Open Access Article
Open Access ArticleMitigation strategies for Li2CO3 contamination in garnet-type solid-state electrolytes: formation mechanisms and interfacial engineering
Bin
Hao
a,
Qiushi
Wang
a,
Fangyuan
Zhao
a,
Jialong
Wu
a,
Weiheng
Chen
b,
Zhong-Jie
Jiang
 *c and
Zhongqing
Jiang
*c and
Zhongqing
Jiang
 *a
*a
aZhejiang Key Laboratory of Quantum State Control and Optical Field Manipulation, Department of Physics, Zhejiang Sci-Tech University, Hangzhou 310018, P. R. China. E-mail: zhongqingjiang@zstu.edu.cn
bVehicle Energy and Safety Laboratory, Department of Mechanical Engineering, Ningbo University of Technology, Ningbo 315336, Zhejiang, P. R. China
cGuangzhou Key Laboratory for Surface Chemistry of Energy Materials, Guangdong Engineering and Technology Research Center for Surface Chemistry of Energy Materials, College of Environment and Energy, South China University of Technology, Guangzhou 510006, P. R. China. E-mail: eszjiang@scut.edu.cn
First published on 13th January 2026
Abstract
Garnet-type solid-state electrolytes (SSEs) are promising candidates for next-generation solid-state batteries (SSBs) owing to their high ionic conductivity, robust mechanical strength, and broad electrochemical stability window. However, exposure to ambient air results in the formation of a Li2CO3 passivation layer on the surface, significantly reducing ionic conductivity and deteriorating interfacial wettability, thereby severely impairing the electrochemical performance of SSBs. This review systematically analyzes the formation mechanisms and influencing factors of Li2CO3 contamination on garnet-type SSE surfaces. It summarizes recent strategies for suppressing Li2CO3 formation, including sintering process optimization, elemental doping, and grain boundary/interface engineering. Among these approaches, interfacial treatments have attracted considerable attention owing to their cost-effectiveness and operational efficiency. This review focuses on categorizing diverse treatment strategies for improving electrode/electrolyte interfacial contact, including physical cleaning, chemical treatment and conversion, and modification with interfacial interlayers—specifically detailing types such as inorganic, organic, and organic–inorganic composite interlayers. Finally, the future prospects of garnet-type SSEs in high-performance SSBs are discussed, pointing out the need for in-depth research into the formation and evolution mechanisms of Li2CO3 and the development of more efficient interface control strategies. This review systematically examines interfacial challenges in garnet-type SSEs, with the ultimate goal of facilitating the development of stable all-solid-state lithium metal batteries and accelerating their commercialization.
1 Introduction
Lithium-ion batteries (LIBs) represent one of the most advanced energy storage technologies and have found widespread applications in daily life.1,2 After three decades of development, the energy density of conventional graphite-anode LIBs is approaching its theoretical limit, with commercial LIBs achieving only 80–240 Wh kg−1. This severely limits their application in electric vehicles and other high-energy-density devices. Furthermore, the flammable organic solvents in liquid electrolytes pose significant safety risks. Lithium dendrite formation and growth in these electrolytes can cause severe safety hazards and performance degradation, ultimately compromising battery longevity and potentially leading to thermal runaway (Fig. 1a).3,4 In recent years, with advancements in new technologies, lithium metal batteries (LMBs), which offer a substantially higher theoretical energy density than LIBs, have regained significant research interest.5 Consequently, solid-state lithium metal batteries (SSLMBs) have emerged as a highly promising alternative to conventional liquid LIBs, combining lithium metal anodes with nonflammable solid electrolytes to simultaneously achieve high energy density and enhanced safety (Fig. 1b).6,7 Recent years have witnessed significant exploration and development of various solid-state electrolytes (SSEs), accelerating the progress of SSLMBs. These electrolytes encompass lithium phosphorus oxynitride (LiPON),8 sodium superionic conductor (NASICON)-type,9 lithium superionic conductor (LISICON)-type,10 sulfide-type,11 perovskite-type,12 and garnet-type materials.13 Among these, garnet-type oxide SSEs have emerged as particularly promising due to their high ionic conductivity (10−4–10−3 S cm−1 at room temperature), wide electrochemical stability window, exceptional shear modulus (56–61 GPa, exceeding that of Li metal by sevenfold), and excellent lithium metal compatibility. These superior characteristics position garnet-type electrolytes as leading candidates for SSLMB applications.14,15 However, numerous studies have shown that the poor interface contact performance of Li/garnet-type SSEs is a major challenge hindering their large-scale application. The poor air stability of garnet-type SSEs is a primary cause of this issue. Exposure to ambient air induces reactions with moisture and CO2, forming lithiophobic contaminants (Li2CO3 and LiOH) on garnet surfaces. These impurities significantly degrade both the lithium metal/garnet electrolyte interfacial contact and lithium-ion transport across the interface, ultimately impairing battery performance.16,17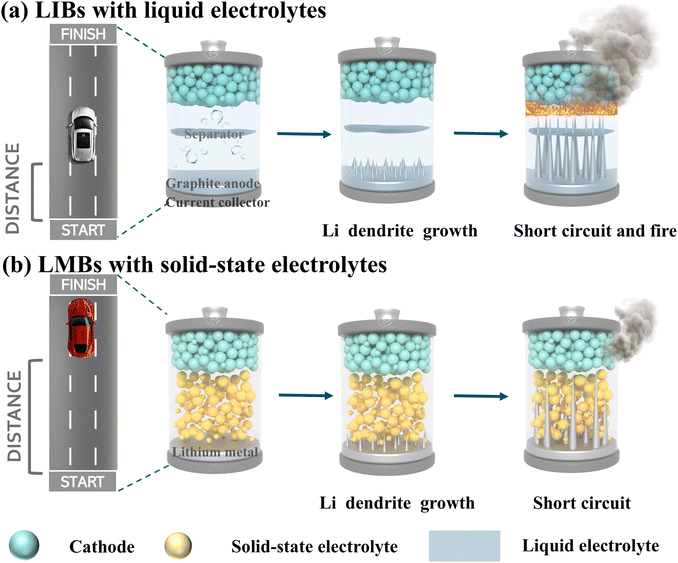 | ||
| Fig. 1 Comparison of energy output and safety performance between (a) conventional LIBs and (b) SSLMBs. | ||
The garnet-type oxide SSE Li7La3Zr2O12 (LLZO) was first reported in 2007. After years of exploration, it was found that substituting part of Zr4+ with Ta5+ cations to form Li7−xLa3Zr2−xTaxO12 (LLZTO) could enhance its lithium-ion conductivity by stabilizing the high-conductivity cubic phase structure, while maintaining its stability with Li metal. Although LLZO is chemically stable in air for short periods, recent studies have demonstrated that prolonged air exposure leads to the gradual formation of a lithiophobic Li2CO3 passivation layer on its surface. The impact of surface Li2CO3 on garnet electrolytes was largely overlooked for a long time. This changed when researchers discovered that Li2CO3 induces lithiophobicity, which contrasts sharply with the lithiophilicity of a pristine garnet surface. Consequently, the formation mechanism and detrimental effects of Li2CO3 began to be widely studied.18 The generation of Li2CO3 depletes lithium from the LLZO structure, degrading the ionic conductivity of the garnet SSE and increasing grain boundary (GB) resistance, which consequently deteriorates the interfacial impedance.19 Numerous modification strategies and interfacial engineering approaches have been developed to mitigate Li2CO3-related issues in garnet-based solid-state batteries (SSBs). Furthermore, Li foil surfaces readily oxidize due to their high reactivity, forming a composite passivation layer (Li2CO3, Li3N, LiOH, and Li2O) during processing and storage. This layer reduces battery energy density by impeding ion transfer at the Li anode/electrolyte interface. Both Li2CO3 contamination on LLZO surfaces and Li foil passivation significantly hinder the advancement of garnet-based SSBs. The main issues are as follows: (1) the lithiophobic nature and low Li+ conductivity of Li2CO3 severely impede intimate contact between Li metal and LLZO, as well as Li+ transport across the interface, thereby reducing the overall energy density of the battery; (2) the lithiophobic nature of Li2CO3 leads to poor molten lithium wettability, causing both high interfacial resistance (ranging from hundreds to thousands of Ω cm2) and lithium dendrite formation, which consequently reduces the critical current density (CCD); (3) due to the poor interface contact, the current or charge distribution at the interface becomes uneven, leading to an uneven point-to-point physical connection between the rigid garnet-type SSE and the lithium metal anode.24,25 Li metal preferentially deposits at interfaces exhibiting high local current densities, generating excessive localized pressure and inducing thermal instability;26 (4) Li2CO3 impurities degrade the microstructure of LLZO, reducing the particle density and inducing the formation of non-negligible electron conduction pathways inside, which promote lithium nucleation and growth, ultimately increasing the risk of battery short-circuiting;27 (5) the opaque nature of garnet SSEs makes it difficult to directly observe the interfacial evolution between the SSE and the electrode using common in situ non-destructive characterization techniques, creating a bottleneck in comprehensively understanding the interface structure. Therefore, constructing effective strategies to eliminate the Li2CO3 passivation layer and exploring diversified interface reconstruction techniques are crucial for enhancing the performance of SSLMBs. These are also key issues that need to be solved to realize the practical application of LLZO.
This review systematically examines the mechanistic impacts of spontaneously formed Li2CO3 layers on the interfacial structure and properties of garnet-type SSEs. We will systematically explain the generation pathways of Li2CO3 and its core determining factors, explaining how it detrimentally limits the electrochemical performance of garnet-based SSLMBs. Furthermore, we will explore effective strategies for improving the air stability of garnet SSBs. Interfacial stability plays a crucial role in the long-term cycling and high-energy-density operation of SSBs, particularly under high-voltage conditions. Effectively suppressing interfacial side reactions and improving electrolyte stability remain pressing challenges to be addressed.28 The review will also analyze the core challenges faced by the Li/LLZO interface and the cathode active material/LLZO interface induced by Li2CO3, and evaluate the diversified interfacial engineering approaches and regulation mechanisms developed in recent years to address these two types of interface issues. At the end of this review, we conclude with forward-looking perspectives on the future research directions for garnet SSBs, aiming to provide fundamental insights into their surface chemistry and theoretical guidance for the precise manipulation of interfacial properties.
2 Interfacial chemistry and degradation mechanisms in garnet-type SSEs
2.1 Interfacial Li2CO3 at the electrode/garnet-type SSE interface
| Li7La3Zr2O12 + xCO2 → Li7−xLa3Zr2O12 + xLi2CO3 | (1) |
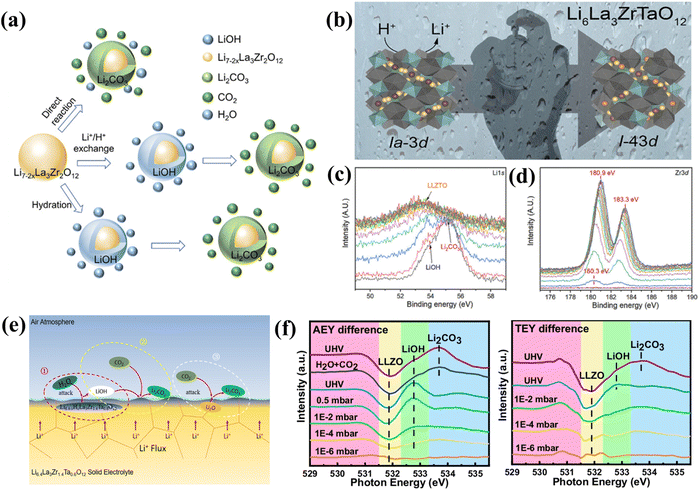 | ||
| Fig. 2 (a) Reaction pathways of garnet electrolytes with air. Reproduced from ref. 20. Copyright 2024, Wiley-VCH GmbH. (b) Schematic illustration of Li6La3ZrTaO12 space group transformation following Li+/H+ exchange. Reproduced with permission from ref. 21. Copyright 2020, American Chemical Society. XPS depth profiling of the freshly dissociated sample after 24 h air exposure: (c) Li 1s and (d) Zr 3d spectra. (e) Proposed APL formation mechanism on LLZTO surfaces. Reproduced with permission from ref. 22. Copyright 2022, American Chemical Society. (f) Comparative AEY and TEY spectra under varying H2O/CO2 pressures. Reproduced from ref. 23. Copyright 2024, Springer Nature. | ||
However, first-principles calculations indicate that the one-step reaction pathway is energetically unfavorable, a result that has been experimentally verified. Therefore, some researchers have proposed a two-step reaction pathway. Specifically, when exposed to humid air, water molecules can either directly insert into the garnet structure or react with LLZO via a Li+/H+ exchange mechanism, resulting in the formation of a surface LiOH layer. The LiOH subsequently reacts with atmospheric CO2 to form Li2CO3 (eqn (2) and (3)). This two-step pathway is considered more prevalent under realistic ambient conditions, which typically contain both moisture and CO2. In environments with higher relative humidity, the proton exchange reaction between the garnet electrolyte and water is facilitated, thereby accelerating Li2CO3 formation. Furthermore, Cheng et al.29 demonstrated that garnet and LiOH form directly through hydration, independent of Li+/H+ proton exchange. This occurs when there is sufficient H2O, leading to the generation of Li2CO3.
| Li7La3Zr2O12 + xH2O → Li7−xHxLa3Zr2O12 + xLiOH | (2) |
| 2LiOH + CO2 → Li2CO3 + H2O | (3) |
Moreover, these reactions are not limited to the surface but can also proceed within the LLZO bulk, propagating along GBs, especially in inadequately densified pellets. Both LiOH and Li2CO3 demonstrate poor lithium-ion conductivity, leading to elevated interfacial resistance at the LLZO/lithium metal interface. Xia et al. investigated Ta-doped LLZO pellets (0.5Ta-LLZO) exposed to dry (∼5% RH) and humid (∼80% RH) air environments for six weeks.30 Raman spectroscopy revealed characteristic peaks of Li2CO3 impurities on the surface of pellets exposed to humid air, whereas no such peaks were detected on those exposed to dry air. Furthermore, Raman mapping images demonstrated that Li2CO3 was not only distributed unevenly across the surface of pellets exposed to humid air but also preferentially accumulated at specific surface sites. It has been substantiated that Li2CO3 tends to nucleate preferentially around GBs, primarily attributable to the inherently high interfacial energy of these regions. Consequently, the areas highlighted in red on the Raman surface maps of pellets exposed to humid air likely correspond to GBs. In contrast, negligible Li2CO3 was observed on the pellets exposed to dry air. These results unequivocally demonstrate that ambient humidity is a critical factor governing the formation of Li2CO3.
In addition, atmospheric exposure significantly alters lattice parameters and lithium site occupancy. Lu et al.31 observed that Nb-LLZO's lattice parameters increased progressively from 12.95753 Å to 12.96244 Å following 1–22 days of environmental exposure. For Li6.5La3Zr1.5Nb0.5O12, atmospheric exposure induced complete redistribution of lithium occupancy between the 24d (Li1) and 96h (Li2) sites, resulting from Li+/H+ exchange and subsequent Li2CO3 formation. Zhang et al.32 employed neutron powder diffraction (NPD) and neutron pair distribution function (nPDF) analyses to demonstrate that Li+ in Li6.25La3Zr2Al0.25O12 crystals is located at the 24d (Li1) and 96h (Li2) positions.
Recently, Redhammer et al. also discovered that Li+/H+ preferentially appears at the 24d (Li1) position of single-crystal LLZTO.21 This is likely because Ta substitution causes Li+ to shift from the 24d position to the 96h position, increasing the mobility of Li+ at the 24d position. The study revealed that Li6La3ZrTaO12 undergoes Li+/H+ exchange primarily at the 24d site during degradation in various environments (air, humid air, water, and acetic acid). As illustrated in Fig. 2b, progressive Li+/H+ exchange induces lattice distortion, driving a structural transition from the Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d to I
d to I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3d space group and accelerating the transformation to the tetragonal phase. This phase transition significantly reduces Li+ conductivity. Therefore, long-term air exposure exacerbates structural decomposition, leading to higher bulk resistance. Leng et al.22 further studied the root cause of the instability of garnet-type SSEs and the characteristics of the air passivation layer (APL). They explored the cross-section of freshly sintered, unpolished Li6.4La3Zr1.4Ta0.6O12 (LLZTO) ceramic pellets and found that LLZTO undergoes passivation when exposed to ambient air. X-ray photoelectron spectroscopy (XPS) depth profiling performed on cross-sections of freshly sintered LLZTO pellets, as shown in Fig. 2c, revealed impurity peaks of LiOH·H2O and Li2CO3 in the Li 1s spectrum on the initial surface of LLZTO. As the etching depth increased, the intensity of Li2CO3 decreased. Correspondingly, LiOH·H2O gradually moved toward the high-energy side and eventually evolved into larger peaks of the underlying LLZTO at the deepest etching levels. Fig. 2d demonstrates a rightward shift in the Zr 3d peak, primarily attributed to compositional changes in LiOH·H2O and H-LLZTO during Li+/H+ exchange. Fig. 2e presents the most probable reaction pathway and formation mechanism of the APL. Fresh LLZTO absorbs water on its surface when exposed to air. Li+/H+ proton exchange then occurs, and specific Li+ sites in LLZTO are replaced by H+ to form H-LLZTO, eventually forming a LiOH·H2O layer on the surface. Notably, the oxygen layer in surface LiOH·H2O serves as a crucial intermediate and precursor for carbonation reactions, where it reacts with atmospheric CO2 to form Li2CO3. To elucidate the Li2CO3 formation mechanism via Li+/H+ exchange, Liu et al.23 employed XPS and X-ray absorption spectroscopy (XAS) to investigate the APL evolution on garnet surfaces. They first obtained an ultra-clean LLZO surface through low-temperature vacuum annealing, then introduced H2O into the LLZO surface and monitored surface composition using ambient pressure XPS (AP-XPS) and ambient pressure XAS (AP-XAS). As shown in Fig. 2f, no O(2−x)− and O(2−x+y)− signals around 530.8 eV were detected in the differential spectra of Auger electron yield (AEY) and total electron yield (TEY), indicating that the oxygen in the LLZO sublayer did not undergo a valence change after exposure to H2O. These results confirm Li+/H+ ion exchange, wherein H+ occupies vacancies created by Li+ migration to the surface. Exposure to a mixed gas (0.5 mbar H2O + 0.5 mbar CO2) yielded distinct Li2CO3 peaks in both AEY and TEY spectra, confirming the surface reaction: 2LiOH + CO2 → Li2CO3 + H2O. These findings provide direct spectroscopic evidence for Li+/H+ exchange and elucidate the critical formation mechanism of the initial surface layer governing air stability.
3d space group and accelerating the transformation to the tetragonal phase. This phase transition significantly reduces Li+ conductivity. Therefore, long-term air exposure exacerbates structural decomposition, leading to higher bulk resistance. Leng et al.22 further studied the root cause of the instability of garnet-type SSEs and the characteristics of the air passivation layer (APL). They explored the cross-section of freshly sintered, unpolished Li6.4La3Zr1.4Ta0.6O12 (LLZTO) ceramic pellets and found that LLZTO undergoes passivation when exposed to ambient air. X-ray photoelectron spectroscopy (XPS) depth profiling performed on cross-sections of freshly sintered LLZTO pellets, as shown in Fig. 2c, revealed impurity peaks of LiOH·H2O and Li2CO3 in the Li 1s spectrum on the initial surface of LLZTO. As the etching depth increased, the intensity of Li2CO3 decreased. Correspondingly, LiOH·H2O gradually moved toward the high-energy side and eventually evolved into larger peaks of the underlying LLZTO at the deepest etching levels. Fig. 2d demonstrates a rightward shift in the Zr 3d peak, primarily attributed to compositional changes in LiOH·H2O and H-LLZTO during Li+/H+ exchange. Fig. 2e presents the most probable reaction pathway and formation mechanism of the APL. Fresh LLZTO absorbs water on its surface when exposed to air. Li+/H+ proton exchange then occurs, and specific Li+ sites in LLZTO are replaced by H+ to form H-LLZTO, eventually forming a LiOH·H2O layer on the surface. Notably, the oxygen layer in surface LiOH·H2O serves as a crucial intermediate and precursor for carbonation reactions, where it reacts with atmospheric CO2 to form Li2CO3. To elucidate the Li2CO3 formation mechanism via Li+/H+ exchange, Liu et al.23 employed XPS and X-ray absorption spectroscopy (XAS) to investigate the APL evolution on garnet surfaces. They first obtained an ultra-clean LLZO surface through low-temperature vacuum annealing, then introduced H2O into the LLZO surface and monitored surface composition using ambient pressure XPS (AP-XPS) and ambient pressure XAS (AP-XAS). As shown in Fig. 2f, no O(2−x)− and O(2−x+y)− signals around 530.8 eV were detected in the differential spectra of Auger electron yield (AEY) and total electron yield (TEY), indicating that the oxygen in the LLZO sublayer did not undergo a valence change after exposure to H2O. These results confirm Li+/H+ ion exchange, wherein H+ occupies vacancies created by Li+ migration to the surface. Exposure to a mixed gas (0.5 mbar H2O + 0.5 mbar CO2) yielded distinct Li2CO3 peaks in both AEY and TEY spectra, confirming the surface reaction: 2LiOH + CO2 → Li2CO3 + H2O. These findings provide direct spectroscopic evidence for Li+/H+ exchange and elucidate the critical formation mechanism of the initial surface layer governing air stability.
 | ||
| Fig. 3 (a) Contact angles of molten Li on LLZO@Li2CO3versus pristine LLZO. (b) Calculated Wad, contact angle (θ), and atomic structure at Li–Li2CO3 (left) and Li–LLZO (right) interfaces. Reproduced with permission from ref. 33. Copyright 2017, American Chemical Society. (c) Schematic of phase-controlled versus diffusion-controlled carbonation processes. Reproduced from ref. 34. Copyright 2023, American Chemical Society. (d) HAADF-STEM image of grains and GBs. Reproduced with permission from ref. 35. Copyright 2024, American Chemical Society. (e) Cryo-TEM Images of LLZO grains and GBs. Reproduced with permission from ref. 36. Copyright 2023, Wiley-VCH GmbH. | ||
The presence of Li2CO3 can undergo a series of detrimental chemical reactions with electrode or electrolyte materials, thus adversely affecting the structural integrity and electrochemical performance of SSBs. Under certain conditions, particularly at high voltages, Li2CO3 can decompose oxidatively, releasing species like CO2 that may react with electrode materials, leading to structural degradation and performance fading.41 Moreover, under low potential and lithium-rich conditions, Li2CO3 may also undergo reduction reactions, which should be of significant concern. Biao et al.36 employed cryogenic transmission electron microscopy (cryo-TEM) to directly observe the chemical behavior of Li2CO3 at the GBs of LLZO during battery cycling, revealing the electrochemical reduction mechanism of Li2CO3 during lithium deposition (Fig. 3e). The study indicates that Li2CO3 located at GBs can be reduced during lithium plating (Li2CO3 + 4Li → 3Li2O + C). The resultant carbon further reacts with Li to form LiCx. Compared to the intrinsically low electronic conductivity of LLZO (<10−10 S cm−1), LiCx exhibits a high electronic conductivity (∼103 S cm−1). This dramatic difference of several orders of magnitude implies that once LiCx forms in the GB regions, it can substantially alter the local charge transport properties.
