 0.5 M H2SO4. (b) Comparison of overpotentials at 10, 100 and 200 mA−2. (c) Tafel plots. (d) Nyquist plots; the inset shows the corresponding equivalent electrical circuit used for fitting. (e) Mass activity at an overpotential of 100 mV. (f) Relationship between TOF values and overpotentials. (g) Stability tests of OsSA–CeO2 and OsNP. (h) Comparison of the stability of OsSA–CeO2 with previously reported Os-based HER catalysts in acidic media.14,15,18–21 | ||
Post-HER XPS analysis (Fig. S15) reveals minimal changes in Os binding energy for OsSA–CeO2, whereas OsNP/CeO2 and OsNP exhibit positive shifts, indicating Os oxidation. Due to the strong interactions between Os and CeO2 in OsSA–CeO2, the electron-buffering effect of CeO2 regulates the electronic structure of Os and prevents its oxidation dissolution.33,39 Controlled-current water electrolysis (Fig. 4g) was further done to test the long-term performance and stability. OsSA–CeO2 maintains stable operation for over 500 h at 100 mA cm−2, far exceeding OsNP and previously reported Os-based HER catalysts (Fig. 4h).
Insights into the origin of enhanced stability at large current densities
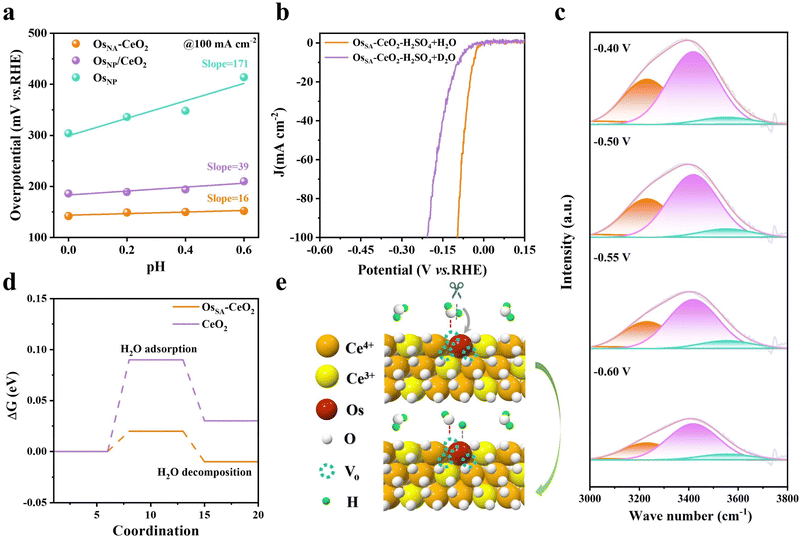
pH-dependent experiments (Fig. S16 and 5a) were performed to simulate the insufficient proton supply conditions that occur during high current operation. The overpotential at 100 mA cm−2 increases sharply with pH for OsNP (slope = 171), indicating strong proton dependence, whereas OsSA–CeO2 shows a much smaller slope (16), suggesting additional water-splitting participation. Kinetic isotope effect (KIE) experiments (Fig. 5b) confirm this: HER performance in 0.5 M H2SO4 with D2O is significantly lower than in 0.5 M H2SO4 with H2O, due to slower D+ migration and higher D–D bond energy, implying that H2O dissociation contributes to H* generation on OsSA–CeO2. Operando infrared spectroscopy (Fig. 5c) further supports this conclusion. The broad band from 3000–3800 cm−1 corresponds to interfacial water species, including 4-hydrogen bonding (HB)-H2O (3231 cm−1), 2-HB-H2O (3418 cm−1), and free H2O (3553 cm−1). With increasing negative potential, the proportion of strongly bound 4-HB-H2O decreases, transforming into free H2O (Table S5), indicating active water participation during the HER.40The facile participation of H2O for the HER on OsSA–CeO2 is probably due to the abundant oxygen vacancies. The oxygen vacancies can act as the strong adsorption sites for the oxygen-containing species like H2O, which is confirmed by the DFT calculations (Fig. 5d). The Gibbs free energy barrier (ΔG) for H2O adsorption decreases from 0.09 eV on CeO2 to 0.02 eV on OsSA–CeO2, greatly facilitating H2O dissociation. As summarized in Fig. 5e, Os doping induces oxygen vacancies that promote H2O adsorption and splitting. The generated H* species migrate to adjacent Os sites, serving as a hydrogen source to support the rapid proton consumption at large current densities, thereby enabling stable HER operation. Also, the generated OH* species are adsorbed by the oxygen vacancies, thus preventing the Os sites from oxidative dissolution.