From the perspective of interface physics, garnet-type SSEs rely on their excellent electronic insulation to prevent electron migration within the electrolyte, thereby suppressing electrochemical lithium deposition at internal interfaces or GBs. However, when Li2CO3 at GBs is reduced and converted into an electronically conductive phase, the originally ion-conducting GB pathways can be activated as localized electron-conducting channels. This electronic percolation effect concentrates current at the GBs and disrupts the uniformity of lithium deposition. Consequently, it significantly lowers the energy barrier for lithium nucleation and growth at the interface, promoting the preferential propagation of lithium dendrites along these boundaries. Notably, this failure mechanism is intricately linked to the spatial distribution of Li2CO3 within the electrolyte, rather than being governed solely by the mere formation of a conductive phase. When Li2CO3 is present in isolated, discrete particles in surface regions, the impact of its reduction products may be locally constrained. In contrast, when Li2CO3 is continuously distributed along the GB network, the reduced LiCx can progressively form interconnected, percolating electron-conducting pathways across GBs and potentially through the electrolyte thickness. Once such percolation paths are established, localized current leakage and rapid lithium penetration are significantly accelerated, ultimately leading to electrolyte piercing and internal short circuits.
In addition to electrochemical reduction, chemical reactions between Li2CO3 and LLZO may also occur at the interface, damaging the local structure of LLZO and impairing its ionic conductivity.42,43 Furthermore, due to the significant mechanical property differences between Li2CO3 and LLZO, the deposition and dissolution of lithium during battery cycling can induce volume changes and stress evolution. The presence of Li2CO3 at the interface exacerbates stress concentration. Such stress concentration can induce interface cracking or delamination, which further compromises mechanical stability and ultimately accelerates structural degradation. During multiple charge–discharge cycles, the continuous generation and accumulation of Li2CO3 not only intensifies the chemical and mechanical mismatch at the interface but also accelerates the structural degradation of both the electrolyte and electrode through the aforementioned electrochemical and mechanical coupling mechanisms. Ultimately, this leads to interface failure and rapid capacity fading, making it difficult to meet the long-term stability requirements for practical applications.
Furthermore, the presence of Li2CO3 severely compromises the interfacial compatibility within the battery. In SSLMBs, optimal compatibility between the electrolyte and electrodes is crucial for achieving high performance. However, Li2CO3 disrupts this compatibility. Its lithiophobic nature results in poor affinity with lithium metal, leading to non-uniform contact, voids, and point contacts at the interface between the Li anode and the garnet-type SSEs. These imperfections cause uneven current distribution, creating localized areas of high current density that initiate the growth of lithium dendrites. The growth of lithium dendrites further compromises the interface stability, increases the interface resistance, and reduces the battery's cycle stability. During repeated cycling, propagating lithium dendrites may ultimately penetrate the SSE, causing an internal short circuit and battery failure.44 The presence of Li2CO3 also adversely affects the long-term cycling stability of the battery. Throughout charge–discharge cycles, Li2CO3 contributes to gradual capacity fade. Its low ionic conductivity hinders Li+ transport, preventing the full utilization of active materials and thereby reducing the achievable capacity. Chang et al.45 proposed a novel and efficient TiO2-induced conversion strategy designed to generate a lithium-ion-conducting phase. This approach simultaneously eliminates pre-existing pores/voids and Li2CO3 contaminants. SSLMBs constructed using LiFePO4 cathodes treated with this strategy exhibited excellent cycling performance, delivering a reversible capacity of 152.9 mAh g−1 at 0.1C with a capacity retention of 96.7% after 100 cycles. In contrast, cells with pristine LLZTO suffered significant degradation, retaining only 127.9 mAh g−1 after 100 cycles.
From the above discussion, we can conclude that the Li2CO3 passivation layer forms on the exterior of LLZO particles, resembling a core–shell structure.46 The formation of Li2CO3 reduces the structural stability and electrochemical performance of the garnet, severely limiting its large-scale application and commercialization. Therefore, a deeper understanding of the mechanism behind the formation of Li2CO3 on the surface of LLZO and its detrimental effects on the interface is of great significance for optimizing the performance of garnet-type SSEs and advancing the development of SSLMBs.
2.2 Synergistic degradation mechanisms of Li2CO3 with other interfacial impurities
While the detrimental impact of the Li2CO3 APL itself on interfacial ion transport and contact has been widely recognized, its harm does not occur in isolation. In the realistic interfacial environment of SSBs, Li2CO3 often coexists with other interfacial impurities or incompatible components. Through coupled processes such as chemical reactions, interfacial transport imbalance, and mechanical mismatch, these factors produce a compounded detrimental effect, significantly exacerbating interfacial failure. Building upon the above-discussed failure mechanism of Li2CO3 in contact with lithium metal, this section further elaborates on the coupling mechanisms between Li2CO3 and other components or processes. Specifically, we analyze these interactions from three key dimensions: the cathode high-voltage environment, the coexistence with surface impurities, and material preparation stages. Finally, we examine their collective role in amplifying interfacial failure.For example, Demuth et al.50 used high-resolution transmission electron microscopy (HRTEM) to study the interface structure evolution during the co-sintering of LLZO and NCM. The study found that even under sintering conditions around 500 °C, the layered structure of NCM near the interface can degrade into a rock-salt-like (NiO-like) structure, and a transition phase resembling LaNiO3 forms in the contact region between LLZO and NCM. These newly formed interfacial phases significantly hinder Li+ transport across the interface and increase interfacial impedance, indicating that unoptimized thermal treatment can induce irreversible chemical and structural changes at the interface, thereby degrading the performance of the electrode/electrolyte interface. Moreover, Li2CO3, acting as a lithium source and reaction medium, can accelerate the diffusion of transition metals (e.g., Co and Ni) towards the LLZTO electrolyte side. This leads to interfacial phase transformations and defect generation, potentially forming ionically insulating phases (e.g., La2Zr2O7), thereby further exacerbating the risk of interfacial failure.51,52 Therefore, during material design and process optimization, special attention must be paid to controlling the temperature and atmosphere during co-sintering. This control is crucial to avoid the formation of unfavorable side phases and to ensure controllable interfacial reactions. Ultimately, these measures are essential for enhancing the long-term stability and performance of the battery.
In conclusion, the hazards of Li2CO3 in garnet-based SSBs arise from the coupling of multiple factors during different stages: cathode high-voltage environments, surface impurity coexistence, and the material preparation stage. The synergistic effects amplify interfacial side reactions, impedance growth, and structural degradation. Consequently, interface optimization strategies should shift from merely removing Li2CO3 towards the systematic and synergistic regulation of interfacial chemistry, transport, and structural stability to enhance long-term stability and application potential.
3 Mitigation of interfacial Li2CO3
3.1 Strategies for enhancing air stability
As detailed above, the formation mechanisms of Li2CO3 and its detrimental effects on electrochemical performance have been thoroughly discussed. This understanding provides critical guidance for developing strategies to enhance LLZO's air stability and prevent or mitigate the formation of the Li2CO3 passivation layer. It is important to note that not only the ambient atmosphere but also the elemental composition and microstructure of the garnet significantly influence its air stability, as the Li+/H+ exchange reaction is prevalent in these materials. In this section, we summarize three principal approaches for enhancing LLZO's air stability: (1) optimization of sintering techniques, (2) elemental doping, and (3) GB engineering.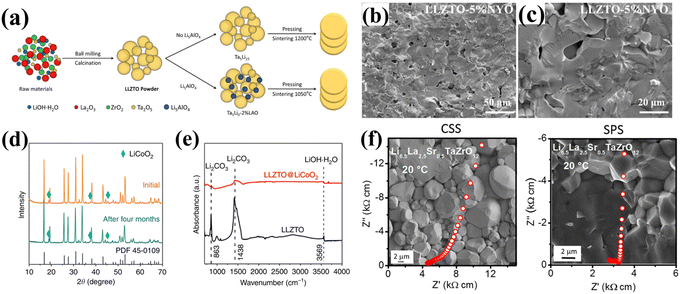 | ||
| Fig. 4 (a) Schematic of Ta5Li15–Li5AlO4 and Ta5Li15 ceramic sample preparation. Reproduced with permission from ref. 53. Copyright 2024, American Chemical Society. (b and c) Cross-sectional SEM images of LLZTO-5% NYO pellets. Reproduced with permission from ref. 54. Copyright 2024, Royal Society of Chemistry. (d) XRD patterns and (e) FTIR spectra comparing LLZTO@LiCoO2 before and after 4-month air exposure, highlighting its enhanced stability. Reproduced from ref. 37. Copyright 2020, Springer Nature. (f) The SEM images of Li6.5La2.5Sr0.5TaZrO12 in the CSS and SPS processes. Reproduced with permission from ref. 55. Copyright 2019, American Chemical Society. | ||
Research indicates that LLZO SSEs prepared via microwave sintering possess more regular crystal structures, fewer internal defects, and reduced surface activation energy, collectively contributing to the effective suppression of Li2CO3 formation. Dabaki et al.59 employed a reduced sintering temperature (1200 °C) and shorter duration (3 h) to limit lithium volatilization, yielding a high ionic conductivity of 4.58 × 10−4 S cm−1 and pellet density of 4.39 g cm−3. Spark plasma sintering (SPS) substantially modifies garnet electrolyte microstructures. As shown in Fig. 4f, Kammampata et al. reported that, compared to traditional solid-state sintering, SPS utilizing lower temperatures and shorter holding times facilitates rapid densification via localized melting induced by electrical discharges between particles and simultaneously reduces active sites for CO2 adsorption. In addition, the increased fine-grain structure provides additional ion transport channels, allowing for faster Li+ hopping and improved ionic conductivity.55 For instance, Li6.5La2.5Sr0.5TaZrO12 synthesized by SPS exhibited an ionic conductivity of 3.08 × 10−4 S cm−1, superior to the 2.12 × 10−4 S cm−1 obtained via traditional solid-state sintering. With the advancement of flash sintering (FS) technology,63,64 many ceramic materials have been sintered using this method. FS is known for its ability to operate at lower temperatures and achieve rapid densification, making it particularly suitable for LLZO materials, effectively reducing lithium evaporation during prolonged high-temperature sintering. FS-prepared LLZO electrolytes exhibit significantly suppressed lithium loss associated with prolonged high-temperature exposure. Yang et al.60 achieved densification by applying a direct current at 700 °C for 5 to 10 s, leveraging the interplay between Joule heating and defect migration, obtaining a garnet pellet with a relative density of 94.72%. More recently, the Randall team70 developed a low-temperature sintering technique termed the cold sintering process (CSP), which can effectively densify various oxide ceramics within the temperature range of 100–350 °C.71–73 Wang et al. demonstrated that CSP can achieve densities up to 87.7% at 350 °C.61 However, CSP-sintered garnets showed increased sensitivity to air and reduced conductivity, attributed to an intergranular phase induced by preferential dissolution of Al and Li, necessitating post-sintering heat treatment to rectify these defects. Table 1 summarizes the characteristics of these four novel sintering techniques and provides a comparative analysis to guide the optimization of garnet-type SSE stability in air.
| Technology | Temperature/time | Relative density | Ionic conductivity (S cm−1) | Advantages | Core challenges | Ref. |
|---|---|---|---|---|---|---|
| Microwave sintering | 1200 °C/3 h | >97% | 4.58 × 10−4 | Fast heating rate, short sintering time, high density | High equipment cost, sensitive process parameters | 59 |
| SPS | 950 °C/10 min | >98.5% | 3.08 × 10−4 | Low sintering temperature (<1000 °C), small grain size (1–2 µm) in preparation, optimized microstructure | Generation of hetero-phases in the high temperature region | 55 |
| FS | 700 °C/5–10 s | 94.72% | 9.8 × 10−4 | Extremely short sintering time, low sintering temperature (<850 °C) | Mechanistic disputes, difficulty in large-scale production | 60 |
| CPS | 350 °C/30–60 min | 87.7% | <1 × 10−4 | Extremely low sintering temperature (100–350 °C) | Grain-boundary phase is sensitive to air | 61 |
Double-element doping, leveraging the synergy between two elements, demonstrates more significant effects in suppressing Li2CO3 formation and improving air stability compared to single-element doping. Fig. 5a demonstrates that Bezabh et al. substantially improved LLZO's ionic conductivity via an optimized Al/Nb dual-doping strategy.65 The substitution of Al3+ for Li+ and Nb5+ for Zr4+, along with the synergistic effect of Al and Nb, effectively minimized Li+/H+ exchange. The dual-doped LLZO particles maintained surface purity after 7-day air exposure, demonstrating exceptional air stability. Huang et al.79 systematically examined dopant effects on moisture resistance in garnet-type SSEs. Relative to Al-LLZO, both Al-LLZTO and Al-LLZNO demonstrated superior air stability. DFT calculations revealed a higher decomposition energy barrier for Al-LLZTO, which effectively suppressed Li2O formation and subsequent decomposition reactions: Li2O + H2O → 2LiOH and 2LiOH + CO2 → Li2CO3 + H2O. Concurrently, dual-doped LLZO also showed advantages in terms of interfacial compatibility with lithium metal anodes, exhibiting reduced interfacial impedance, suppressed lithium dendrite growth, and improved charge/discharge performance, indicating multi-scale performance optimization. Research by Kobi et al.66 revealed that in the Al and Mg co-doped LLZO system, a clean, nearly perfect, and stable interface was formed, which was distinctly different from the material obtained with Al doping alone. Furthermore, studies found that lithium dendrite propagation primarily occurred through Al-LLZO, but not through Al/Mg-LLZO (Fig. 5b). The cubic LLZO with a garnet structure possesses three-dimensional lithium channels and a sufficient amount of mobile Li+ to meet high conductivity requirements.
 | ||
| Fig. 5 (a) Raman spectra of pristine and (Al, Nb)-doped LLZO before and after 7-day air exposure. Reproduced with permission from ref. 65. Copyright 2024, Elsevier. (b) Mechanism of Al/Mg co-doping effects on LLZO performance. Reproduced with permission from ref. 66. Copyright 2023, American Chemical Society. (c) 3D Raman spectra of LLZO and LGLZTSNO electrolyte pellets following 10-day air exposure. Reproduced with permission from ref. 67. Copyright 2024, Elsevier. (d) Proposed water stabilization mechanism in LLZO pellets. Reproduced with permission from ref. 68. Copyright 2022, Elsevier. (e) Nanoscale LiF coating distribution on LLZTO grains. Reproduced with permission from ref. 56. Copyright 2024, Tsinghua University Press. (f) Variation in the Li-ion conductivities as a function of the aging behavior of samples. Reproduced with permission from ref. 69. Copyright 2023, Wiley-VCH GmbH. | ||
Recent studies have shown that garnet structures synthesized using multi-element high-entropy strategies exhibit good tolerance to various species and demonstrate significantly enhanced ionic conductivity, leading to improved overall material performance.80–82 Feng et al. developed an ultrafast synthesis method of high-entropy garnet-type SSEs with the composition Li7+a−c−2dLa3(Aa3+Bb4+Cc5+Dd6+)O12 (A = Sc, Y, Bi; B = Zr, Mo, Sn, Te, Hf; C = Nb, Sb, Ta; D = W).83 Among these compositions, the pentanary garnet Li6.6La3Zr0.4Sn0.4Sc0.4Ta0.4-Nb0.4O12 demonstrated optimal performance, achieving the highest ionic conductivity (3.57 × 10−3 S cm−1) and the lowest electronic conductivity (5.26 × 10−9 S cm−1). Fig. 5c illustrates that Yu et al. successfully synthesized a Li5.75Ga0.25La3Zr0.5Ti0.5Sn0.5Nb0.5O12 (LGLZTSNO) garnet SSE via solid-state sintering.67 After exposing both LLZO and LGLZTSNO to air for 10 days, the formation of Li2CO3 was detected using three-dimensional (3D) Raman mapping. The results indicated that the green areas in the 3D Raman map of LLZO-10d were associated with Li2CO3, whereas the blue areas in the map of LGLZTSNO-10d corresponded to the LLZO phase itself. The high-entropy LGLZTSNO exhibits substantially enhanced air stability compared to conventional LLZO. Thermogravimetric analysis (TGA) reveals that the weight loss between 380 and 710 °C predominantly results from Li+/H+ exchange, with the exchange extent quantifiable by the weight loss percentage. Notably, LGLZTSNO shows reduced weight loss relative to LLZO, demonstrating that the high-entropy effect effectively inhibits Li+/H+ exchange and consequently improves electrolyte stability. In summary, high-entropy doping or multi-element co-doping utilizes the complex interactions of various elements, demonstrating unique advantages in improving air stability and overall performance. The lattice distortion and atomic disorder induced by the high-entropy effect effectively suppress Li2CO3 formation while simultaneously improving ionic conductivity and battery cycling performance. However, high-entropy doping systems are more complex, requiring stricter control over synthesis parameters and elemental ratios, and related theoretical understanding is still inadequate. Future efforts need to overcome limitations in solid solubility and processing bottlenecks, combining multi-scale simulations and dynamic regulation techniques to facilitate the translation of doping strategies from laboratory research to large-scale application.84,85
3.2 Removal of Li2CO3 at the lithium anode interface
The Li2CO3 contamination layer on LLZO surfaces leads to poor anode/garnet interfacial contact, typically exhibiting impedance values >1000 Ω cm2. As previously discussed, neither additives nor elemental doping can fully prevent Li2CO3 formation. Consequently, post-synthetic garnet interface treatment has emerged as a cost-effective approach for obtaining Li2CO3-free electrolytes. Current interface enhancement strategies primarily fall into three categories: (1) physical cleaning; (2) chemical treatment and conversion; (3) interface layer modification.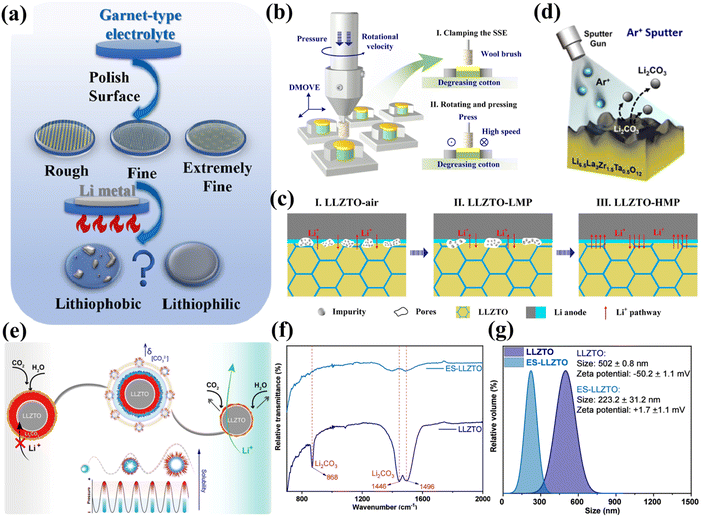 | ||
| Fig. 6 (a) Schematic of garnet electrolyte surface polishing. Reproduced with permission from ref. 86. Copyright 2023, Elsevier. (b) Ultra-clean SSE surface obtained via HMP with a wool brush. (c) LLZTO interface evolution under varying rotation speeds. Reproduced with permission from ref. 87. Copyright 2022, Elsevier. (d) LLZTO surface cleaning mechanism using Ar+ sputtering. Reproduced with permission from ref. 88. Copyright 2021, American Chemical Society. (e) Scalable sonication method for controlled Li2CO3 layer thickness (<10 nm) on LLZTO. (f) Comparative FTIR spectra of LLZTO and ES-LLZTO NPs. (g) DLS analysis of particle size and zeta potential changes of LLZTO NPs post-sonication. Reproduced with permission from ref. 89. Copyright 2025, American Chemical Society. | ||
In addition, vacuum heat treatment is another viable method for cleaning the LLZO surface, utilizing high temperature and vacuum to remove Li2CO3 at the lithium anode interface. This approach leverages the decomposition characteristics of Li2CO3 at elevated temperatures and the promoting effect of vacuum on the volatilization of decomposition products. Vema et al. found that LiOH and Li2CO3 begin to decompose at 500 °C and completely disappear by 800 °C under vacuum.98 The decomposition reaction is Li2CO3 → Li2O + CO2↑. The resulting Li2O and CO2 can volatilize rapidly in the vacuum environment. However, excessively high temperatures can cause Li loss and the formation of La2Zr2O7 impurity phases, disrupting the cubic LLZO structure and reducing ionic conductivity.
To lower the high treatment temperatures, plasma treatment has been explored as a physical cleaning method, utilizing the interaction between energetic particles in the plasma and Li2CO3. As shown in Fig. 6d, Li2CO3 and LiOH can be effectively removed under Ar+ sputtering conditions at a relatively low temperature of 227 °C.88 Energetic electrons collide with Li2CO3 molecules, inducing decomposition. Simultaneously, oxygen radicals (O˙) react with carbon in Li2CO3, generating CO2 and facilitating its breakdown. Furthermore, plasma treatment under different atmospheres can achieve surface doping effects. Chen et al.99 proposed that N2 plasma treatment effectively cleans and dopes the LLZTO electrolyte surface by etching away Li2CO3 and LiOH, while forming Ta–N bonds with surface/subsurface Ta atoms, significantly improving the ionic conductivity to 9.92 × 10−4 S cm−1. The in situ formed Li3N, upon contact with molten Li, drastically reduced the interfacial resistance from 125.8 Ω cm2 to 3.50 Ω cm2. Laser cleaning shares a similar principle with plasma etching. High-energy nanosecond laser scanning can effectively remove the surface impurity layer from air-exposed LLZTO electrolytes.100
Compared to other methods like heat treatment and mechanical polishing, laser cleaning requires significantly less time and energy. Additionally, Srivastava et al. developed a scalable, green, ultrasound-assisted technique for precisely controlling the thickness (<10 nm) of the Li2CO3 layer on LLZTO nanoparticles (NPs).89 As depicted in Fig. 6e, this method uses local mechanical energy generated by the ultrasonic cavitation effect to selectively peel off the Li2CO3 passivation layer from the surface of LLZTO NPs, creating an ultra-thin and structurally stable modified Li2CO3 interface, which significantly improves the air stability of LLZTO NPs. FTIR analysis confirmed the effectiveness of the ultrasound treatment: the characteristic Li2CO3 vibration peaks at 868, 1446, and 1496 cm−1 were significantly attenuated in the extensively sonicated LLZTO NPs (labeled ES-LLZTO), indicating a reduced Li2CO3 layer thickness (Fig. 6f). Dynamic light scattering (DLS) analysis revealed that sonication reduced the average hydrodynamic diameter of LLZTO NPs from 502 nm to 223 nm, accompanied by a zeta potential shift from −50.2 ± 1.1 mV to +1.7 ± 1.1 mV (Fig. 6g). The initially negative zeta potential originated from the insulating Li2CO3 surface layer, which was substantially reduced in the ES-LLZTO. This direct, efficient in situ modulation strategy, free from chemical additives, effectively suppressed the ongoing passivation reaction of LLZTO in air. For practical applications, future efforts should focus on enhancing the feasibility of physical cleaning methods for large-scale production. Developing equipment and processes suitable for industrial manufacturing is crucial to ensure the stable and reliable removal of Li2CO3 from LLZO surfaces, thereby guaranteeing battery consistency and performance stability. Table 2 summarizes various physical methods for Li2CO3 removal and compares the performance of batteries assembled with the treated LLZTO.
| Chemical formula | Method | Ionic conductivity (S cm−1) | Interfacial resistance (Ω cm2) | CCD (mA cm−2) | Ref. |
|---|---|---|---|---|---|
| Li6.5La3Zr1.5Ta0.5O12 | Polishing and spreading | — | 17.5 | 2.8 | 42 |
| Li6.25Al0.2La3Zr2O11.85Br0.15 | Polish treatment | 8.01 × 10−4 | 20.9 | 1.1 | 86 |
| Li6.5La3Zr1.5Ta0.5O12 | High-speed mechanical polishing | 7.3 × 10−4 | 28.15 | 1.91 | 87 |
| Al0.36Li5.92La3Zr2O12 | Vacuum heat treatment | — | <10 | >1 | 98 |
| Li6.5La3Zr1.5Ta0.5O12 | Argon-ion sputtering | 1.3 × 10−4 | 18 | 0.9 | 88 |
| Li6.5La3Zr1.5Ta0.5O12 | N2 plasma | 9.92 × 10−4 | 3.5 | 1.6 | 99 |
| Li6.4La3Zr1.4Ta0.6O12 | Laser cleaning | 7.3 × 10−4 | 76.4 | — | 100 |
| Li6.75La3Zr1.75Ta0.25O12 | Sonication-assisted | 1.5 × 10-4 | — | 1.5 | 89 |
Researchers have been actively seeking effective methods to remove Li2CO3 from the surface of garnet-type SSEs to improve their stability in air. A promising approach involves the chemical reaction-based removal of Li2CO3. Given the alkaline nature of Li2CO3, treatment with acidic compounds offers a straightforward and effective solution. Ruan et al.108 developed a facile phosphoric acid (H3PO4) treatment to convert detrimental Li2CO3/LiOH passivation layers on LLZTO surfaces into a lithiophilic Li3PO4 modification layer. This in situ formed Li3PO4 layer simultaneously (1) reduces the Li/LLZTO interfacial energy to enhance contact and (2) establishes a robust solid electrolyte interphase (SEI) that effectively blocks Li dendrite penetration. The treated interfaces achieved an ultra-low resistance of 7 Ω cm2, enabling stable cycling of Li symmetric cells for >1600 h at 0.1 mA cm−2. Beyond H3PO4, alternative precursors can generate similar Li3PO4 protective layers, as demonstrated by Liu et al. who employed molten NaH2PO2 salts to drive the Li2CO3-to-Li3PO4 conversion reaction on garnet surfaces.101 As shown in Fig. 7a, the Li2CO3 passivation layer was converted to ion-conductive Li3PO4 under mild conditions (200 °C) using a simple gas molecule release and cleaning strategy. The reaction process is as follows:
| 2NaH2PO2 → Na2HPO4 + PH3 (g) | (4) |
| 2PH3 + 3Li2CO3 → 2Li3PO4 + 2C + CH4 (g) + H2O (g) | (5) |
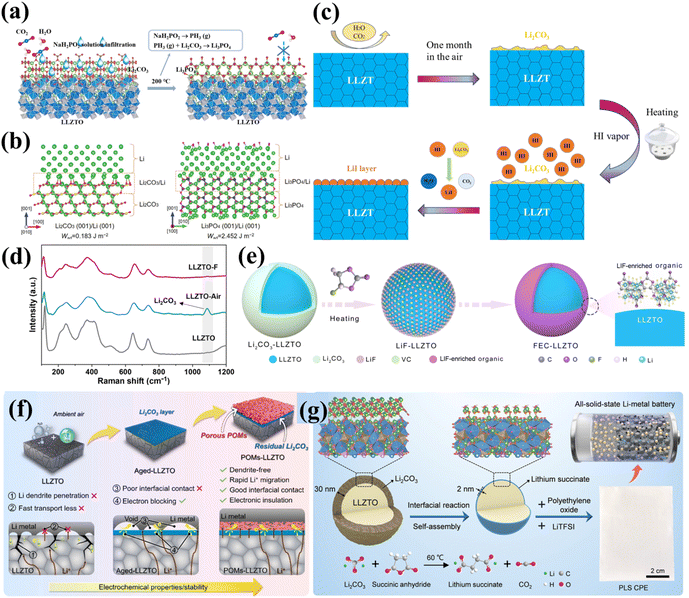 | ||
| Fig. 7 (a) Schematic diagram of the conversion of the Li2CO3 passivation layer on LLZTO into a Li3PO4 layer through the thermal decomposition of the infiltrated NaH2PO2 salt. Reproduced with permission from ref. 101. Copyright 2022, Elsevier. (b) Atomic configurations of fully relaxed supercells of Li2CO3 (001)/Li (001) and Li3PO4 (001)/Li (001) interfaces. Reproduced with permission from ref. 102. Copyright 2022, Wiley-VCH GmbH. (c) Li2CO3 removal from LLZT surfaces using HI vapor. Reproduced with permission from ref. 103. Copyright 2024, Elsevier. (d) Comparative Raman spectra of pristine LLZTO, LLZTO-air and LLZTO-F pellets. Reproduced with permission from ref. 104. Copyright 2024, Royal Society of Chemistry. (e) Synthesis mechanism of FEC-LLZTO. Reproduced with permission from ref. 105. Copyright 2025, Elsevier. (f) Schematic illustration and interfacial characteristics of the Li/LLZTO, Li/Aged-LLZTO, and Li|POMs-LLZTO interfaces. Reproduced with permission from ref. 106. Copyright 2025, American Chemical Society. (g) Schematic diagram of interfacial reconstruction of LLZTO through mild liquid-phase reaction and its further application in SSBs. Reproduced with permission from ref. 107. Copyright 2024, Wiley-VCH GmbH. | ||
Furthermore, DFT calculations were performed to evaluate the adhesion work (Wad) of different interfaces and assess the adhesion strength of the interfaces. Wad refers to the energy required to separate two components at the interface, where a higher Wad value corresponds to stronger interface adhesion. As shown in Fig. 7b, the Wad of the Li3PO4 (001)/Li (001) interface is significantly higher than that of the Li2CO3 (001)/Li (001) interface, with values of 2.452 and 0.183 J m−2, respectively.102 This suggests that the in situ chemical transformation of the Li2CO3 layer on the LLZTO and Li metal surface strengthens the interface contact. Based on both experimental and theoretical research, the Li3PO4 protective layer demonstrates potential advantages in optimizing the interfacial performance of SSLMBs. Similarly, Wang et al. utilized acid vapor to remove Li2CO3 impurities from the surface of Li6.4La3Zr1.4Ta0.6O12 (LLZT).103 The surface alkali species readily react with volatile HI gas, simultaneously generating a protective LiI layer (Fig. 7c). Due to its low electronic conductivity, the LiI buffer layer effectively prevents electron tunneling and attack on LLZT. The LiI layer also mitigates side decomposition reactions between the lithium metal and the electrolyte. In traditional LIBs, gas–solid reactions involving iodine and lithium metal have been used to create LiI interface layers, proven to enhance electrochemical performance. In SSLMBs, LiI demonstrates exceptional interfacial stability between the Li anode and SSE. This additive reduces the LLZT/Li interfacial resistance from 6160 Ω cm2 to 30 Ω cm2 while enabling a CCD of 1.5 mA cm−2 at room temperature (RT). Moreover, Li symmetric cells maintained stable cycling for >1000 h at 0.6 mA cm−2. In a similar approach, Chen et al. treated Li2CO3 impurities on the LLZTO surface with a LiPF6 solution at 60 °C.109 The Li2CO3 impurities in situ converted to Li3PO4 and LiF, achieving good interfacial contact. The presence of Li3PO4 effectively enhanced the ionic conductivity at the interface, improving battery performance, while the protective nature of the LiF layer safeguarded the underlying LLZTO from solvent decomposition. Both LiI and LiF, as primary components of lithium halides, exhibit excellent characteristics in SSLMBs.
Additionally, Cai et al.142 used an aqueous H3BO3 solution and HF vapor to form a nanoporous interlayer in situ on the LLZTO surface. The vigorous chemical reaction between the lithium salt and lithium, characterized by a relatively small Gibbs free energy (ΔG = −208.84 kcal mol−1), combined with the capillary force of the nanoporous layer, resulted in excellent lithiophilicity. The high surface energy and electronic insulation suppress dendrite formation.
Regarding chemical treatment methods, surface coating can effectively inhibit the formation of Li2CO3. Hu et al. proposed an unconventional interface reaction strategy where the typically undesirable Li2CO3 is intentionally retained and cleverly utilized.104 A solvent-free trifluoroacetic acid (TFA) treatment strategy was employed to successfully construct a LiF-rich interlayer on the LLZTO surface. This process achieves in situ conversion of the Li2CO3 contaminant without damaging the structural integrity of the LLZTO bulk. The introduction of the solvent-free system effectively avoids parasitic reactions between organic media and LLZTO, preventing the generation of secondary impurities. Raman spectroscopy confirmed the presence of Li2CO3 in LLZTO exposed to air (LLZTO-air) through characteristic peaks at 158 cm−1 (weak peak, assigned to CO32−ν1 symmetric stretching vibration) and 1090 cm−1 (strong peak, assigned to CO32−ν4 out-of-plane bending vibration). In contrast, these carbonate signals completely disappeared in fluorinated LLZTO (LLZTO-F), indicating the complete conversion of harmful Li2CO3 without detectable byproducts (Fig. 7d). Similarly, as shown in Fig. 7f, a multi-metallic polyoxometalate (POM) layer, Li3PW12O40, was constructed on the LLZTO surface through the controlled reaction of phosphotungstic acid (PTA) with Li2CO3.106 This design selectively retains an appropriate thickness of the Li2CO3 layer through precise control of the reaction process, forming a unique LLZTO/Li2CO3/POMs sandwich structure. The POMs layer significantly enhances the lithium metal wettability and constructs a 3D ionic diffusion channel, while the residual Li2CO3, with its intrinsic low electronic conductivity, serves as an effective electronic barrier to prevent interface electron leakage. This strategy transforms the traditional harmful phase into a functional interface component, offering a new path for the large-scale development of garnet-type SSLMBs.
By combining chemical treatment and transformation methods, the advantages of both can be leveraged to generate a synergistic effect, more effectively suppressing the formation of Li2CO3 and improving the performance of garnet-type SSEs. Lu et al.110 designed a surface layer on LLZTO using ultrathin, super-lithiophilic but hydrophobic AlF3. Through reactive modification using LiOH, Al(NO3)3, and HF, the LLZTO surface acquired excellent hydrophobicity, effectively inhibiting Li2CO3 formation and significantly improving air durability even under humid conditions (25 °C, 60% humidity). Reaction with molten Li yielded a composite interfacial layer comprising Li–Al alloy, Li, and LiF species, demonstrating superior interfacial compatibility. The Li–Al alloy promotes homogeneous distribution of both electrons and Li+, while the LiF phase serves as an electron-blocking barrier due to its high ionic diffusivity and low electronic conductivity. This dual mechanism effectively suppresses Li dendrite formation at both LLZTO GBs and interfaces. As shown in Fig. 7e, Li et al. achieved multifunctional interfacial modification of LLZTO through a surface conversion reaction between fluoroethylene carbonate (FEC) and LLZTO, constructing an organic layer rich in lithiophilic LiF in situ on the LLZTO surface.105 FEC undergoes defluorination at high temperatures, generating HF and vinylene carbonate (VC). The HF then eliminates Li2CO3 impurities on the LLZTO surface via an acid–base neutralization reaction, producing lithiophilic LiF. VC undergoes polymerization catalyzed by Lewis acids and bases, forming a LiF-rich organic layer that enhances both the air stability and lithiophilicity of LLZTO. Symmetric cells incorporating this modified interface demonstrated exceptional cycling stability, maintaining operation for >2000 h at 0.1 mA cm−2.
Surface coating technology can form a physical barrier on garnet-type SSEs, preventing direct contact between air (H2O and CO2) and the electrolyte, thereby reducing Li2CO3 generation. In situ chemical conversion technology, on the other hand, can construct a stable and lithiophilic interface layer, further improving the surface properties of the electrolyte, reducing interfacial impedance, and enhancing battery stability. Similarly, Zhan et al.107 developed an interface reconstruction approach utilizing mild liquid-phase chemistry and self-assembly processes (Fig. 7g). This strategy utilizes a controlled chemical reaction between succinic anhydride (SA) and Li2CO3 on the LLZTO surface to synchronously construct an ultrathin, nanoscale lithium succinate (SALi) ion conductor interface layer. The in situ generated SALi layer serves a dual function: effectively isolating the LLZTO from gas–solid reactions with environmental components and maintaining excellent lithium-ion transport kinetics. More importantly, the SALi-modified LLZTO (LLZTO-SALi) composite with polyethylene oxide (PEO) as the SSE (PEO/LLZTO-SALi, PLS) exhibits outstanding compatibility with lithium metal interfaces. The assembled Li/PLS/LiFePO4 all-solid-state battery demonstrated 84.3% capacity retention after 1400 cycles at 1C and room temperature. Table 3 compares recent chemical treatment approaches for Li2CO3 surface conversion, highlighting their substantial improvements in interfacial stability and electrochemical performance.
| Driver reagent | Conversion product | Interfacial resistance (Ω cm2) | CCD (mA cm−2) | Cycling performance | Ref. |
|---|---|---|---|---|---|
| H3PO4 | Li3PO4 | 7 | 0.8 | 1600 h (J = 0.1 mA cm−2, 25 °C) | 108 |
| NaH2PO2 | Li3PO4 | 2 | 2.6 | 1500 h (J = 0.1 mA cm−2, 25 °C) | 101 |
| HI | LiI | 30 | 1.5 | 1050 h (J = 0.5 mA cm−2) | 103 |
| LiPF6 | LiF/Li3PO4 | 32.4 | 1.1 | 700 h (J = 0.2 mA cm−2, 60 °C) | 109 |
| TFA | LiF | — | 2.0 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h (J = 0.1 mA cm−2, RT) 000 h (J = 0.1 mA cm−2, RT) |
104 |
| PTA | Li3PW12O40 | 5.8 | 1.5 | 3000 h (J = 0.5 mA cm−2) | 106 |
| HF | Li–Al/LiF | 1.3 | 1.2 | 3000 h (J = 0.3 mA cm−2, 25 °C) | 110 |
| FEC | LiF | 75.8 | — | 2500 h (J = 0.1 mA cm−2, 25 °C) | 105 |
| SA | SALi | 40 | 0.7 | 2000 h (J = 0.2 mA cm−2, RT) | 107 |
| HCOOH | HCOOLi | 3 | 1.7 | 1000 h (J = 0.5 mA cm−2) | 111 |
| HCl–HF | LiF–LiCl | 11.6 | 1.8 | 1000 h (J = 0.5 mA cm−2, 25 °C) | 112 |
| NaBF4 | LiF/LiBO2/NaF | 6 | 2.0 | 3000 h (J = 0.3 mA cm−2, RT) | 113 |
Although the above in situ chemical conversion strategies effectively transform Li2CO3 into functional interfacial phases like Li3PO4, LiF, and LiI, representing a promising approach for interface engineering, the chemical-potential stability of these newly formed interlayers under extreme electrochemical conditions requires a more comprehensive understanding. This is particularly crucial when assessing their compatibility with Li metal anodes and diverse cathode chemistries. Taking phosphate-based interphases as an example, Li3PO4 is thermodynamically susceptible to reduction toward phosphides like Li3P under extremely low lithium chemical potentials. However, both theoretical and experimental studies indicate that this deep reduction process is typically subject to significant kinetic limitations. On one hand, Li3PO4 itself possesses strong ionic bonding and low electronic conductivity, necessitating electron transport across the dense interphase to drive the reduction reaction. On the other hand, once localized reduction occurs at the interface, the resulting reaction products establish a new electron-ion transport boundary, thereby suppressing further progression of the reaction and resulting in a self-limiting rather than complete conversion process (e.g., Li3PO4 → Li3P).143
This self-limiting conversion can exert dual effects on the long-term evolution of interfacial impedance. On the positive side, when the reaction-derived interphase formed upon contact between Li3PO4 and lithium metal is dense and exhibits moderate Li+ conductivity, it can rapidly passivate the interface, forming a composite interfacial structure reminiscent of LiPON-like layers. This stabilizes the interfacial impedance and promotes Li+ transport across the interface.102 In fact, components such as Li3P and Li2O, which may be present within the interphase layer, are known to exhibit good ionic conductivity and can remain stable within the SEI/interphase. In conventional ionic battery systems, they can also facilitate Li+ desolvation and interfacial migration, thereby positively contributing to reduced interfacial polarization and enhanced cycling performance.144,145 Conversely, if the reaction-formed interphase layer is thick, porous, or accompanied by significant volume changes and microstructural damage, it may lead to a gradual increase in interfacial impedance and reduced interfacial stability over long-term cycling. Therefore, the evolution of interfacial impedance depends not merely on whether reduction occurs, but also on the depth of the reduction, the structural compactness of the interphase layer, and its conductive properties.
Furthermore, the electrochemical stability windows of different cathode materials impose distinct requirements on the applicability of these in situ formed interphases. For high-voltage cathode systems, such as high-nickel NCM series materials, the interphase layer must possess sufficient oxidative stability to withstand high potentials while maintaining ionic conductivity and suppressing detrimental side reactions.146 Generally, LiF-rich interphases exhibit a wider oxidative stability window under high-voltage conditions owing to their excellent chemical inertness and electronic insulation. However, their limited ionic conductivity may introduce additional polarization if the layer is overly thick. In contrast, phosphate-based interphases offer favorable ion transport and film-forming properties, though their composition and stability may adjust according to the interfacial chemical environment. Accordingly, rational interphase design should integrate considerations of electrochemical stability, transport properties, and structural integrity to realize persistently stable interfacial impedance and robust long-term cycling performance.
| Categorization | Materials | Method | Interfacial resistance (Ω cm2) | CCD (mA cm−2) | Cycling performance | Ref. |
|---|---|---|---|---|---|---|
| Inorganic composite interlayers | Bi2Te3 | Solid-state polishing | 2.0 | 5.2 | 1500 h (J = 0.5 mA cm−2) | 114 |
| KF | Vacuum thermal evaporation | 5.9 | 1.0 | 3000 h (J = 0.2 mA cm−2, 25 °C) | 115 | |
| Te/Se | Chemical vapor deposition | 2.5/3.3 | 0.6/1.3 | 500 h (J = 0.2 mA cm−2, 25 °C) | 116 | |
| 1200 h (J = 0.3 mA cm−2, 25 °C) | ||||||
| SbI3 | Drop-casting | 139 | 0.8 | 5000 h (J = 0.1 mA cm−2) | 117 | |
| HI | Facile liquid phase | 10.3 | 2.3 | 800 h (J = 1 mA cm−2) | 118 | |
| Urea | Drop-casting | 85 | 1.7 | 600 h (J = 0.5 mA cm−2) | 119 | |
| BiCl3 | Facile liquid phase | 7.46 | 2.9 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h (J = 0.3 mA cm−2, RT) 000 h (J = 0.3 mA cm−2, RT) |
120 | |
| ZnO | Ultrasonic spraying | 36 | 2.15 | 600 h (J = 2 mA cm−2) | 121 | |
| SnCl2 | Facile liquid phase | 9.4 | 0.8 | 6000 h (J = 0.2 mA cm−2) | 122 | |
| Ag | Magnetron sputtering | 2 | 5.1 | 300 h (J = 0.1 mA cm−2) | 123 | |
| SnO2-Al | Direct-current/radio-frequency plasma magnetron co-sputtering | 9 | 5.4 | 8700 h (J = 0.2 mA cm−2, 30 °C) | 124 | |
| Ga2O3 | Liquid metal painting | 5 | 1.7 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h (J = 0.2 mA cm−2, 60 °C) 000 h (J = 0.2 mA cm−2, 60 °C) |
125 | |
| GaIn | Liquid metal painting | 52.5 | 2.7 | 1400 h (J = 1 mA cm−2, 25 °C) | 126 | |
| SiO2 | Ultrasonic spraying | 24.2 | 1.2 | 1000 h (J = 0.2 mA cm−2) | 127 | |
| InCl3 | Facile liquid phase | 10 | 1.4 | 4000 h (J = 0.2 mA cm−2, 25 °C) | 128 | |
| Organic compound interlayers | A solid polymer electrolyte (SPE) | Drop-casting | — | 0.3 | 700 h (J = 0.2 mA cm−2, 90 °C) | 129 |
| PEO | Spin-coating | 177 | — | 200 h (J = 0.2 mA cm−2) | 130 | |
| ETPTA | In situ thermal polymerizing | 88 | 1.1 | 400 h (J = 0.1 mA cm−2, 30 °C) | 131 | |
| PDA and PEO | Solution oxidation method and spin-coating | 218 | 1.1 | 800 h (J = 0.15 mA cm−2, 80 °C) | 132 | |
| Azo compound | Acid–base neutralization | — | 1.4 | >1500 h (J = 0.1 mA cm−2, 50 °C) | 133 | |
| PLSS | Spin-coating | 9 | 1.1 | 400 h (J = 0.5 mA cm−2, 25 °C) | 134 | |
| PEGMEMA and PVDF-HFP with PFPE additive | Electrostatic spinning and UV-irradiation methods | 142.1 | 3.6 | 400 h (J = 1.0 mA cm−2, 25 °C) | 135 | |
| poly(MAAc-co-MMA-co-NMMAm) in G4/LITFSI | UV-irradiation method | 38 | 1.58 | 1500 h (J = 0.2 mA cm−2, 25 °C) | 136 | |
| P1444FSI | Spin-coating | 120 | 0.15 | — | 137 | |
| Inorganic–organic composite interlayers | COMF | Spin-coating | — | >1 | 1000 h (J = 0.1 mA cm−2) | 138 |
| PVDF-HFP and BaTi2O5 | Pasting | — | 0.678 | 0.05 h (J = 0.1 mA cm−2) | 139 | |
| (C6H5)3Sb | Liquid metal painting | 6 | 1.3 | 500 h (J = 0.1 mA cm−2, 60 °C) | 140 | |
| MMA/EGDMA/AIBN + LLZTO + LiPF6-LE | in situ thermal polymerization | — | 1.9 | 600 h (J = 1.5 mA cm−2) | 141 |
3.2.3.1 Construction of inorganic interlayers. Introducing an inorganic interfacial interlayer between the Li anode and the garnet-type SSE is an effective strategy for inhibiting Li2CO3 formation and improving air stability. The inorganic interlayer can enhance the interfacial stability by modifying the physicochemical properties of the interface, indirectly suppressing Li2CO3 formation.148,149 Ji et al. employed a solid-state polishing technique, rubbing Bi2Te3 powder (particle size ∼1 µm) between two sandpapers to polish LLZTO ceramic pellets.114 This process yielded a uniform, dense Bi2Te3 coating on LLZTO surfaces (Fig. 8a). The modified interface demonstrated enhanced lithiophilicity upon molten Li contact while preventing electron accumulation at surface defects, thereby achieving optimal Li/LLZTO interfacial contact with superior dendrite suppression. The solid-state polishing approach completely filled surface cracks and pores, outperforming wet-chemical methods in preventing interfacial Li dendrite growth. Zhang et al.115 employed vacuum thermal evaporation to deposit a 50-nm KF layer on LLZTO. The excellent Li/KF wettability enabled effective void repair at the Li/LLZTO interface, achieving an interfacial impedance of 5.9 Ω cm2. As illustrated in Fig. 8b, the resulting dense LiF/KF layer completely coated the LLZTO surface, functioning as: (1) an electron-blocking barrier to minimize leakage currents and (2) an electronic conductivity suppressor. This dual functionality effectively prevented Li dendrite propagation along GBs and pores while eliminating current hotspots, thereby enhancing the CCD. Liu et al.116 proposed an innovative strategy to suppress dendrite penetration by depositing Se/Te nanofilms on the LLZTO surface via chemical vapor deposition, followed by an in situ reaction with molten lithium to form a tightly bonded interface with highly ion-conductive Li2Se/Li2Te interlayers. As illustrated in Fig. 8c, the core mechanism lies in a solid-state physical effect: the Li2Se/Li2Te semiconductor layers (with high work function characteristics) form a p-type Schottky contact with lithium metal. The resulting Schottky barrier effectively blocks electron migration towards the LLZTO electrolyte. Theoretical analysis revealed that without an interlayer, the Fermi level difference between Li and LLZTO drives spontaneous electron injection, forming a highly conductive inversion layer (n-type ohmic contact) on the LLZTO surface that promotes Li penetration. In contrast, the Schottky barrier significantly inhibits electron leakage, fundamentally suppressing dendrite growth. Furthermore, some materials can effectively wet molten lithium via conversion reactions with low ΔG. Liu et al.117 successfully introduced a dense LiI/Li–Sb layer through a conversion reaction between SbI3 and Li to construct ionically and electronically conductive layers. This led to a more uniform distribution of electric field and current density on the surface, effectively regulating Li deposition (Fig. 8d). Additionally, various materials such as LiI/ZnLix,118 Li–N,119 BiCl3,120 ZnO,121 and SnCl2 (ref. 122) have been prepared as lithiophilic interfacial layers. Wu et al. deposited a 20 nm Ag layer on the LLZTO surface via magnetron sputtering.123 The subsequent in situ electrochemical reaction formed a Li–Ag alloy that filled interfacial voids and defects, achieving intimate contact between the electrolyte and lithium metal. This resulted in a significant reduction in interfacial impedance, effectively preventing the deposition of Li atoms and dendrite formation on the metal surface. Hao et al.124 proposed a multi-target co-sputtering strategy, which allows for more precise control over the interfacial composition compared to single-target deposition. Using direct-current/radio-frequency magnetron co-sputtering, they deposited a nanoscale SnO2/Al (SA) multilayer interlayer on the LLZTO surface. Reaction with molten lithium formed a gradient artificial SEI structure with a triple-functional design (Fig. 8e): (1) surface interface layer: highly ion-conductive LiAlO2 ensures efficient Li+ transport; (2) middle regulation layer: lithiophilic Li9Al4 alloy induces uniform Li deposition, suppressing dendrite nucleation; (3) substrate bonding layer: a mixed conductive phase of Li2O/LixSn balances ion/electron transport. This spatially graded structure reduced the interfacial impedance from 764.5 Ω cm2 to 9 Ω cm2, and the synergistic effect increased the CCD to 5.4 mA cm−2. The Li/SA-LLZTO-SA/Li symmetric cell exhibited dendrite-free cycling for >8700 h at 0.1–0.2 mA cm−2, providing a new paradigm for SSB interface engineering.
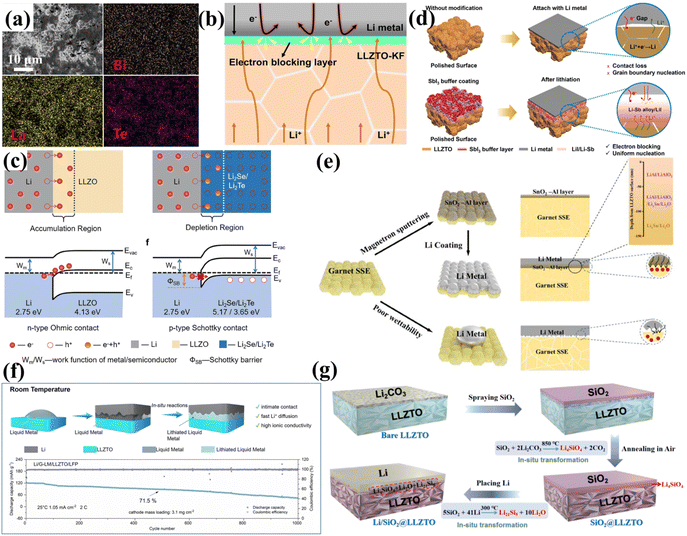 | ||
| Fig. 8 (a) Top-view SEM and energy dispersive X-ray spectroscopy (EDS) elemental mapping (Bi, Te, and La) of LLZTO@Bi2Te3. Reproduced with permission from ref. 114. Copyright 2025, Elsevier. (b) Li|LLZTO-KF interface demonstrating dendrite-free cycling enabled by an electron-blocking interlayer. Reproduced with permission from ref. 115. Copyright 2024, Springer Nature. (c) Formation mechanisms of n-type ohmic and p-type Schottky contacts. Reproduced with permission from ref. 116. Copyright 2025, Wiley-VCH GmbH. (d) Ion-electron composite layer enhancing Li/LLZTO interfacial contact while suppressing Li penetration. Reproduced with permission from ref. 117. Copyright 2025, American Chemical Society. (e) Lithium wettability characteristics on garnet SSE surfaces. Reproduced with permission from ref. 124. Copyright 2025, Wiley-VCH GmbH. (f) Fabrication process of Li/G-LM/LLZTO and cycling performance of Li/G-LM/LLZTO/LFP cells at 1.05 mA cm−2. Reproduced with permission from ref. 126. Copyright 2025, Elsevier. (g) In situ SiO2-mediated Li2CO3 elimination creating a stabilized multifunctional interlayer. Reproduced with permission from ref. 127. Copyright 2025, Elsevier. | ||
In contrast to methods involving additional artificial lithiophilic layers or removal of lithium contaminants, Meng et al.125 applied a simple functional liquid metal (FLM) coating on the LLZTO surface. This process allowed the FLM droplets to fully wet Li2CO3, reducing its size and tearing it into Li2CO3 nanodomains surrounded by FLM nanoparticles. The dissipation effect enabled the lithiated FLM nanodomains to act as alternative lithium flux carriers at the Li/garnet interface. This simple method requires no additional deposition equipment or precise control of alloy composition. Meanwhile, the native oxide layer of the FLM allows for smooth coating on the solid electrolyte surface, preventing further exposure of the garnet to H2O and CO2. Similarly, Ji et al.126 proposed an innovative interface engineering strategy based on GaIn liquid metal (G-LM), constructing a multifunctional gradient interface through an electrochemically driven in situ lithiation process. This strategy transformed G-LM into LiGaO2/LiInO2 compounds with high dielectric constants during the initial lithiation stage, while retaining an electronically conductive phase. The unique structure achieved a triple synergistic effect: (1) the adaptive deformation capability of the liquid metal ensured intimate interfacial contact; (2) the high-dielectric LiGaO2/LiInO2 layer homogenized the electric field distribution; (3) the in situ formed electronically conductive phase created selective ion/electron transport channels. This design simultaneously addressed issues of interfacial resistance, dendrite growth, and side reactions, enabling stable cycling of SSLMBs for 1000 cycles at 1.05 mA cm−2, breaking the performance limits of existing liquid metal interface designs (Fig. 8f). Furthermore, Yu et al. sprayed SiO2 material onto the LLZTO surface.127 A high-temperature reaction with the surface Li2CO3 generated Li4SiO4, which subsequently underwent a conversion reaction with Li to form a multi-component buffer layer comprising Li4SiO4, Li2O, and Li21Si5. This approach successfully transformed the detrimental Li2CO3 passivation layer into a beneficial lithiophilic layer, achieving a waste-to-wealth valorization (Fig. 8g). The introduced Li4SiO4/Li2O/Li21Si5 nano-multifunctional interlayer significantly improved the air stability and interfacial contact of LLZTO/Li, reducing the interfacial impedance to 24.2 Ω cm2 and enabling stable operation for 2400 h and 1000 h at current densities of 0.05 mA cm−2 and 0.2 mA cm−2, respectively. From the perspective of physical isolation, the inorganic interlayer can form an effective physical barrier between the Li anode and garnet-type SSEs, preventing direct contact with H2O and CO2 in air. From a chemical adsorption and reaction perspective, inorganic interfacial materials can passivate the impurity passivation layer on the garnet surface through specific chemical reactions, converting it into harmless substances or immobilizing them within the interlayer, thereby reducing their impact on the garnet-type SSEs.
3.2.3.2 Construction of organic interlayers. Modification with organic interfacial interlayers involves introducing a layer of organic material between the Li anode and the garnet-type SSEs, forming a dual physical and chemical barrier. This approach effectively inhibits Li2CO3 formation and improves contact with the Li anode. An effective method involves depositing a soft solid polymer electrolyte (SPE) interfacial layer onto solid electrolytes like LLZTO, significantly reducing interfacial resistance and enhancing air stability. For instance, Chi et al. proposed a strategy of depositing an SPE interfacial layer on the LLZTO surface.129 This SPE layer, based on a poly(ethylene oxide) (PEO) polymer, was achieved by directly drop-casting an SPE solution onto the solid electrolyte to ensure uniform coverage. The soft SPE layer forms a continuous contact interface between LLZTO and the Li metal, effectively resolving interfacial issues (Fig. 9a). Similarly, as shown in Fig. 9c, Bi et al.131 employed an in situ thermal polymerization method, combining ethoxylated trimethylolpropane triacrylate (ETPTA) monomer with a lithium salt (LiPF6) to prepare a multifunctional gel polymer electrolyte (GPE). This GPE not only served as an interfacial interlayer to address the interface problem between the Li anode and LLZTO but also acted as a binder to connect garnet pellets, enabling the construction of flexible and large-scale SSBs. In terms of interface engineering, the liquid electrolyte precursor effectively wets the Li/LLZTO interface and penetrates into the bulk of the electrolyte before polymerization. After thermal polymerization, the precursor conformally solidifies at the Li/LLZTO interface, forming uniform Li/LLZTO contact. Furthermore, the GPE with uniform Li distribution ensures uniform lithium deposition and stripping, leading to the formation of a stable SEI layer and effectively suppressing lithium dendrite formation. In inhibiting Li2CO3 formation and improving air stability, polydopamine (PDA) coatings have shown exceptional effectiveness. Chen et al. demonstrated the introduction of a dual interfacial layer at the electrode/garnet interface, consisting of a PDA film and a PEO-based composite electrolyte layer.132 The adhesive nature of the PDA film facilitates intimate interfacial bonding and improves the interfacial wettability between the PEO layer and the garnet-type SSE. Research indicates that PDA molecules contain numerous polar groups, such as amino and hydroxyl groups, which can interact with water and CO2 molecules, hindering their diffusion towards the garnet solid electrolyte surface. The introduction of the PEO layer helps establish continuous interfacial contact and regulates uniform Li transport and current distribution at the Li/garnet interface. Additionally, the dual interfacial layer effectively isolates GBs from the Li metal, suppressing Li dendrite nucleation. Whether using soft SPEs, GPEs, or dual interfacial layers, these organic interlayers exhibit good compactness and continuity, tightly covering the garnet solid electrolyte surface. When exposed to air, they effectively prevent direct contact between H2O/CO2 and the electrolyte. During diffusion, water and CO2 molecules are blocked by the organic layer, cutting off the source of reactants needed for Li2CO3 formation.
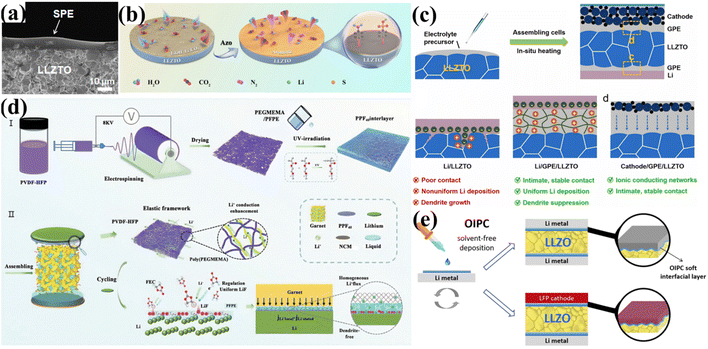 | ||
| Fig. 9 (a) Cross-sectional SEM images demonstrating intimate interfacial contact via SPE deposition on LLZTO. Reproduced with permission from ref. 129. Copyright 2018, Elsevier. (b) Azo-compound conversion mechanism transforming Li-contaminated LLZTO to aromatic-coated surfaces. Reproduced with permission from ref. 133. Copyright 2022, Elsevier. (c) In situ formed GPE interfacial modification layer on garnet electrolytes. Reproduced with permission from ref. 131. Copyright 2021, Elsevier. (d) Polymer interlayer synthesis and interfacial interactions. Reproduced with permission from ref. 135. Copyright 2023, Wiley-VCH GmbH. (e) OIPC layer deposition process and surface characteristics. Reproduced with permission from ref. 137. Copyright 2021, American Chemical Society. | ||
Organic substances can also react with Li2CO3 on the LLZO surface, converting it into a lithiophilic interlayer. Li et al.133 proposed a mechanism where azo compounds, under certain conditions, remove contaminants from the LLZTO surface and form a high-coverage aromatic layer (Fig. 9b). The azo compounds undergo acid–base neutralization reactions with LiOH and Li2CO3, generating aromatic radicals that improve the garnet/lithium interface properties. Furthermore, through the in situ construction of a high-coverage organic layer, these radicals can transfer to the LLZTO surface, reducing the resistance to lithium-ion transport. After exposing the treated LLZTO sample to air for one week, no Li2CO3 formation was detected by XRD and FTIR spectroscopy, further indicating the significant positive role of azo compounds in suppressing Li2CO3 formation on the LLZTO surface and improving air stability.
Studies have found that organic interlayers can also improve interfacial compatibility by interacting with active sites on the garnet-type SSE surface. Research by Yang et al.134 confirmed the ability of poly(lithium 4-styrenesulfonate) (PLSS) introduced between LLZTO and the lithium metal to improve interfacial performance. PLSS contains abundant lithium sulfonate groups (–SO3Li), which form strong coordination interactions with metal atoms (e.g., La, Zr, Ta, and Li) exposed on the LLZTO surface. This atomic-level interaction successfully builds an efficient Li+ migration channel, exhibiting low energy barriers and high Li+ diffusion coefficients at the LLZTO/PLSS interface. This transport mechanism not only enhances Li+ transport efficiency but also reduces Li+ accumulation and uneven distribution at the interface, thereby lowering the possibility of Li2CO3 formation. It is precisely this atomic-level interaction that maintains a robust and seamless interface contact between LLZTO and the Li anode during long-term cycling. The interfacial resistance dropped to 9 Ω cm2 at 25 °C, and the prepared symmetric cell stably cycled for 4800 h at 0.1 mA cm−2. As shown in Fig. 9d, Zheng et al.135 developed a novel elastic conductive interlayer. This interlayer was formed by UV-curing of poly(ethylene glycol) methyl ether methacrylate (PEGMEMA) and poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP), creating an elastic skeleton, with perfluoropolyether (PFPE) added to form an interlayer structure. The elastic skeleton provides effective transport paths for Li+ and enhances deformation resistance and interfacial contact quality through its unique viscoelastic properties. Furthermore, the ether oxygen atoms in PEGMEMA can coordinate with lithium ions, promoting Li+ migration. Simultaneously, fluoroethylene carbonate (FEC) molecules can be reduced by acquiring Li+ and electrons, forming an LiF-rich SEI layer. This multifunctional organic interlayer helps improve interfacial contact and significantly enhances air stability. Furthermore, Zhao et al. designed a multifunctional interlayer with both ionic conductivity and self-healing capabilities (ICSHI).136 This layer is based on a copolymer network of methacrylic acid (MAAc), N-methyl methacrylamide (NMMAm), and methyl methacrylate (MMA), whose abundant hydrogen bonds confer excellent self-healing properties and flexibility. Tetraethylene glycol dimethyl ether (G4) containing dissolved lithium bis(fluorosulfonyl)imide (LiTFSI) was used to ensure ion transport. Placed between LLZTO and the lithium anode, the ICSHI utilizes its flexibility to conform tightly to the interface, repairing mechanical damage during cycling in real-time and improving interfacial contact. In situ observation directly confirmed that without ICSHI, LLZTO cracked rapidly at high current and was penetrated by lithium dendrites, whereas the ICSHI-protected interface significantly suppressed dendrite growth, maintaining the structural integrity of LLZTO for over 50 min and achieving uniform and stable lithium deposition. Given that most organic solvents, even in trace residues, readily undergo side reactions with lithium metal, significantly deteriorating interfacial Li+ transport kinetics and overall battery performance, a solvent-free deposition technique was used to construct a flexible interfacial layer of triisobutylmethylphosphonium bis(trifluoromethane)sulfonimide (P1444TFSI) organic ionic plastic crystals (OIPCs) on the surface of the lithium metal and LLZO solid electrolyte (Fig. 9e). This flexible OIPC layer effectively enhanced Li+ conduction at the interface, increasing the room-temperature ionic conductivity of the composite OIPC-LLZO solid electrolyte to 1.1 × 10−3 S cm−1 and reducing the interfacial impedance by five times compared to the untreated interface.137
Regarding the enhancement of air stability, traditional physical and chemical methods often only address already formed Li2CO3 and have limited effectiveness in preventing its regeneration. After mechanical or chemical treatments, Li2CO3 can rapidly regenerate when the garnet-type SSE is re-exposed to air. In contrast, the stable interfacial layer formed by organic interlayer modification can effectively maintain the interfacial stability between the Li anode and the garnet SSE, even under prolonged air exposure, thereby significantly improving the storage and operational stability of the battery in air. Although traditional methods can mitigate the impact of Li2CO3 on battery performance to some extent, their ability to comprehensively enhance overall battery performance is limited. Mechanical treatments may introduce new interfacial issues that impede Li+ transport, while chemical treatment may alter the chemical composition and structure of materials, adversely impacting the long-term stability of the battery. In comparison, organic interlayer modification not only suppresses the formation of Li2CO3 but also improves the interfacial compatibility between the Li anode and the garnet-type SSE, optimizes Li+ transport kinetics, and thus holistically enhances battery performance. The organic interlayer can effectively reduce interfacial resistance, improve charge/discharge efficiency, regulate Li+ distribution, inhibit the growth of lithium dendrites, and enhance cycling stability and safety.
3.2.3.3 Construction of organic/inorganic composite interlayers. Polymeric interlayers are increasingly emerging as a promising solution to address the interfacial challenges between lithium anodes and electrolytes in advanced battery systems, improving interfacial stability by effectively suppressing Li2CO3 formation. On one hand, flexible polymer interlayers can form composite layers with good flexibility and denseness, combined with the lithium anode, thereby mitigating the accumulation of interfacial voids during lithium deposition and subsequently reducing the growth of lithium dendrites near the interface. On the other hand, polymer interlayers also face numerous challenges, such as low ionic conductivity and weak mechanical properties, which may lead to aggravated interfacial decomposition and side reactions during battery cycling, ultimately resulting in battery failure. Therefore, achieving high ionic conductivity, excellent mechanical strength, superior electrochemical performance, and effective suppression of interfacial Li2CO3 formation remain significant challenges in the design of polymer interlayers.
As shown in Fig. 10a, Wang et al. coated a carbonized metal–organic framework (CMOF) interlayer on the LLZTO surface to ensure good interfacial contact between Li metal and LLZTO.138 The abundant zinc clusters within the CMOF layer interact with molten lithium metal (forming Li-CMOF), creating an intimate contact interface between the lithium metal and LLZTO. The CMOF interfacial layer serves as a lithiophilic nucleation template, directing uniform Li deposition by regulating both electric field distribution and Li+ flux, thereby effectively suppressing dendrite formation. Consequently, the Li-CMOF/LLZTO/Li-CMOF symmetric cell could cycle stably for over 1000 h at a current density of 0.1 mA cm−2 without short-circuiting. Furthermore, Li et al.139 proposed incorporating the ferroelectric material barium titanate BaTi2O5 (BT) nanorods into a PVDF-HFP matrix, successfully preparing an inorganic-modified polymer composite (CPE-BT). The ferroelectric additive simultaneously enhanced polymer electrolyte performance through two mechanisms: (1) plasticization effects increasing chain mobility and (2) spontaneous polarization facilitating Li+ transport, yielding significantly improved ionic conductivity and transference numbers (Fig. 10b). Complementary studies have demonstrated that NP incorporation or high-valence cation doping can further strengthen mechanical properties while maintaining electrochemical stability in polymer interlayers. In an innovative study, Liu et al.140 developed an alloyable viscous interfacial wetting layer for garnet electrolytes to enable highly reversible SSBs. Upon contact with lithium, triphenyl antimony (TPA, (C6H5)3Sb) self-assembled into a fluid, liquid-metal-like interlayer. The in situ process generated a mixed-conducting interphase comprising Sb, Li3Sb, LiSbO3, and Li2C2, which stabilized the Li/LLZTO interface during cycling (Fig. 10c). This modification reduced the interfacial impedance to 6 Ω cm2 and enabled stable symmetric cell operation for >500 h at 0.1 mA cm−2, with a maximum current density tolerance of 1.3 mA cm−2. Separately, Zhang et al. demonstrated a scalable thermal polymerization approach to fabricate an organic–inorganic gel interlayer (GI) at the LLZTO/Li interface (Fig. 10d).141 The GI forms via in situ polymerization of LLZTO particles with methyl methacrylate (MMA) and ethylene glycol dimethacrylate (EGDMA) monomers, initiated by 2,2′-azobisisobutyronitrile (AIBN) in a LiPF6-containing liquid electrolyte (LiPF6-LE). The design leverages the intrinsic lithiophilicity of LLZTO particles to significantly optimize interfacial physical contact and electrochemical compatibility. The 3D cross-linked network within the GI, synergizing with the LLZTO particles, constructs continuous lithium-ion channels, achieving uniform ion flux distribution at the interface. Its innovative rigid–flexible coupled structure is characterized by a rigid inorganic-rich composition near the lithium side to suppress dendrites and a flexible organic-rich composition near the LLZTO side to accommodate volume fluctuations. The GI enhanced the CCD to 1.9 mA cm−2 while demonstrating exceptional cycling stability (>2000 h at 0.1 mA cm−2 and >600 h at 1.5 mA cm−2), confirming its superior dendrite suppression capability. Research has also found that charge transfer and interdiffusion at the interface significantly influence the modification by organic–inorganic interlayers.150 When organic and inorganic materials contact, charge transfer may occur due to differences in their electron cloud distributions. This charge transfer can alter the electronic structure at the interface, thereby affecting the electrical properties and chemical reactivity of the materials. Simultaneously, interdiffusion of molecules may occur at the interface between the organic and inorganic materials. Under specific conditions, segments of organic molecular chains might diffuse into the lattice interstices of the inorganic material, or atoms/ions from the inorganic material might diffuse between the molecular chains of the organic material. This interdiffusion can enhance the bonding between organic and inorganic materials, improving interfacial compatibility.141,151 The preparation of unique organic/inorganic composite interlayers demonstrates multifaceted advantages, particularly at high current densities and during long-term battery cycling, fully proving their potential benefits in improving interfacial contact, suppressing dendrite growth, and enhancing air stability.
 | ||
| Fig. 10 (a) Casting process of pure Li and Li-CMOF on LLZTO. Reproduced with permission from ref. 138. Copyright 2024, American Chemical Society. (b) Electrochemical behavior of the CPE-BT modified Li/LLZO interface. Reproduced with permission from ref. 139. Copyright 2023, American Chemical Society. (c) Blade-coating and thermal lithiation of a-TPA on LLZTO. Reproduced with permission from ref. 140. Copyright 2022, Wiley-VCH GmbH. (d) In situ thermal polymerization for GI-modified SSLMB fabrication. Reproduced with permission from ref. 141. Copyright 2025, Elsevier. | ||
In summary, the organic/inorganic composite interlayer not only improves the chemical interfacial contact between LLZO and electrodes, but also regulates the modulus gradient and mitigates local stress concentration. This dual functionality opens up new avenues for designing and fabricating stable ultrathin garnet-type SSEs.
3.3 Coupling of the surface state and mechanical reliability in roll-to-roll LLZO membrane manufacturing
Garnet-type SSEs feature high elastic and shear moduli, yet their intrinsically low fracture toughness renders them highly sensitive to surface and near-surface flaws under tensile or bending loads.152 When LLZO is transitioned from bulk or thick-sheet forms to ultrathin ceramic membranes or free-standing composite films, this flaw sensitivity is markedly amplified during roll-to-roll (R2R) processing. As illustrated by the process-evolution model in Fig. 11, membrane transport along an R2R line is not merely physical conveyance, but rather a process accompanied by dynamic evolution of mechanical reliability. During this process, the membrane is no longer a static elastic body but a dynamic system defined jointly by its surface state and external stress. Under bending conditions, the outer surface of the membrane experiences tensile stress, while the inner surface is under compression, causing surface defects to become primary sites for stress concentration. Consequently, the effective bending reliability of the membrane is governed not by its bulk mechanical properties but by defect-controlled fracture behavior.156,157Surface post-treatments used to remove Li2CO3 or improve interfacial wettability (e.g., chemical etching, halogenation reactions, plasma treatment, and mechanical polishing) can optimize interfacial chemistry, but they often inevitably modify the surface topography and near-surface microstructure. Non-uniform chemical etching may introduce micron-scale pits, plasma or ion treatments can create amorphous damaged layers or residual stress fields in the subsurface region, and improper mechanical polishing can easily produce directional scratches. This progression corresponds to the surface-state evolution depicted at the top of Fig. 11, where the membrane surface accumulates damage as processing proceeds, transitioning from a pristine state to a degraded state containing microcracks and defects. While such defects may not be prominent in static characterization, they can substantially reduce the effective fracture resistance under bending or cyclic loading, serving as critical triggers for crack initiation and propagation.
As the LLZO thickness is reduced to tens of micrometers or below, the tensile strain at the outer surface can become significant even at relatively large bending radii, allowing micron-scale surface flaws to trigger catastrophic fracture. Moreover, the coupling of web tension and bending in R2R manufacturing, repetitive low-strain cycling, and modulus mismatch within composite architectures may further accelerate subcritical crack growth and amplify failure risk.158–160 This underpins the defect–stress coupling mechanism highlighted in Fig. 11: surface defects induce local stress concentration, while the tensile and bending moments imposed by R2R processing, in turn, promote defect growth. This adverse feedback loop leads to a rapid deterioration of mechanical reliability along the processing line (as schematically shown by the dashboard at the bottom of Fig. 11), ultimately resulting in a sharply increased fracture risk. In this context, organic/inorganic composite interlayers serve a dual purpose. They improve electrochemical performance by enhancing interfacial contact. Simultaneously, the compliant organic phase helps redistribute tensile stress and blunts crack tips. These combined effects thereby improve the processing tolerance and structural robustness of ultrathin LLZO membranes during practical manufacturing.
In this section, we have systematically summarized a range of interfacial and surface engineering techniques. These strategies primarily aim to reduce the interfacial impedance between LLZO and Li and suppress the formation of Li2CO3, thereby enhancing interfacial stability and electrochemical performance. Meanwhile, with the development of garnet electrolytes toward ultrathin ceramic membranes and free-standing composite films, manufacturing-induced surface-state changes and their impacts on mechanical reliability have become increasingly important. Different technical approaches possess distinct advantages and limitations regarding interfacial chemistry regulation, structural integrity, and manufacturing compatibility. Therefore, rational selection for specific application scenarios is particularly crucial. Based on available studies, the following principles may serve as practical guidance for researchers:
(1) Technical effectiveness: priority should be given to technologies that have been experimentally validated to significantly reduce interfacial impedance and efficiently remove or convert Li2CO3. For instance, attention should be paid to quantitative metrics such as the actual reduction magnitude of interfacial resistance and the residual amount of Li2CO3 after implementing the technology. A technology demonstrating a consistent reduction of interfacial impedance by more than 50% across multiple experimental sets, coupled with minimal residual Li2CO3, can be considered highly effective.
(2) Compatibility considerations: the selected technology must exhibit good chemical and physical compatibility with both the LLZO electrolyte and the Li electrode. On one hand, the technology itself should not undergo chemical reactions with LLZO or Li that could damage their structure and electrochemical properties. On the other hand, any newly introduced materials should coexist stably with these two components. For example, when selecting a coating material, its coefficient of thermal expansion should be matched with those of LLZO and Li to avoid interfacial failure or delamination induced by temperature fluctuations.
(3) Alignment with application scenario requirements: the choice of technology should be tailored to the specific operating conditions of the battery, such as temperature, voltage, and humidity. In high-temperature or high-pressure environments, priority should be given to inorganic interlayers or high-entropy doped materials with good thermal stability. For flexible or wearable devices, organic interlayers designed for enhanced flexibility to accommodate mechanical deformation are preferable. In high-humidity environments, hydrophobic interlayers with a contact angle greater than 110° should be used to block moisture infiltration, thereby preventing the formation of Li2CO3.
(4) Cost-effectiveness and resource constraints: a balance between performance enhancement and cost input is necessary, prioritizing cost-effective solutions. For applications where performance requirements are not stringent, low-cost chemical treatment strategies may be more suitable. In scenarios demanding high performance, although techniques like plasma cleaning or composite interlayer design involve higher costs, they can effectively achieve a Li2CO3-free interface, significantly mitigate interfacial issues, and markedly improve battery cycle life.
(5) Manufacturing compatibility and mechanical reliability: for ultrathin LLZO membranes or self-supporting composite electrolytes, the selection of interface engineering strategies requires careful prioritization. Strategies should be chosen primarily for their ability to enhance interfacial electrochemical performance. Crucially, they must also avoid introducing significant surface damage or stress concentration risks. Particularly under R2R processing conditions, their impact on bending stability and fracture tolerance must be thoroughly evaluated.
By comprehensively weighing the above principles, researchers can make more informed decisions when choosing suitable interface engineering strategies, thereby accelerating the development of high-performance garnet-based SSLMBs.
3.4 Mitigation of Li2CO3 at the cathode interface
In garnet-type SSLMBs, the stability and ion transport efficiency of the cathode/LLZO interface are critical factors determining the energy density, rate performance, and long-term reliability of the battery. Among these, the formation, evolution, and high-voltage reactivity of Li2CO3 at the cathode interface have increasingly been recognized as key scientific and engineering bottlenecks affecting the intrinsic properties, electrochemical stability, and even device lifetime. Li2CO3 primarily originates from the unavoidable surface chemical reactions of the garnet electrolyte in air, and its presence at the cathode interface exerts multidimensional and multiscale effects on the interface. From a relatively static physicochemical perspective, Li2CO3 is an electronic insulator with extremely low ionic conductivity. When it exists as a continuous or discontinuous thin layer between the cathode active material (e.g., high-nickel NCM, LiCoO2) and the LLZO electrolyte, it introduces a significant energy barrier for Li+ migration from the electrolyte bulk into the cathode interior. This leads to a marked increase in interfacial ion transport resistance, manifesting as elevated initial polarization and poor first-cycle efficiency.Under high-voltage charge/discharge conditions, the electrochemical instability of Li2CO3 exacerbates the above issues. When the cell operating voltage exceeds its stability window (generally considered to start around 3.8–4.0 V vs. Li+/Li), Li2CO3 at the interface undergoes oxidative decomposition, releasing O2 and CO2. In solid-state systems lacking the re-wetting capability of liquid electrolytes, these gases accumulate within the cathode composite layer, at GBs, and at solid–solid interfaces, forming microscopic gas cavities. This physically disrupts the initially intimate contact between cathode active particles and LLZO particles, blocking ion transport pathways. The contact failure caused by gas generation in solid-state systems is highly irreversible and is a more destructive degradation mechanism compared to traditional liquid cells. Moreover, the decomposition of Li2CO3 generates active oxygen species. These species further oxidize and erode the cathode material surface, triggering the continuous growth of a thicker and higher-impedance cathode electrolyte interphase (CEI) layer.161 Concurrently, they promote the leaching and migration of transition metal ions, collectively establishing a vicious cycle of continuous interface degradation. By using cycling and potential-resolved electrochemical impedance spectroscopy (PR-EIS), measurements clearly demonstrate that the abrupt increase in interfacial impedance is strongly correlated with gas evolution during the operation of LiMn2O4 (LMO)/LLZO/Li batteries within the 4–4.6 V range. This correlation confirms the central role of Li2CO3 decomposition in cathode interface degradation, as illustrated in Fig. 12c.48
 | ||
| Fig. 12 (a) In situ Li2CO3-to-LiF conversion at cathode/LLZTO interfaces. Reproduced with permission from ref. 153. Copyright 2023, Wiley-VCH GmbH. (b) Comparative electrode/LLZTO interface engineering for high-voltage SSBs. Reproduced with permission from ref. 154. Copyright 2025, American Chemical Society. (c) Potential-resolved impedance spectra of LMO/LLZO/Li cells (4.6 V cutoff), with the inset showing 100 mV interval data from 4.0–4.6 V. Reproduced with permission from ref. 48. Copyright 2021, Wiley-VCH GmbH. (d) In situ engineered NCM811/LLZTO interface. Reproduced with permission from ref. 155. Copyright 2020, Elsevier. (e) UHS-processed HE-DRX|LLZTO interface stabilization. Reproduced with permission from ref. 81. Copyright 2024, Springer Nature. | ||
During the material preparation stage, Li2CO3 can also have a profound impact on the compatibility of the cathode/LLZO interface. During the high-temperature co-sintering of composite cathodes, Li2CO3 on the LLZO surface can react with the cathode material. This reaction may lead to the formation of electrochemically inert impurity phases, such as La2Zr2O7, or promote the diffusion of transition metals like Co and Ni into the electrolyte. These processes compromise the structural integrity of both the cathode and the electrolyte. Traditional long-duration high-temperature sintering processes often exacerbate these side reactions.47,50
Faced with the systematic challenges posed by Li2CO3 at the cathode interface, current research has shifted from passively avoiding its formation to actively utilizing and engineering its conversion, developing multi-level and multi-approach interface regulation strategies.
For interface failure issues under high-voltage conditions, constructing an artificial LiF-rich interface layer is considered a more targeted solution. Guo et al.153 developed a LiPF6-mediated decarbonization–fluorination reaction at 60 °C (Li2CO3 → LiF), constructing a crystalline LiF-rich cathode electrolyte interphase at the interface. This stable layer effectively suppresses the high-voltage decomposition and gas generation problems of Li2CO3 (Fig. 12a). Similarly, as shown in Fig. 12b, Yin et al.154 proposed a synergistic in situ polymerization–fluorination strategy (Poly-FR), using a bifunctional initiator to simultaneously trigger polymer matrix formation and the LiF interface. Both strategies, by converting detrimental Li2CO3 into a stable LiF layer, fundamentally circumvent the regeneration limitations of traditional calcination methods, offering universal solutions for high-voltage SSB interface engineering.
In summary, suppressing the formation of Li2CO3 and reducing ion transport resistance at the interface are crucial for maintaining the structural stability and electrochemical compatibility of the cathode/LLZO interface. The synergistic application of interface wetting, in situ chemical conversion, and advanced preparation processes can alleviate Li2CO3-induced interface failure issues across different scales, providing a reliable interfacial foundation for the stable operation of high-voltage, high-energy-density SSLMBs. Furthermore, starting from the perspective of stabilizing the interface and near-interface transport structure, Fan et al. regulated ion migration and interfacial compatibility through confined interaction networks, offering new ideas and directions for interface design under high-voltage conditions.166 Notably, the high-voltage stability of LiF-rich interfaces has gradually become a common recognition across different systems. Recent research on high-voltage electrolyte design also emphasizes achieving more stable interface structures through fluorine-containing chemistry, which indirectly confirms the universal value of LiF-rich interlayers in high-voltage stabilization.167
4 Conclusion and perspective
Garnet-type SSEs, particularly LLZO, exhibit exceptional promise for all-solid-state batteries owing to their high ionic conductivity, superior chemical stability, and broad electrochemical window. However, air exposure induces the formation of insulating Li2CO3/LiOH passivation layers on particle surfaces, as confirmed by both experimental and computational studies. These surface layers not only create resistive core–shell architectures but also promote deleterious phase transformations during synthesis, generating impurity phases (e.g., La2Zr2O7) that degrade bulk ionic conductivity by up to 2–3 orders of magnitude. More critically, the surface Li2CO3 layer severely deteriorates the electrochemical performance of SSLMBs. Its detrimental effects are primarily manifested in a dramatic increase in interfacial resistance, hindered lithium-ion transport, and accelerated lithium dendrite growth. Furthermore, within composite cathodes, Li2CO3 contaminants impede effective contact between active materials and LLZO electrolyte particles, leading to diminished capacity retention. The ultra-low ionic conductivity and lithiophobic nature of these contaminants exert a profoundly negative impact on Li+ migration and solid–solid interfacial contact, posing a significant bottleneck for the commercialization of garnet-type SSBs. Therefore, achieving the scalable production, storage, and application of LLZO electrolytes without reaction with ambient air remains a critical challenge to be addressed. This paper systematically reviews the inherent causes of the instability of garnet electrolytes in air, summarizes recent strategies to improve their air stability (including controlling external environments and regulating internal microstructures), and delves into interfacial engineering methods to solve the electrode/garnet electrolyte interface issues. These strategies aim to collaboratively enhance the ionic conductivity, air stability, dendrite suppression ability, and interfacial compatibility of garnet electrolytes (Fig. 13). By offering profound insights into the Li2CO3 contamination problem, this review is intended to facilitate the development of high-performance and highly stable garnet-type SSLMBs.4.1 Enhancing air stability and suppressing Li2CO3 formation
While sulfide-type SSEs generally exhibit higher sensitivity to air compared to garnet electrolytes, relatively mature strategies have been established to enhance their air stability, notably through rational elemental doping. Inspired by this, utilizing elemental doping to improve the air stability of garnet-type SSEs and inhibit Li2CO3 formation has become a prominent research avenue. However, a deep understanding of the reaction mechanisms of dopant elements is crucial for their rational selection. Current research hotspots focus on high-entropy doping strategies, which aim to enhance the thermodynamic stability of the electrolyte by introducing multi-component elements to lower the system's ΔG. Looking ahead, the combination of machine learning (ML) and automated reaction screening holds promise as a novel approach for guiding element selection prior to experimental doping, enabling rapid screening of target products. ML can not only analyze the effects of different dopants but also integrate electrochemical data and structural characterization results. Subsequently, artificial intelligence (AI) could be leveraged to predict the formation mechanisms of Li2CO3. Building on this, inverse materials design can be further explored: intelligently designing novel high-entropy doped garnet electrolyte materials based on predefined target performance parameters (e.g., ionic conductivity and interfacial stability).4.2 Developing multifunctional composite interface modification strategies
Current mainstream interface modification methods primarily focus on introducing single protective layers (e.g., AlF3, Zn, and Ag). However, such single-layer structures struggle to effectively address the multiple complex challenges at solid–solid interfaces. Therefore, there is an urgent need to develop multifunctional composite interface modification strategies. Examples include synergistically constructing interface layers with mixed ionic/electronic conductive properties and designing composite anode structures, aiming to achieve more stable and efficient interfacial ion transport kinetics and mechanical stability. A core future research direction will be to intensively investigate how such composite strategies effectively suppress lithium dendrite growth and increase the CCD, ultimately constructing an optimal Li/LLZO interface.4.3 Optimizing the cathode/electrolyte interface
Compared to the electrolyte/lithium metal anode interface, optimizing the cathode/electrolyte interface requires greater emphasis. Current research often relies on introducing liquid electrolytes to address challenges like poor solid–solid interfacial compatibility and limited ion transport on the cathode side, which deviates from the fundamental goal of an all-solid-state solution. Although introducing sintering aids can effectively improve the contact and stability at the cathode/garnet electrolyte interface, systematic studies in this area remain limited. Optimizing the composite cathode interface requires a multi-dimensional collaborative effort, including but not limited to increasing the effective solid–solid contact area, maintaining interfacial structural/chemical stability, and constructing a continuous and efficient mixed ionic/electronic conductive network. Therefore, deeply analyzing the internal hierarchical structure and key interfacial issues of LLZO-based composite cathodes is a decisive factor for achieving the large-scale application of high-performance garnet-type SSBs, holding significant fundamental research value and practical guidance.4.4 Reconsidering the role of Li2CO3
Currently, numerous studies report comprehensive strategies to inhibit Li2CO3 formation, such as optimizing sintering processes and tailoring microstructures or using physical/chemical methods to remove Li2CO3 contaminants after air exposure. However, these strategies often struggle to effectively improve interfacial wettability or require expensive equipment, presenting significant limitations. In light of this, rather than focusing solely on the complete eradication of Li2CO3 from garnet particle interfaces, it is worthwhile to shift perspective and explore the possibility of its rational utilization. Li2CO3 at the interface can provide an alkaline environment, and its properties could be harnessed to construct lithiophilic, hydrophobic, or wetting layers between phases. Furthermore, the inherent chemical inertness of Li2CO3 allows it to potentially serve as a stable interfacial layer. Under proper storage conditions, its negative impact on the structure and electrochemical performance of the electrolyte material can be effectively controlled. From this viewpoint, the traditionally perceived Li2CO3 layer as an insulating barrier might instead offer new opportunities and possibilities for achieving intimate solid–solid interfacial contact, enhancing material air stability, and optimizing battery electrochemical performance.4.5 Advanced characterization for interface evolution
Building on a deep understanding of the formation mechanisms of Li2CO3 on the LLZO surface and multi-scale interface regulation strategies, research finds that breakthroughs in core issues like tracing the origin of contaminants and interface engineering optimization highly depend on the precise analysis of material dynamic evolution processes. The current research frontier is undergoing a crucial shift from static component analysis to the visual tracking of dynamic processes. There is an urgent need to develop advanced characterization techniques with spatiotemporal resolution capabilities to enable the full-cycle dynamic monitoring of ion transport, stress distribution, and chemical reconstruction behaviors at solid–solid interfaces. Representative techniques include: synchrotron-based in situ XAS, which can resolve the real-time evolution of the local electronic structure and coordination environment of transition metal elements (e.g., Ni, Co, and Mn) in electrode materials; three-dimensional layer-by-layer analysis using Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS), capable of precisely revealing the gradient distribution characteristics of elements in the interface region; and the combination of atomic-level interface imaging via cryo-TEM with chemical fingerprint identification by Raman spectroscopy, which can construct quantitative correlation models between interfacial reaction kinetics and macroscopic electrochemical performance at the mesoscale. The synergistic application and innovation of these multi-dimensional, high-resolution characterization techniques not only provide critical experimental criteria for evaluating the effectiveness of interface modification strategies but also reveal the complex mechanisms behind interface modifications at a deeper level. This promotes a paradigm shift in solid electrolyte research from empirical optimization to mechanism-driven design, laying a scientific foundation for the multi-scale, dynamic, and precise regulation of SSB interfaces.4.6 From lab to fab: the scalability and manufacturability of Li2CO3 mitigation strategies
While significant progress has been made at the laboratory scale in understanding the formation mechanisms, interfacial degradation, and mitigation strategies for Li2CO3, its translation into practical applications hinges critically on manufacturability and cost control during the industrial-scale production process. Unlike processing single pellet samples under controlled lab conditions, mass production emphasizes process consistency, continuity, and robustness against environmental fluctuations. Therefore, future developments in Li2CO3-related interface engineering need to shift from performance optimization to design for manufacturability.At the scale-up preparation level, garnet-type SSEs are highly sensitive to exposure to H2O/CO2, making atmosphere control and process integration critical challenges. Post-treatment steps such as polishing, chemical conversion, or surface modification risk having their effectiveness rapidly diminished during transfer and storage if they cannot be integrated in situ or in a closed-loop manner with subsequent manufacturing steps. Furthermore, many laboratory techniques, such as acid treatment, fluorination, or phosphate conversion strategies, require comprehensive evaluation for their safety, solvent recovery, by-product management, and large-area uniformity control when scaled up. In contrast, physical processes such as plasma, laser, or ultrasound could potentially offer advantages in process controllability if they can be adapted to online, continuous operation. However, these processes still require careful consideration of equipment costs and energy consumption per unit output.
On the other hand, industrialization demands that interface performance enhancement be translated into actionable quality control (QC) metrics. For example, the residual levels of Li2CO3/LiOH, interface roughness, defect density, and the statistical distribution of key interface impedance need to be characterized using rapid, low-cost, online or spot-checking methods to ensure consistency and yield in mass production. Interface engineering strategies without clear process windows and quality thresholds will struggle to be reliably reproduced in actual production lines. From a cost and system integration perspective, more practical Li2CO3 control strategies typically feature low temperature, short time, and low environmental sensitivity, while being compatible with existing slurry coating, roll-to-roll lamination, co-sintering, or rapid sintering processes. Future research should place greater emphasis on establishing quantifiable trade-offs between interface stability improvements and manufacturing complexity, energy consumption, and unit kWh costs.
Overall, solving the Li2CO3 issue is not only a material or interface chemistry problem but also a systemic engineering challenge that spans material synthesis, interface engineering, and process scaling. Only through the collaborative optimization of mechanistic understanding and manufacturability design can the interface control strategies for Li2CO3 in garnet-type LMBs be truly scaled for practical applications.
In conclusion, garnet-type SSEs show considerable promise for enabling high-energy-density and high safety in SSLMBs. However, the interface chemical, electrochemical, and mechanical failure issues caused by Li2CO3 are not isolated phenomena but represent a systemic challenge that spans material synthesis, interface construction, and device manufacturing. In recent years, significant progress has been made in understanding the formation mechanisms of Li2CO3, its high-voltage failure behavior, and interface control strategies. The research focus has gradually expanded from material optimization to the synergistic design of interface engineering and process strategies. Looking to the future, the scalability of garnet-type SSLMBs will not only depend on further improvements in interface performance but also on the comprehensive balance of manufacturability, consistency, and cost controllability of the related strategies. With the deepening understanding of interfacial evolution mechanisms and the introduction of advanced preparation and engineering approaches, garnet-type SSLMBs are poised to overcome existing bottlenecks and advance steadily toward practical application and commercialization.
Author contributions
B. Hao: investigation, writing – original draft and format analysis; Q. Wang: investigation; F. Zhao: formal analysis; J. Wu: formal analysis; W. Chen: formal analysis; Z.-J. Jiang: supervision and writing – review & editing; Z. Jiang: resources, writing – review & editing, and supervision.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
The authors acknowledge support from the Zhejiang Provincial Natural Science Foundation (No. LRG26E070001 and LR22E070001), the National Natural Science Foundation of China (No. 12275239), and the Guangdong Basic and Applied Basic Research Foundation (No. 2025A1515010349).Notes and references
- Z. Deng, Y. Liu, L. Wang, N. Fu, Y. Li, Y. Luo, J. Wang, X. Xiao, X. Wang, X. Yang, X. He and H. Zhang, Challenges of Thermal Stability of High-Energy Layered Oxide Cathode Materials for Lithium-Ion Batteries: A Review, Mater. Today, 2023, 69, 236–261 CrossRef CAS.
- J. Biao, C. Bai, J. Ma, M. Liu, F. Kang, Y. Cao and Y.-B. He, Perspectives on Li Dendrite Penetration in Li7La3Zr2O12-Based Solid-State Electrolytes and Batteries: Materials, Interfaces, and Charge Transfer, Adv. Energy Mater., 2024, 14, 2303128 CrossRef.
- H. Wan, J. Xu and C. Wang, Designing Electrolytes and Interphases for High-Energy Lithium Batteries, Nat. Rev. Chem., 2024, 8, 30–44 CrossRef CAS PubMed.
- C. Bai, Y. Li, G. Xiao, J. Chen, S. Tan, P. Shi, T. Hou, M. Liu, Y.-B. He and F. Kang, Understanding the Electrochemical Window of Solid-State Electrolyte in Full Battery Application, Chem. Rev., 2025, 125, 6541–6608 CrossRef CAS PubMed.
- J. Lee, S. Zhou, V. C. Ferrari, C. Zhao, A. Sun, S. Nicholas, Y. Liu, C. Sun, D. Wierzbicki, D. Y. Parkinson, J. Bai, W. Xu, Y. Du, K. Amine and G.-L. Xu, Halide Segregation to Boost All-Solid-State Lithium-Chalcogen Batteries, Science, 2025, 388, 724–729 CrossRef CAS PubMed.
- S. C. Kim, J. Wang, R. Xu, P. Zhang, Y. Chen, Z. Huang, Y. Yang, Z. Yu, S. T. Oyakhire, W. Zhang, L. C. Greenburg, M. S. Kim, D. T. Boyle, P. Sayavong, Y. Ye, J. Qin, Z. Bao and Y. Cui, High-Entropy Electrolytes for Practical Lithium Metal Batteries, Nat. Energy, 2023, 8, 814–826 CrossRef CAS.
- L. Chen, P. Shi, T. Gu, J. Mi, K. Yang, L. Zhao, J. Lv, M. Liu, Y.-B. He and F. Kang, Strategies of Constructing Highly Stable Interfaces with Low Resistance in Inorganic Oxide-Based Solid-State Lithium Batteries, eScience, 2025, 5, 100277 CrossRef.
- P. López-Aranguren, M. Reynaud, P. Głuchowski, A. Bustinza, M. Galceran, J. M. López del Amo, M. Armand and M. Casas-Cabanas, Crystalline LiPON as a Bulk-Type Solid Electrolyte, ACS Energy Lett., 2021, 6, 445–450 CrossRef.
- Z. Jian, Y.-S. Hu, X. Ji and W. Chen, NASICON-Structured Materials for Energy Storage, Adv. Mater., 2017, 29, 1601925 CrossRef PubMed.
- S. Woo and B. Kang, Superior Compatibilities of a LISICON-Type Oxide Solid Electrolyte Enable High Energy Density All-Solid-State Batteries, J. Mater. Chem. A, 2022, 10, 23185–23194 RSC.
- D. Zeng, J. Yao, L. Zhang, R. Xu, S. Wang, X. Yan, C. Yu and L. Wang, Promoting Favorable Interfacial Properties in Lithium-Based Batteries using Chlorine-Rich Sulfide Inorganic Solid-State Electrolytes, Nat. Commun., 2022, 13, 1909 CrossRef CAS PubMed.
- X. Zhou, C. Gao, D. Wang, S. Peng, L. Huang, W. Yang, W.-H. Zhang and X. Gao, Revealing the Dominant Factor of Domain Boundary Resistance on Bulk Conductivity in Lanthanum Lithium Titanates, J. Energy Chem., 2022, 73, 354–359 CrossRef CAS.
- Z. Jin, X. Kong, H. Huang, Y. Jiang, W. Xiang, Y. Xu, L. Zhang, R. Peng and C. Wang, Garnet-Type Solid-State Mixed Ionic and Electronic Conductor, Energy Storage Mater., 2023, 59, 102788 CrossRef.
- C. Wang, K. Fu, S. P. Kammampata, D. W. McOwen, A. J. Samson, L. Zhang, G. T. Hitz, A. M. Nolan, E. D. Wachsman, Y. Mo, V. Thangadurai and L. Hu, Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries, Chem. Rev., 2020, 120, 4257–4300 Search PubMed.
- X. He, F. Yan, M. Gao, Y. Shi, G. Ge, B. Shen and J. Zhai, Cu-Doped Alloy Layer Guiding Uniform Li Deposition on a Li-LLZO Interface under High Current Density, ACS Appl. Mater. Interfaces, 2021, 13, 42212–42219 Search PubMed.
- J.-F. Wu, B.-W. Pu, D. Wang, S.-Q. Shi, N. Zhao, X. Guo and X. Guo, In Situ Formed Shields Enabling Li2CO3-Free Solid Electrolytes: A New Route to Uncover the Intrinsic Lithiophilicity of Garnet Electrolytes for Dendrite-Free Li-Metal Batteries, ACS Appl. Mater. Interfaces, 2019, 11, 898–905 CrossRef CAS PubMed.
- B. Chen, J. Zhang, T. Zhang, R. Wang, J. Zheng, C. Liu and X. Liu, Directly Using Li2CO3 as a Lithiophobic Interlayer to Inhibit Li Dendrites for High-Performance Solid-State Batteries, ACS Energy Lett., 2023, 8, 2221–2231 CrossRef CAS.
- A. Sharafi, S. Yu, M. Naguib, M. Lee, C. Ma, H. M. Meyer, J. Nanda, M. Chi, D. J. Siegel and J. Sakamoto, Impact of Air Exposure and Surface Chemistry on Li-Li7La3Zr2O12 Interfacial Resistance, J. Mater. Chem. A, 2017, 5, 13475–13487 Search PubMed.
- C. Cao, L. Zhao, Y. Zhong, J. Simi and Z. Shao, Optimization of Garnet-Type Solid-State Lithium Batteries via Synergistic Integration of an Advanced Composite Interface for Elevated Performance, Chem. Eng. J., 2024, 495, 153649 CrossRef CAS.
- Y. Wang, Z. Chen, K. Jiang, Z. Shen, S. Passerini and M. Chen, Accelerating the Development of LLZO in Solid-State Batteries Toward Commercialization: A Comprehensive Review, Small, 2024, 20, 2402035 CrossRef CAS.
- G. J. Redhammer, P. Badami, M. Meven, S. Ganschow, S. Berendts, G. Tippelt and D. Rettenwander, Wet-Environment-Induced Structural Alterations in Single- and Polycrystalline LLZTO Solid Electrolytes Studied by Diffraction Techniques, ACS Appl. Mater. Interfaces, 2021, 13, 350–359 CrossRef CAS PubMed.
- J. Leng, H. Wang, H. Liang, Z. Xiao, S. Wang, Z. Zhang and Z. Tang, Storage of Garnet Solid Electrolytes: Insights into Air Stability and Surface Chemistry, ACS Appl. Energy Mater., 2022, 5, 5108–5116 CrossRef CAS.
- N. Zhang, G. Ren, L. Li, Z. Wang, P. Yu, X. Li, J. Zhou, H. Zhang, L. Zhang, Z. Liu and X. Liu, Dynamical Evolution of CO2 and H2O on Garnet Electrolyte Elucidated by Ambient Pressure X-ray Spectroscopies, Nat. Commun., 2024, 15, 2777 CrossRef CAS PubMed.
- Q. Zhao, S. Stalin, C.-Z. Zhao and L. A. Archer, Designing Solid-State Electrolytes for Safe, Energy-Dense Batteries, Nat. Rev. Mater., 2020, 5, 229–252 CrossRef CAS.
- M. Du, Y. Sun, B. Liu, B. Chen, K. Liao, R. Ran, R. Cai, W. Zhou and Z. Shao, Smart Construction of an Intimate Lithium | Garnet Interface for All-Solid-State Batteries by Tuning the Tension of Molten Lithium, Adv. Funct. Mater., 2021, 31, 2101556 CrossRef CAS.
- K. Yoon, S. Lee, K. Oh and K. Kang, Challenges and Strategies towards Practically Feasible Solid-State Lithium Metal Batteries, Adv. Mater., 2022, 34, 2104666 CrossRef CAS PubMed.
- L. Zhang, Q. Meng, Y. Dai, X. Feng, M. Shen, Q. Zhuang, Z. Ju, R. Zheng, Z. Wang, Y. Cui, H. Sun and Y. Liu, Ion/Electron Conductive Layer with Double-Layer-Like Structure for Dendrite-Free Solid-State Lithium Metal Batteries, Nano Energy, 2023, 113, 108573 CrossRef CAS.
- F. Wang, Z. Jiang, Y. Zhang, Y. Zhang, J. Li, H. Wang, Y. Jiang, G. Xing, H. Liu and Y. Tang, Revitalizing Sodium-Ion Batteries Via Controllable Microstructures and Advanced Electrolytes for Hard Carbon, eScience, 2024, 4, 100181 CrossRef.
- L. Cheng, E. J. Crumlin, W. Chen, R. Qiao, H. Hou, S. Franz Lux, V. Zorba, R. Russo, R. Kostecki, Z. Liu, K. Persson, W. Yang, J. Cabana, T. Richardson, G. Chen and M. Doeff, The Origin of High Electrolyte-Electrode Interfacial Resistances in Lithium Cells Containing Garnet Type Solid Electrolytes, Phys. Chem. Chem. Phys., 2014, 16, 18294–18300 RSC.
- W. Xia, B. Xu, H. Duan, X. Tang, Y. Guo, H. Kang, H. Li and H. Liu, Reaction Mechanisms of Lithium Garnet Pellets in Ambient Air: The Effect of Humidity and CO2, J. Am. Ceram. Soc., 2017, 100, 2832–2839 CrossRef CAS.
- W. Lu, T. Wang, M. Xue and C. Zhang, Improved Li6.5La3Zr1.5Nb0.5O12 Electrolyte and Effects of Atmosphere Exposure on Conductivities, J. Power Sources, 2021, 497, 229845 CrossRef CAS.
- Z. Zhang, E. Zhao, W. Yin, B. Wang, Y. Li and F. Wang, Visualization and Quantification of Li Distribution in Garnet Solid Electrolytes Li6.25La3Zr2Al0.25O12, Appl. Phys. Lett., 2024, 125, 243903 CrossRef CAS.
- A. Sharafi, E. Kazyak, A. L. Davis, S. Yu, T. Thompson, D. J. Siegel, N. P. Dasgupta and J. Sakamoto, Surface Chemistry Mechanism of Ultra-Low Interfacial Resistance in the Solid-State Electrolyte Li7La3Zr2O12, Chem. Mater., 2017, 29, 7961–7968 CrossRef CAS.
- M. Nakayama, T. Horie, R. Natsume, S. Hashimura, N. Tanibata, H. Takeda, H. Maeda and M. Kotobuki, Reaction Kinetics of Carbonation at the Surface of Garnet-Type Li7La3Zr2O12 as Solid Electrolytes for All-Solid-State Li Ion Batteries, J. Phys. Chem. C, 2023, 127, 7595–7601 CrossRef CAS.
- A. Kim, K. Song, M. Avdeev and B. Kang, High Energy Density Ultra-thin Li Metal Solid-State Battery Enabled by a Li2CO3-Proof Garnet-Type Solid Electrolyte, ACS Energy Lett., 2024, 9, 1976–1983 CrossRef CAS.
- J. Biao, B. Han, Y. Cao, Q. Li, G. Zhong, J. Ma, L. Chen, K. Yang, J. Mi, Y. Deng, M. Liu, W. Lv, F. Kang and Y.-B. He, Inhibiting Formation and Reduction of Li2CO3 to LiCx at Grain Boundaries in Garnet Electrolytes to Prevent Li Penetration, Adv. Mater., 2023, 35, 2208951 CrossRef CAS PubMed.
- Y.-N. Yang, Y.-X. Li, Y.-Q. Li and T. Zhang, On-Surface Lithium Donor Reaction Enables Decarbonated Lithium Garnets and Compatible Interfaces within Cathodes, Nat. Commun., 2020, 11, 5519 Search PubMed.
- T. Zhang, T. D. Christopher, S. Huang, Y. g. Liu, W. Gao, T. Söhnel and P. Cao, Pressureless Sintering of Al-free Ta-Doped Lithium Garnets Li7-xLa3Zr2-xTaxO12 and the Degradation Mechanism in Humid Air, Ceram. Int., 2019, 45, 20954–20960 CrossRef CAS.
- M. W. Swift, J. W. Swift and Y. Qi, Modeling the Electrical Double Layer at Solid-State Electrochemical Interfaces, Nat. Comput. Sci., 2021, 1, 212–220 CrossRef PubMed.
- Z. Guo, Q. Li, X. Li, Z. Wang, H. Guo, W. Peng, G. Li, G. Yan and J. Wang, Uniform Densification of Garnet Electrolyte for Solid-State Lithium Batteries, Small Methods, 2023, 7, 2300232 CrossRef CAS PubMed.
- D. Cao, C. Tan and Y. Chen, Oxidative Decomposition Mechanisms of Lithium Carbonate on Carbon Substrates in Lithium Battery Chemistries, Nat. Commun., 2022, 13, 4908 CrossRef CAS PubMed.
- H. Zheng, G. Li, R. Ouyang, Y. Han, H. Zhu, Y. Wu, X. Huang, H. Liu and H. Duan, Origin of Lithiophilicity of Lithium Garnets: Compositing or Cleaning?, Adv. Funct. Mater., 2022, 32, 2205778 Search PubMed.
- D. Wang, H. Shi, S. Liang, R. Shao, S. Wang, W. Cui, W. Wang and Z. Xu, Evolution of the Cell Structure, Ionic Conductivity, and Elastic Modulus of the γ-Irradiated LLZTO Electrolyte via Neutron Diffraction and Nanoindentation, J. Phys. Chem. C, 2024, 128, 1911–1920 CrossRef CAS.
- P. Chen, P. Guo, W. Guo, B. Ding, H. Dou and X. Zhang, Identifying Interface Evolutions for Achieving Stable Solid-State Li Metal Batteries, J. Energy Chem., 2025, 110, 363–371 CrossRef CAS.
- C.-Y. Chang, C.-C. Wang, C.-H. Cheng, Y.-L. Lu, S.-H. Lin, J. Granwehr, A. Windmüller, C.-L. Tsai, R.-A. Eichel and K.-F. Chiu, Enhanced Stability and High Rate Capability of Garnet Solid-State Electrolyte Interface through Integration of Nanoscale Li4Ti5O12 for Li Battery Applications, J. Power Sources, 2025, 652, 237593 Search PubMed.
- C. Tang, Z. Fan, B. Ding, C. Xu, H. Wu, H. Dou and X. Zhang, Functional Separator with Poly(Acrylic Acid)-Enabled Li2CO3-Free Garnet Coating for Long-Cycling Lithium Metal Batteries, Small, 2025, 21, 2407558 Search PubMed.
- S. Weinmann, H. Gobena, L. Quincke, J. J. Hinricher, S. Merk, H. Chu, T. Prein, J. L. M. Rupp and K. J. Kim, Stabilizing Interfaces of All-Ceramic Composite Cathodes for Li-Garnet Batteries, Adv. Energy Mater., 2025, 15, 2502280 Search PubMed.
- A. A. Delluva, J. Kulberg-Savercool and A. Holewinski, Decomposition of Trace Li2CO3 During Charging Leads to Cathode Interface Degradation with the Solid Electrolyte LLZO, Adv. Funct. Mater., 2021, 31, 2103716 Search PubMed.
- Q. Gan, N. Qin, H. Yuan, L. Lu, Z. Xu and Z. Lu, Critical review on the degradation mechanisms and recent progress of Ni-rich layered oxide cathodes for lithium-ion batteries, EnergyChem, 2023, 5, 100103 CrossRef CAS.
- T. Demuth, T. Fuchs, F. Walther, A. Pokle, S. Ahmed, M. Malaki, A. Beyer, J. Janek and K. Volz, Influence of the sintering temperature on LLZO-NCM cathode composites for solid-state batteries studied by transmission electron microscopy, Matter, 2023, 6, 2324–2339 CrossRef CAS.
- Y. Kim, I. Waluyo, A. Hunt and B. Yildiz, Avoiding CO2 Improves Thermal Stability at the Interface of Li7La3Zr2O12 Electrolyte with Layered Oxide Cathodes, Adv. Energy Mater., 2022, 12, 2102741 Search PubMed.
- W. S. Scheld, K. Kim, C. Schwab, A. C. Moy, S.-K. Jiang, M. Mann, C. Dellen, Y. J. Sohn, S. Lobe, M. Ihrig, M. G. Danner, C.-Y. Chang, S. Uhlenbruck, E. D. Wachsman, B. J. Hwang, J. Sakamoto, L. F. Wan, B. C. Wood, M. Finsterbusch and D. Fattakhova-Rohlfing, The Riddle of Dark LLZO: Cobalt Diffusion in Garnet Separators of Solid-State Lithium Batteries, Adv. Funct. Mater., 2023, 33, 2302939 CrossRef CAS.
- H. Zhou, Y. Zhou, X. Li, X. Huang and B. Tian, Li5AlO4-Assisted Low-Temperature Sintering of Dense Li7La3Zr2O12 Solid Electrolyte with High Critical Current Density, ACS Appl. Mater. Interfaces, 2024, 16, 5989–5998 CrossRef CAS.
- H. Zhang, Y. Wu, J. Zhu, X. Xie, Z. Liu, Z. Zhang, Y. Ma, T. Huang, L. Wang, J. Lin, Q. Xie and D.-L. Peng, Fusing Ta-Doped Li7La3Zr2O12 Grains Using Nanoscale Y2O3 Sintering Aids for High-Performance Solid-State Lithium Batteries, Nanoscale, 2024, 16, 14871–14878 RSC.
- S. P. Kammampata, R. H. Basappa, T. Ito, H. Yamada and V. Thangadurai, Microstructural and Electrochemical Properties of Alkaline Earth Metal-Doped Li Garnet-Type Solid Electrolytes Prepared by Solid-State Sintering and Spark Plasma Sintering Methods, ACS Appl. Energy Mater., 2019, 2, 1765–1773 CrossRef CAS.
- X. Xiang, Z. Fang, C. Du, Z. Zhao, J. Chen, Y. Zhang, H. Wang, C. Wei, F. Chen and Q. Shen, Constructing Electron-Blocking Grain Boundaries in Garnet to Suppress Lithium Dendrite Growth, J. Adv. Ceram., 2024, 13, 166–175 CrossRef CAS.
- R. A. Jonson and P. J. McGinn, Effects of High Energy Ball Milling on Phase Stability of Li7La3Zr2O12, ECS Meet. Abstr., 2016, MA2016-03, 673 CrossRef.
- X. Feng, L. Zhang, C. Li, M. Shen, R. Zheng, Z. Wang, H. Sun and Y. Liu, Al-Ta Dual-Substituted Li7La3Zr2O12 Ceramic Electrolytes with Two-Step Sintering for Stable All-Solid-State Lithium Batteries, Ceram. Int., 2024, 50, 38999–39009 Search PubMed.
- Y. Dabaki, M. Kassem, A. Sammoury, M. Bokova, M. Fourmentin, O. El-Kedim and E. Bychkov, Influence of Microwave Sintering and Pellet Pressure on the Phase Formation, Microstructure and Lithium-Ion Conductivity of the Li6.28Al0.24La3Zr2O12 Solid Electrolyte, J. Mater. Eng. Perform., 2025, 34, 6733–6742 CrossRef CAS.
- Y. Yang, Z. Zhang, T. Ma, S. Jia and C. Huang, Low-Temperature Flash Sintering of Dense Ta-Doped Li7La3Zr2O12 Solid Electrolyte for Solid-State Lithium Batteries, Ionics, 2025, 31, 1341–1350 CrossRef CAS.
- X. Wang, J. Wang, F. Li, F. Zhu and C. Ma, Influence of Cold Sintering Process on the Structure and Properties of Garnet-Type Solid Electrolytes, Ceram. Int., 2020, 46, 18544–18550 Search PubMed.
- A. C. Moy, G. Häuschen, D. Fattakhova-Rohlfing, J. B. Wolfenstine, M. Finsterbusch and J. Sakamoto, The Effects of Aluminum Concentration on the Microstructural and Electrochemical Properties of Lithium Lanthanum Zirconium Oxide, J. Mater. Chem. A, 2022, 10, 21955–21972 Search PubMed.
- R. Shi, Y. Pu, W. Wang, Y. Shi, J. Li, X. Guo and M. Yang, Flash Sintering of Barium Titanate, Ceram. Int., 2019, 45, 7085–7089 Search PubMed.
- M. Biesuz and V. M. Sglavo, Flash Sintering of Ceramics, J. Eur. Ceram. Soc., 2019, 39, 115–143 Search PubMed.
- H. K. Bezabh, L. H. Abrha, S.-F. Chiu, Y. Nikodimos, T. M. Hagos, M.-C. Tsai, W.-N. Su and B. J. Hwang, Enhancing Ionic Conductivity and Air Stability of Li7La3Zr2O12 Garnet-Based Electrolyte through Dual-Dopant Strategy, J. Alloys Compd., 2025, 1010, 177277 CrossRef CAS.
- S. Kobi, A. Sharma and A. Mukhopadhyay, Low Interfacial Resistance and Superior Suppression to Li-Dendrite Penetration Facilitated by Air-Stable and Mechanically Robust Al/Mg-Co-Doped Li-La-Zirconate as Electrolyte for Li-Based Solid-State Cells, ACS Appl. Mater. Interfaces, 2023, 15, 39276–39290 Search PubMed.
- S. Yu, Y. Li, J. Luo, D. Chen, L. Yang, Y. Wei, D. Li, Y. Li and Y. Chen, Reasonable Design a High-Entropy Garnet-Type Solid Electrolyte for All-Solid-State Lithium Batteries, J. Energy Chem., 2024, 96, 414–423 Search PubMed.
- H. Zheng, G. Li, J. Liu, S. Wu, X. Zhang, Y. Wu, H. Zhu, X. Huang, H. Liu and H. Duan, A rational Design of Garnet-Type Li7La3Zr2O12 with Ultrahigh Moisture Stability, Energy Storage Mater., 2022, 49, 278–290 Search PubMed.
- M. Nasir, J. Y. Park, P. Heo, K. H. Choi and H. J. Park, Li-La-Zr-O Garnets with High Li-Ion Conductivity and Air-Stability by Microstructure-Engineering, Adv. Funct. Mater., 2023, 33, 2303397 Search PubMed.
- J. Guo, H. Guo, A. L. Baker, M. T. Lanagan, E. R. Kupp, G. L. Messing and C. A. Randall, Cold Sintering: A Paradigm Shift for Processing and Integration of Ceramics, Angew. Chem., Int. Ed., 2016, 55, 11457–11461 Search PubMed.
- B. Nie, T.-W. Wang, S. W. Lee, J. Zhang and H. Sun, Probing Cold Sintering-Regulated Interfaces and Integration of Polymer-in-Ceramic Solid-State Electrolytes, Mater. Today Energy, 2025, 49, 101829 Search PubMed.
- H. Salazar, B. F. Gonçalves, A. Valverde, R. Gonçalves, C. M. Costa, L. P. Cavalcanti, J. M. Porro, V. Petrenko, S. Lanceros-Mendez and Q. Zhang, High-Performance Composite Solid-State Electrolyte Combining NASICON-Type Li1.5Al0.5Ti1.5(PO4)3 with Ionic Liquid and Polymeric Binders, Electrochim. Acta, 2025, 509, 145299 CrossRef CAS.
- H. Leng, J. Huang, J. Nie and J. Luo, Cold Sintering and Ionic Conductivities of Na3.256Mg0.128Zr1.872Si2PO12 Solid Electrolytes, J. Power Sources, 2018, 391, 170–179 CrossRef CAS.
- Y. Chen, T. Wang, H. Chen, W. H. Kan, W. Yin, Z. Song, C. Wang, J. Ma, W. Luo and Y. Huang, Local Structural Features of Medium-Entropy Garnet with Ultra-Long Cycle Life, Matter, 2023, 6, 1530–1541 CrossRef.
- R. Jalem, Y. Morishita, T. Okajima, H. Takeda, Y. Kondo, M. Nakayama and T. Kasuga, Experimental and First-Principles DFT Study on the Electrochemical Reactivity of Garnet-Type Solid Electrolytes with Carbon, J. Mater. Chem. A, 2016, 4, 14371–14379 RSC.
- D. Rettenwander, C. A. Geiger, M. Tribus, P. Tropper and G. Amthauer, A Synthesis and Crystal Chemical Study of the Fast Ion Conductor Li7–3xGaxLa3Zr2O12 with x = 0.08 to 0.84, Inorg. Chem., 2014, 53, 6264–6269 Search PubMed.
- H. Shiiba, M. Koyama, N. Zettsu and K. Teshima, Li-Ion Conduction Characteristics at Grain Boundaries in Garnet Li7–xLa3Zr2–xNbxO12 (0 ≤ x ≤ 2), Chem. Mater., 2024, 36, 6370–6380 CrossRef CAS.
- A. Kim, J.-H. Kang, K. Song and B. Kang, Simultaneously Improved Cubic Phase Stability and Li-Ion Conductivity in Garnet-Type Solid Electrolytes Enabled by Controlling the Al Occupation Sites, ACS Appl. Mater. Interfaces, 2022, 14, 12331–12339 CrossRef CAS PubMed.
- L. Huang, J. Gao, Z. Bi, N. Zhao, J. Wu, Q. Fang, X. Wang, Y. Wan and X. Guo, Comparative Study of Stability against Moisture for Solid Garnet Electrolytes with Different Dopants, Energies, 2022, 15, 3206 Search PubMed.
- S. Wang, X. Wen, Z. Huang, H. Xu, F. Fan, X. Wang, G. Tian, S. Liu, P. Liu, C. Wang, C. Zeng, C. Shu and Z. Liang, High-Entropy Strategy Flattening Lithium Ion Migration Energy Landscape to Enhance the Conductivity of Garnet-Type Solid-State Electrolytes, Adv. Funct. Mater., 2025, 35, 2416389 Search PubMed.
- X. Kong, R. Gu, Z. Jin, L. Zhang, C. Zhang, W. Xiang, C. Li, K. Zhu, Y. Xu, H. Huang, X. Liu, R. Peng and C. Wang, Maximizing Interface Stability in All-Solid-State Lithium Batteries through Entropy Stabilization and Fast Kinetics, Nat. Commun., 2024, 15, 7247 Search PubMed.
- Y. Zeng, B. Ouyang, J. Liu, Y.-W. Byeon, Z. Cai, L. J. Miara, Y. Wang and G. Ceder, High-Entropy Mechanism to Boost Ionic Conductivity, Science, 2022, 378, 1320–1324 Search PubMed.
- Y. Feng, L. Yang, Z. Yan, D. Zuo, Z. Zhu, L. Zeng, Y. Zhu and J. Wan, Discovery of High Entropy Garnet Solid-State Electrolytes via Ultrafast Synthesis, Energy Storage Mater., 2023, 63, 103053 CrossRef.
- E. Anderson, E. Zolfaghar, A. Jonderian, R. Z. Khaliullin and E. McCalla, Comprehensive Dopant Screening in Li7La3Zr2O12 Garnet Solid Electrolyte, Adv. Energy Mater., 2024, 14, 2304025 Search PubMed.
- M. Ali, M. Saleem, T. Sattar, M. Z. Khan, J. H. Koh, O. Gohar, I. Hussain, Y. Zhang, M. B. Hanif, G. Ali and M. F. Khan, High-Entropy Battery Materials: Revolutionizing Energy Storage with Structural Complexity and Entropy-Driven Stabilization, Mater. Sci. Eng., R, 2025, 163, 100921 Search PubMed.
- X. Ma and Y. Xu, Effects of Polishing Treatments on the Interface Between Garnet Solid Electrolyte and Lithium Metal, Electrochim. Acta, 2023, 441, 141789 Search PubMed.
- Z. Qin, Y. Xie, X. Meng, D. Qian, C. Shan, D. Mao, G. He, Z. Zheng, L. Wan and Y. Huang, Interface Engineering for Garnet-Type Electrolyte Enables Low Interfacial Resistance in Solid-State Lithium Batteries, Chem. Eng. J., 2022, 447, 137538 CrossRef CAS.
- S. Rajendran, A. Pilli, O. Omolere, J. Kelber and L. M. R. Arava, An All-Solid-State Battery with a Tailored Electrode-Electrolyte Interface Using Surface Chemistry and Interlayer-Based Approaches, Chem. Mater., 2021, 33, 3401–3412 Search PubMed.
- P. Srivastava, B. Bazri, D. K. Maurya, Y.-T. Hung, D.-H. Wei and R.-S. Liu, Green Strategy for Li2CO3 Regulation in Garnet-Type Solid-State Electrolytes via Acoustic Cavitation, ACS Energy Lett., 2025, 10, 1725–1732 CrossRef CAS.
- T. Sun, X. Cheng, T. Cao, M. Wang, J. Tian, T. Yan, D. Qin, X. Liu, J. Lu and Y. Zhang, Optimizing Li Ion Transport in a Garnet-Type Solid Electrolyte via a Grain Boundary Design, Batteries, 2023, 9, 526 CrossRef CAS.
- W. Jeong, S. S. Park, J. Yun, H. R. Shin, J. Moon and J.-W. Lee, Tailoring Grain Boundary Structures and Chemistry of Li7La3Zr2O12 Solid Electrolytes for Enhanced Air Stability, Energy Storage Mater., 2023, 54, 543–552 Search PubMed.
- K. Ma, B. Chen, C.-X. Li and V. Thangadurai, Improvement of the Li-Ion Conductivity and Air Stability of the Ta-doped Li7La3Zr2O12 Electrolyte via Ga Co-Doping and Its Application in Li–S batteries, J. Mater. Chem. A, 2024, 12, 3601–3615 RSC.
- G. Zhao, C. Luo and Q. Hua, Enhanced Comprehensive Performance of the LLZO Series Solid Electrolyte via Multifunctional Additive, J. Eur. Ceram. Soc., 2024, 44, 2251–2260 CrossRef CAS.
- Y. Luo, J. Dong, Y. Wang, Z. Wang, Z. a. Chen and H. Zhang, Enhanced Electrochemical Performance of Garnet Li7La3Zr2O12 Electrolyte by Efficient Incorporation of LiCl, Ceram. Int., 2024, 50, 51055–51064 CrossRef CAS.
- X. Huang, C. Liu, Y. Lu, T. Xiu, J. Jin, M. E. Badding and Z. Wen, A Li-Garnet composite ceramic electrolyte and its solid-state Li-S battery, J. Power Sources, 2018, 382, 190–197 Search PubMed.
- Y.-G. Lee, S. Hong, B. Pan, X. Wu, E. C. Dickey and J. F. Whitacre, Improving Bulk and Interfacial Lithium Transport in Garnet-Type Solid Electrolytes through Microstructure Optimization for High-Performance All-Solid-State Batteries, ACS Appl. Mater. Interfaces, 2024, 16, 60340–60347 CrossRef CAS PubMed.
- Z. Qin, Y. Xie, X. Meng, D. Qian, D. Mao, Z. Zheng, L. Wan and Y. Huang, Grain Boundary Engineering in Ta-Doped Garnet-Type Electrolyte for Lithium Dendrite Suppression, ACS Appl. Mater. Interfaces, 2022, 14, 40959–40966 CrossRef CAS PubMed.
- S. Vema, F. N. Sayed, S. Nagendran, B. Karagoz, C. Sternemann, M. Paulus, G. Held and C. P. Grey, Understanding the Surface Regeneration and Reactivity of Garnet Solid-State Electrolytes, ACS Energy Lett., 2023, 8, 3476–3484 Search PubMed.
- Y. Chen, B. Ouyang, X. Li, W. Liu, B. Yang, P. Ning, Q. Xia, F. Zan, E. Kan, J. Xu and H. Xia, Gradient Nitrogen Doping in the Garnet Electrolyte for Highly Efficient Solid-State-Electrolyte/Li Interface by N2 Plasma, ACS Appl. Mater. Interfaces, 2023, 15, 44962–44973 Search PubMed.
- L. Chen, Y. Su, J. Zhang, H. Zhang, B. Fan, G. Shao, M. Zhong and C.-A. Wang, Nanosecond Laser Cleaning Method to Reduce the Surface Inert Layer and Activate the Garnet Electrolyte for a Solid-State Li Metal Battery, ACS Appl. Mater. Interfaces, 2021, 13, 37082–37090 Search PubMed.
- Y. Liu, M. Lei, C. Lai, J. Meng, X. Wu, Y. Yu, Y. Zhang and C. Li, Enable High Reversibility of Fe/Cu Based Fluoride Conversion Batteries via Interfacial Gas Release and Detergency of Garnet Electrolytes, Mater. Today, 2022, 61, 65–77 CrossRef CAS.