Conclusions
In summary, we have successfully synthesized an OsSA–CeO2 catalyst composed of atomically dispersed Os single atoms strongly anchored on a porous CeO2 support. The porous CeO2 architecture provides abundant anchoring sites that stabilize Os atoms and strengthen the Os–CeO2 interfacial coupling. This interaction optimizes the electronic structure of Os, moderates H* binding strength, and induces abundant oxygen vacancies that activate CeO2 toward enhanced H2O dissociation. Benefiting from these synergistic effects, the OsSA–CeO2 catalyst exhibits outstanding HER activity in acidic electrolytes, surpassing commercial Pt/C at high current densities. It delivers a remarkable mass activity of 13.25 A mg−1 at 100 mV overpotential and maintains stable operation for over 500 h at 100 mA cm−2 with negligible degradation. This work provides a new design strategy to enhance the stability of noble-metal catalysts without sacrificing activity by engineering strong metal–support interfacial interactions.Author contributions
J. Y. conceptualized the project. J. Y. and L. Z. supervised the work. Q. L. performed catalyst synthesis and general characterization. Q. L., M. L., W. L., and Y. H. contributed to experimental design and data analysis. Q. L. and J. Y. wrote the original manuscript draft. All authors contributed to discussions on the data and to the development of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental methods, additional DFT calculations, structure models for DFT calculations; SEM, TEM, schematic illustration of the synthesis route, EXAFS, XPS and ICP-OES; additional electrochemical data and summarized table for situ FTIR spectroscopy. See DOI: https://doi.org/10.1039/d5sc09741j.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 22472101), the Guangdong Science and Technology Department Program (2025A1515010431, 2024A1515011543, 2021QN02L252), the Shenzhen Science and Technology Programs (20231120181703001), the Research Team Cultivation Program of Shenzhen University (2023QNT007), and the Scientific Foundation for Youth Scholars of Shenzhen University (868000001032086). The authors sincerely acknowledge the Instrumental Analysis Center of Shenzhen University (Xili Campus) and Electron Microscope Center of Shenzhen University.Notes and references
- N. Johnson, M. Liebreich, D. M. Kammen, P. Ekins, R. McKenna and I. Staffell, Nat. Rev. Clean Technol., 2025, 1, 1–21 Search PubMed.
- M. Jin, J. Chen, X. Han, Y. Guo, Y. Liu, J. Wang, J. Li, Y. Yuan, H. Jin, S. Wang and X. Huang, Nat. Commun., 2025, 16, 11615 CrossRef CAS PubMed.
- Q. Zhang, Y. Dou, C. Liu, H. Fan, M. Cui, P. Liu, H. K. Liu, S. X. Dou and D. Yuan, Electrochem. Energy Rev., 2025, 8, 36 CrossRef CAS.
- Y. Guan, X. Yao, C. Xue, Z. Song, X. Ren, T. K. Sham, L. M. Liu, L. Zhang and X. Sun, Adv. Funct. Mater., 2025, 35, 202524427 Search PubMed.
- S. Riyajuddin, M. Pahuja, P. K. Sachdeva, K. Azmi, S. Kumar, M. Afshan, F. Ali, J. Sultana, T. Maruyama and C. Bera, ACS Nano, 2022, 16, 4861–4875 CrossRef CAS PubMed.
- A. I. Feidenhans, Y. N. Regmi, C. Wei, D. Xia, J. Kibsgaard and L. A. King, Chem. Rev., 2024, 124, 5617–5667 CrossRef CAS PubMed.
- C. Hu, K. Yue, J. Han, X. Liu, L. Liu, Q. Liu, Q. Kong, C.-W. Pao, Z. Hu, K. Suenaga, D. Su, Q. Zhang, X. Wang, Y. Tan and X. Huang, Sci. Adv., 2023, 9, eadf9144 CrossRef CAS PubMed.
- M. F. Iqbal, M. Li, T. Xu, J. Yu, D. Xiong, Z. Mao, E. Hu, J. Zhang, P. Xu and Z. Chen, Renewables, 2024, 2, 111–137 CrossRef.
- G. Chen, J.-h. Zhang, K.-L. Zhou, Y. Yang, H. Ma, Y. Jin, J. Liu and H. Wang, Front. Energy, 2024, 18, 369–377 CrossRef CAS.
- Y. Zhu, F. Guo, S. Zhang, Z. Wang, R. Chen, G. He, X. Sun and N. Cheng, Electrochem. Energy Rev., 2025, 8, 18 CrossRef CAS PubMed.
- J. Zhang, Z. Song, X. Yao, Y. Guan, Z. Huo, N. Chen, L. Zhang and X. Sun, Chem, 2025, 11, 102498 CAS.
- Y. Guan, Z. Song, C. Xue, X. Yao, M. L. Zheng, J. Fu, W. Li, Y. Li, X. Ren, L. Zhang, L. M. Liu and X. Sun, Adv. Energy Mater., 2024, 14, 202402714 Search PubMed.
- M. Liao, T. Wu, Y. Zhong, Q. Lin, J. Qiu and L. Zhang, Chem. Eng. J., 2025, 520, 165284 CrossRef CAS.
- D. Cao, H. Xu, H. Li, C. Feng, J. Zeng and D. Cheng, Nat. Commun., 2022, 13, 5843 CrossRef CAS.
- Y. Li, C.-K. Peng, H. Hu, S.-Y. Chen, J.-H. Choi, Y.-G. Lin and J.-M. Lee, Nat. Commun., 2022, 13, 1143 CrossRef CAS.
- Y. Gao, Y. Xue, H. Wu, S. Chen, X. Zheng, C. Xing and Y. Li, J. Am. Chem. Soc., 2024, 146, 10573–10580 CrossRef CAS.
- D. Chen and S. Mu, Energy Rev., 2023, 2, 100053 CrossRef.
- D. Chen, R. Lu, R. Yu, Y. Dai, H. Zhao, D. Wu, P. Wang, J. Zhu, Z. Pu, L. Chen, J. Yu and S. Mu, Angew. Chem., Int. Ed., 2022, 61, e202208642 CrossRef CAS.
- J. Zhu, F. Xia, Y. Guo, R. Lu, L. Gong, D. Chen, P. Wang, L. Chen, J. Yu, J. Wu and S. Mu, ACS Catal., 2022, 12, 13312–13320 CrossRef CAS.
- M. N. Krstajić Pajić, A. S. Dobrota, A. Mazare, S. Đurđić, I. Hwang, N. V. Skorodumova, D. Manojlovic, R. Vasilic, I. A. Pasti and P. Schmuki, ACS Appl. Mater. Interfaces, 2023, 15, 31459–31469 CrossRef.
- M. Liu, K. Shi, Z. Duan, M. Zhang, Y. Xu, Z. Wang, X. Li, L. Wang and H. Wang, J. Mater. Chem. A, 2022, 10, 13042–13047 RSC.
- L. Yang, S. Hou, S. Zhu, Z. Shi, X. Wang, J. Jiang, Y. Chu, J. Bai, Y. Wang, L. Zhang, Z. Jiang, C. Liu, W. Xing and J. Ge, ACS Catal., 2022, 12, 13523–13532 CrossRef CAS.
- F. Shen, Z. Zhang, Z. Wang, H. Ren, X. Liang, Z. Cai, S. Yang, G. Sun, Y. Cao, X. Yang, M. Hu, Z. Hao and K. Zhou, Nat. Commun., 2024, 15, 448 CrossRef CAS PubMed.