- Z. Bi, Q. Sun, M. Jia, M. Zuo, N. Zhao and X. Guo, Molten Salt Driven Conversion Reaction Enabling Lithiophilic and Air-Stable Garnet Surface for Solid-State Lithium Batteries, Adv. Funct. Mater., 2022, 32, 2208751 CrossRef CAS.
- Z. Wang, W. Li, S. Jiao, J. Zhu, Z. Wang, J. Peng, W. Gong, J. Wang, H. Huang, H. Song and M. Yu, An in Situ-Formed LiI Interface Layer for Garnet-Based Lithium Metal Batteries, Electrochim. Acta, 2024, 488, 144242 Search PubMed.
- X. Hu, Y. Wang, W. Guo, Y. Tian, X. Zhang, F. Kang, D. Zhou and B. Li, Garnet-Based Solid Lithium Metal Batteries with Ultralong Lifespan Enabled by Solvent-Free Trifluoroacetic Acid-Induced Interfacial Engineering, J. Mater. Chem. A, 2024, 12, 13830–13840 Search PubMed.
- Q. Li, Z. Xiao, K. Pu, Y. Ma, C. Xian, W. Sun, M. Xu and S.-J. Bao, FEC-Driven Surface Conversion Reaction to Construct Lithiophilic and Air-Stabilized LLZTO for Durable Lithium Battery, Chem. Eng. J., 2025, 507, 160413 CrossRef CAS.
- W. Liu, B. Zhang, Q. Zhu, H. Qin, Z. Xiao, X. He, F. Zheng and X. Ou, Contradictory Structure Design with Li2CO3 Retention for Garnet-Based Solid-State Lithium Metal Batteries, Nano Lett., 2025, 25, 7818–7825 Search PubMed.
- X. Zhan, X. Pang, F. Mao, J. Lin, M. Li, Y. Zhao, P. Xu, Z. Xu, K. Liao, Q. Zhang and L. Zhang, Interfacial Reconstruction Unlocks Inherent Ionic Conductivity of Li-La-Zr-Ta-O Garnet in Organic Polymer Electrolyte for Durable Room-Temperature All-Solid-State Batteries, Adv. Energy Mater., 2024, 14, 2402509 CrossRef CAS.
- Y. Ruan, Y. Lu, X. Huang, J. Su, C. Sun, J. Jin and Z. Wen, Acid Induced Conversion Towards a Robust and Lithiophilic Interface for Li-Li7La3Zr2O12 Solid-State Batteries, J. Mater. Chem. A, 2019, 7, 14565–14574 Search PubMed.
- N. Chen, B. Gui, B. Yang, C. Deng, Y. Liang, F. Zhang, B. Li, W. Sun, F. Wu and R. Chen, LiPF6 Induces Phosphorization of Garnet-Type Solid-State Electrolyte for Stable Lithium Metal Batteries, Small, 2024, 20, 2305576 Search PubMed.
- G. Lu, W. Liu, Z. Yang, Y. Wang, W. Zheng, R. Deng, R. Wang, L. Lu and C. Xu, Superlithiophilic, Ultrastable, and Ionic-Conductive Interface Enabled Long Lifespan All-Solid-State Lithium-Metal Batteries under High Mass Loading, Adv. Funct. Mater., 2023, 33, 2304407 CrossRef CAS.
- H. Zhao, M. Du, H. Mo, C. Wang, W. Zhou, K. Liao and Z. Shao, Garnet-Based Solid Li-Metal Batteries Operable under High External Pressure with HCOOH-Induced Electron-Blocking and Lithiophilic Interlayer, ACS Appl. Mater. Interfaces, 2024, 16, 44997–45005 Search PubMed.
- Y. Ruan, Y. Lu, Y. Li, C. Zheng, J. Su, J. Jin, T. Xiu, Z. Song, M. E. Badding and Z. Wen, A 3D Cross-Linking Lithiophilic and Electronically Insulating Interfacial Engineering for Garnet-Type Solid-State Lithium Batteries, Adv. Funct. Mater., 2021, 31, 2007815 Search PubMed.
- L. Wang, Y. Lu, C. Zheng, M. Cai, F. Xu and Z. Wen, In Situ Construction of a Lithiophilic and Electronically Insulating Multifunctional Hybrid Layer Based on the Principle of Hydrolysis for a Stable Garnet/Li Interface, Adv. Funct. Mater., 2024, 34, 2402971 Search PubMed.
- C. Ji, S. Zhou, L. Cai, Y. Yuan, X. Liu, P. Huang and X. Xiong, Minimizing Surface Defects for High-Performance Garnet-Based Solid-State Li Metal Batteries, Chem. Eng. J., 2025, 506, 160161 Search PubMed.
- C. Zhang, J. Yu, Y. Cui, Y. Lv, Y. Zhang, T. Gao, Y. He, X. Chen, T. Li, T. Lin, Q. Mi, Y. Yu and W. Liu, An Electron-Blocking Interface for Garnet-based Quasi-Solid-State Lithium-Metal Batteries to Improve Lifespan, Nat. Commun., 2024, 15, 5325 Search PubMed.
- J. Liu, S. Song, J. Wang, X. Mei and H. Zhao, Electron Insulative Interface Based on Schottky Contact Enabling Dendrite-free Solid-state Lithium Metal Batteries, Adv. Funct. Mater., 2025, 35, 2505836 Search PubMed.
- J. Liu, K. Zha, S. Zhang, Q. Deng, N. Zhang, X. Lu and B. Wei, Interfacial Engineering of Garnet-Type Electrolytes for Solid-State Lithium Metal Batteries, ACS Appl. Energy Mater., 2025, 8, 7300–7309 Search PubMed.
- L. Zhai, J. Wang, X. Zhang, X. Zhou, F. Jiang, L. Li and J. Sun, Interface Engineering of Li6.75La3Zr1.75Ta0.25O12 via in situ Built LiI/ZnLix Mixed Buffer Layer for Solid-State Lithium Metal Batteries, Chem. Sci., 2024, 15, 7144–7149 Search PubMed.
- J. Wang, X. Han, Y. Feng, S. Chen, H. Yuan, R. Yang, W. Du, C. Hou, X. Liu, T. Tong, W. Zhang, F. Jiang, J. Sun and X. Zhang, In-Situ Construction of a Composite Interlayer for Dendrite-Free Li6.75La3Zr1.75Ta0.25O12 Solid-State Batteries, Compos. Commun., 2024, 46, 101851 Search PubMed.
- X. Bai, G. Zhao, G. Yang, M. Wang, Q. Lin and N. Zhang, Multifunctional Double Layer Based on Regional Segregation for Stabilized and Dendrite-Free Solid-State Li Batteries, Adv. Energy Mater., 2024, 14, 2304112 CrossRef CAS.
- M. Gao, P. Li, S. Fu, S. Yu, Y. Hu, D. Chen, Y. Wei, D. Li, N. Wang, L. Yang and Y. Chen, Constructing a Li2O/LiZn Mixed Ionic Electron Conductive Layer by Ultrasonic Spraying to Enhance Li/Garnet Solid Electrolyte Interface Stability for Solid-State Batteries, ACS Appl. Mater. Interfaces, 2024, 16, 69253–69261 CrossRef CAS PubMed.
- Y. Yuan, Q. Liu, C. Ji, Z. Xiang, S. Feng and X. Xiong, Artificial Interface Engineering to Achieve High-Performance Garnet-Based Solid-State Lithium Metal Batteries, J. Mater. Chem. A, 2025, 13, 6804–6812 Search PubMed.
- M. Wu, G. Xu, F. Liu, J. Ke, H. Bao, Q. Wang and Z. Ali, Alleviating the Local Charge Accumulation at Li/Garnet Interface Enabling Solid-State Lithium Batteries, Chem. Eng. J., 2024, 500, 157128 CrossRef CAS.
- B. Hao, W. Chen, J. Wu, Z.-J. Jiang, X. Chen and Z. Jiang, Long-Term Cycling Stability and Dendrite Suppression in Garnet-Type Solid-State Lithium Batteries via Plasma-Induced Artificial SEI Layer Formation, Adv. Funct. Mater., 2025, 35, 2502429 Search PubMed.
- J. Meng, Y. Zhang, X. Zhou, M. Lei and C. Li, Li2CO3-Affiliative Mechanism for Air-Accessible Interface Engineering of Garnet Electrolyte via Facile Liquid Metal Painting, Nat. Commun., 2020, 11, 3716 Search PubMed.
- W. Ji, B. Luo, Z. Zhang, Y. Tian, Q. Wang, G. Yu, X. He, Z. Liu, Z. Zhao, R. Zhao, S. Wang, J. Zheng, X. Wang, B. Zhang, J. Zhang, J. Liang and R. Jin, Liquid Metal-Driven Interfacial Electrochemistry: Phase Evolution Mechanisms for Enhanced Li/LLZTO Contact at Room Temperature, Energy Storage Mater., 2025, 81, 104488 Search PubMed.
- S. Yu, Z. Gong, M. Gao, J. Li, W. Xie, Y. Wei, D. Li, L. Yang, D. Chen, Y. Li and Y. Chen, In-Situ Construction of Nano-Multifunctional Interlayer to Obtain Intimate Li/Garnet Interface for Dendrite-Free All Solid-State Battery, J. Mater. Sci. Technol., 2025, 206, 248–256 Search PubMed.
- J. Leng, H. Liang, H. Wang, Z. Xiao, S. Wang, Z. Zhang and Z. Tang, A Facile and Low-Cost Wet-Chemistry Artificial Interface Engineering for Garnet-Based Solid-State Li Metal Batteries, Nano Energy, 2022, 101, 107603 CrossRef CAS.
- S.-S. Chi, Y. Liu, N. Zhao, X. Guo, C.-W. Nan and L.-Z. Fan, Solid Polymer Electrolyte Soft Interface Layer with 3D Lithium Anode for All-Solid-State Lithium Batteries, Energy Storage Mater., 2019, 17, 309–316 CrossRef.
- X. Ma, Y. Xu, B. Zhang, X. Xue, C. Wang, S. He, J. Lin and L. Yang, Garnet Si-Li7La3Zr2O12 Electrolyte with a Durable, Low Resistance Interface Layer for All-Solid-State Lithium Metal Batteries, J. Power Sources, 2020, 453, 227881 CrossRef CAS.
- Z. Bi, W. Huang, S. Mu, W. Sun, N. Zhao and X. Guo, Dual-Interface Reinforced Flexible Solid Garnet Batteries Enabled by In-Situ Solidified Gel Polymer Electrolytes, Nano Energy, 2021, 90, 106498 Search PubMed.
- L. Chen, Z. Huang, W. Pang, Z. Jin, Y. Li and C.-A. Wang, Dual Interface Layers for Solid-State Li Metal Battery with Low Interfacial Resistance and Small Polarization Based on Garnet Electrolyte, Electrochim. Acta, 2020, 330, 135352 CrossRef CAS.
- J. Li, H. Zhang, Y. Cui, H. Da, Y. Cai and S. Zhang, Ultra-Stable High Voltage Lithium Metal Batteries Enabled by Solid Garnet Electrolyte Surface-Engineered with a Grafted Aromatics Layer, Chem. Eng. J., 2022, 450, 138457 Search PubMed.
- G. Yang, X. Bai, Y. Zhang, Z. Guo, C. Zhao, L. Fan and N. Zhang, A Bridge between Ceramics Electrolyte and Interface Layer to Fast Li+ Transfer for Low Interface Impedance Solid-State Batteries, Adv. Funct. Mater., 2023, 33, 2211387 CrossRef CAS.
- C. Zheng, Y. Lu, Q. Chang, Z. Song, T. Xiu, J. Jin, M. E. Badding and Z. Wen, High-Performance Garnet-Type Solid-State Lithium Metal Batteries Enabled by Scalable Elastic and Li+-Conducting Interlayer, Adv. Funct. Mater., 2023, 33, 2302729 CrossRef CAS.
- G. Zhao, W. Liu, B. Zhang, Z. Zhao, W. Huang, Y. Xu, J. Fang, T. Li and Y. Xu, Ion-Conducting Self-Healing Interlayer Enabling Ultrastable Li7La3Zr2O12-Lithium Interphase for High Performance Solid Lithium Metal Batteries, Renewables, 2025, 3, 21–30 Search PubMed.
- A. Gutiérrez-Pardo, F. Aguesse, F. Fernández-Carretero, A. I. Siriwardana, A. García-Luis and A. Llordés, Improved Electromechanical Stability of the Li Metal/Garnet Ceramic Interface by a Solvent-Free Deposited OIPC Soft Layer, ACS Appl. Energy Mater., 2021, 4, 2388–2397 CrossRef.
- G. Wang, C. Wei, X. Liu and W. Ding, Efficient Lithium Dendrite Control and Intimate Li/Garnet Interface Built through a Carbonized Metal-Organic Framework Layer, ACS Appl. Energy Mater., 2024, 7, 2278–2284 CrossRef CAS.
- Z. Li, X. Wang, X. Lin, X. Ou, J. Luo, Z. Chen, A. Li, J. Zhang, X. Wang and R. Zhao, Ferroelectric Nanorods as a Polymer Interface Additive for High-Performance Garnet-Based Solid-State Batteries, ACS Appl. Mater. Interfaces, 2023, 15, 35684–35691 Search PubMed.
- Y. Liu, J. Meng, M. Lei, Y. Yu, C. Lai and C. Li, Alloyable Viscous Fluid for Interface Welding of Garnet Electrolyte to Enable Highly Reversible Fluoride Conversion Solid State Batteries, Adv. Funct. Mater., 2023, 33, 2208013 Search PubMed.
- L. Zhang, N. Wang, J. Wang, Y. Dang, R. Zheng, Z. Wang, J. Jiang, Y. Cui, Y. Wu, H. Sun, Q. Zhuang, Y. Liu and X. Sun, A Rigid-Flexible Gel Interlayer with Li+-Conducting/e−-Insulating Properties for Dendrite-Free Solid-State Lithium Metal Batteries, Mater. Today, 2025, 87, 77–89 Search PubMed.
- M. Cai, J. Jin, T. Xiu, Z. Song, M. E. Badding and Z. Wen, In-situ Constructed Lithium-Salt Lithiophilic Layer Inducing Bi-Functional Interphase for Stable LLZO/Li Interface, Energy Storage Mater., 2022, 47, 61–69 Search PubMed.
- K. Wang and D. Mollenhauer, First-Principles Studies on the Structure and Stability of the Solid Electrolyte Interphase with LiPON in Solid-State Batteries, J. Phys. Chem. C, 2025, 129, 13160–13168 Search PubMed.
- S. J. Turrell, Y. Liang, T. Cai, B. Jagger and M. Pasta, Origin of Stability in the Solid Electrolyte Interphase formed between Lithium and Lithium Phosphorus Oxynitride, Chem. Mater., 2025, 37, 3504–3518 CrossRef CAS PubMed.
- S. Tu, B. Zhang, Y. Zhang, Z. Chen, X. Wang, R. Zhan, Y. Ou, W. Wang, X. Liu, X. Duan, L. Wang and Y. Sun, Fast-charging capability of graphite-based lithium-ion batteries enabled by Li3P-based crystalline solid–electrolyte interphase, Nat. Energy, 2023, 8, 1365–1374 CrossRef CAS.
- Y. Xia, G. Mao, T. Yao, J. Yang, Y. Zhou, K. Lin, Z. Xu, D. Chen, H. Shao and L. Shen, One-Step Biphasic Interfacial Engineering Stabilizes Single-Crystal Ultrahigh-Nickel Cathodes, Adv. Funct. Mater., 2025, e13107 CrossRef.
- Z. Gu, D. Song, S. Luo, H. Liu, X. Sun, L. Zhu, W. Ma and X. Zhang, Insights into the Anode-Initiated and Grain Boundary-Initiated Mechanisms for Dendrite Formation in All-Solid-State Lithium Metal Batteries, Adv. Energy Mater., 2023, 13, 2302945 CrossRef CAS.
- M. Wu, J. Ke, C. Tan, F. Liu, X. Sun and G. Xu, Depolarization-Induced Potential Equilibrium Reduces Electron Accumulation to Improve Interfacial Compactness of Garnet Electrolyte with Li Metal, Electrochim. Acta, 2025, 536, 146822 CrossRef CAS.
- Y. Zhao, X. Lin, Y. Yang, X. Wu, Z. Hu, M. Hua and P. Si, Economic and Effective Construction of a P/Sn Multifunctional Layer on the Li/Garnet Interface via Transformation Reactions, ACS Sustainable Chem. Eng., 2024, 12, 7092–7104 Search PubMed.
- Y. Xu, Y. Guo, X. Zhang, G. Zhang, K. Fang, Q. Peng, X. Zhang, X. Sun, K. Wang and Y. Ma, Tailoring Highly Ion-Conductive and Stabled PVDF-Based Solid Electrolyte via Surface Coordination Chemistry, Adv. Funct. Mater., 2025, 35, 2422461 CrossRef CAS.
- L. Zhao, Y. Du, C. Wang, D. Li, H. Li and Y. Zhao, Rationally Coordinating Polymer Enabling Effective Li-Ion Percolation Network in Composite Electrolyte for Solid-State Li-Metal Batteries, Energy Storage Mater., 2024, 68, 103360 CrossRef.
- M. B. Dixit, B. S. Vishugopi, W. Zaman, P. Kenesei, J.-S. Park, J. Almer, P. P. Mukherjee and K. B. Hatzell, Polymorphism of garnet solid electrolytes and its implications for grain-level chemo-mechanics, Nat. Mater., 2022, 21, 1298–1305 CrossRef CAS PubMed.
- Y. Guo, S. Pan, X. Yi, S. Chi, X. Yin, C. Geng, Q. Yin, Q. Zhan, Z. Zhao, F.-M. Jin, H. Fang, Y.-B. He, F. Kang, S. Wu and Q.-H. Yang, Fluorinating All Interfaces Enables Super-Stable Solid-State Lithium Batteries by In Situ Conversion of Detrimental Surface Li2CO3, Adv. Mater., 2024, 36, 2308493 CrossRef CAS PubMed.
- X. Yin, Y. Guo, S. Chi, Y. Jia, F. Li, J. Qi, X. Yi, S. Wu and Q.-H. Yang, Beyond Polymerization: In Situ Coupled Fluorination Enables More Stable Interfaces for Solid-State Lithium Batteries, J. Am. Chem. Soc., 2025, 147, 4393–4402 CrossRef CAS PubMed.
- Z. Zhao, Z. Wen, X. Liu, H. Yang, S. Chen, C. Li, H. Lv, F. Wu, B. Wu and D. Mu, Tuning a Compatible Interface with LLZTO Integrated on Cathode Material for Improving NCM811/LLZTO Solid-State Battery, Chem. Eng. J., 2021, 405, 127031 CrossRef CAS.
- Y. Fu, X. C. Zhang, F. Z. Xuan, S. T. Tu and Z. D. Wang, Multiple cracking of thin films due to residual stress combined with bending stress, Comput. Mater. Sci., 2013, 73, 113–119 CrossRef CAS.
- L. Guan, Y. Shi, C. Gao, T. Wang, J. Zhou and R. Cai, Interfacial contact loss and bending effects on electrochemical-mechanical modeling for all-solid-state Li-ion batteries, Electrochim. Acta, 2023, 440, 141669 CrossRef CAS.
- S. H. Kang, H. Lee, Y.-J. Hong, S. Myoung, H. Seo, J. Choi, S. Yoon, J. Y. Kim, D. O. Shin, M. J. Lee, Y.-S. Park, Y.-G. Lee and Y. M. Lee, High-Performance, Roll-to-Roll Fabricated Scaffold-Supported Solid Electrolyte Separator for Practical All-Solid-State Batteries, Small, 2025, 21, 2502996 CrossRef CAS PubMed.
- J. Park, J. Kim, J. Kim, M. Kim, T. Song and U. Paik, Sustainable and cost-effective electrode manufacturing for advanced lithium batteries: the roll-to-roll dry coating process, Chem. Sci., 2025, 16, 6598–6619 Search PubMed.
- K. H. Ayalew, N. Palaniyandy, M. K. Mathe and P. F. Msomi, Garnet-type LLZO electrolytes for solid-state lithium batteries: Interfaces, conductivity, in-situ processing, and industrial prospects, Chem. Eng. J., 2025, 524, 168098 Search PubMed.
- W. Huang, Z. Bi, N. Zhao, Q. Sun and X. Guo, Chemical Interface Engineering of Solid Garnet Batteries for Long-Life and High-Rate Performance, Chem. Eng. J., 2021, 424, 130423 Search PubMed.
- X. Liu, X. Kong, W. Xiang, Y. Jiang, B. Xiong, W. Ping, C. Xia, D. Huan and C. Wang, LiCoO2 Sintering Aid Towards Cathode-Interface-Enhanced Garnet Electrolytes, J. Energy Chem., 2023, 84, 181–188 CrossRef CAS.
- F. Han, J. Yue, C. Chen, N. Zhao, X. Fan, Z. Ma, T. Gao, F. Wang, X. Guo and C. Wang, Interphase Engineering Enabled All-Ceramic Lithium Battery, Joule, 2018, 2, 497–508 Search PubMed.
- H. Hosokawa, A. Takeda, R. Inada and Y. Sakurai, Tolerance for Li Dendrite Penetration in Ta-Doped Li7La3Zr2O12 Solid Electrolytes Sintered with Li2.3C0.7B0.3O3 Additive, Mater. Lett., 2020, 279, 128481 Search PubMed.