- J. Yu, J. Wang, X. Long, L. Chen, Q. Cao, J. Wang, C. Qiu, J. Lim and S. Yang, Adv. Energy Mater., 2021, 11, 2002731 CrossRef CAS.
- X. Liu, Y. Zhou, J. Lin, X. Xiao, Z. Wang, L. Jia, M. Li, K. Yang, J. Fan and W. Yang, Angew. Chem., 2024, 136, e202406650 CrossRef.
- N. P. de Moraes, M. B. de Campos Sanmartin, R. da Silva Rocha, A. de Siervo, M. R. de Vasconcelos Lanza, D. A. Reddy, L. Yu and L. A. Rodrigues, J. Rare Earths, 2024, 42, 314–322 CrossRef CAS.
- Y. Jiang, H. Fu, Z. Liang, Q. Zhang and Y. Du, Chem. Soc. Rev., 2024, 53, 714–763 RSC.
- H. Liu, Q. Zhou, J. Yu, M. Nakabayashi, Y.-T. Lee, N. Shibata, Y. Li and J.-J. Delaunay, ACS Catal., 2025, 15, 8511–8521 CrossRef CAS.
- C.-H. Yan and X. Huang, J. Rare Earths, 2025, 43, 2029–2052 CrossRef.
- B. H. Yan, B. R. Xu, Y. Jin, H. Xiao, S. C. Luo, R. H. Duan, H. Li, X. Q. Yan, B. Lin and G. D. Yang, cMat, 2024, 1, e28 CrossRef.
- H. Liu, H. Duan, J. Yu, C. Qiu, R. Yu, J. Gao, S. Li, X. Du, Z. Si and S. Yang, ACS Mater. Lett., 2022, 4, 2572–2578 CrossRef CAS.
- H. Liu, J. Yu, J. Lin, B. Feng, M. Sun, C. Qiu, K. Qian, Z. Si, B. Huang and J.-J. Delaunay, EES Catal., 2023, 1, 720–729 RSC.
- Y. Li, J. Zou, L. Sun, S. Liu, H. Li, Z. Song, J. Yu, L. Zhang and Z. Guo, Adv. Funct. Mater., 2025, 2509899 CrossRef CAS.
- J. Yu, Q. Cao, Y. Li, X. Long, S. Yang, J. K. Clark, M. Nakabayashi, N. Shibata and J.-J. Delaunay, ACS Catal., 2019, 9, 1605–1611 CrossRef CAS.
- W. Tan, S. Xie, D. Le, W. Diao, M. Wang, K.-B. Low, D. Austin, S. Hong, F. Gao and L. Dong, Nat. Commun., 2022, 13, 7070 CrossRef CAS.
- W. Liang, Y. Zhang, D. Wang, J. Peng, J. Zou, H. Li, Z. Song, M. Tan, J. Yu and L. Zhang, J. Phys. Chem. Lett., 2025, 16, 12589–12595 CrossRef CAS.
- X. Zheng, X. Miao, Z. Yang, Z. Luo, J. Yu, H. Li and L. Zhang, Chem. Sci., 2025, 16, 19820–19829 RSC.
- R. Wei, M. Liao, L. Sun, Q. Zhang, H. Zhang, L. Zhang and Z. Song, ACS Appl. Mater. Interfaces, 2024, 16, 7141–7151 CrossRef CAS.
- Z. Dong, C. Zhou, W. Chen, F. Lin, H. Luo, Z. Sun, Q. Huang, R. Zeng, Y. Tan, Z. Xiao, H. Huang, K. Wang, M. Luo, F. Lv and S. Guo, Adv. Funct. Mater., 2024, 34, 2400809 CrossRef CAS.
- Y. Xu, J. Du, J. Jiang, Y. Miao, Z. Zhuang, Z. Liu, Y. Yan, R. Pan, J. Yang, M. Wang, S. Gu, L. Kang and D. Wang, Angew. Chem., Int. Ed., 2025, 64, e202502227 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |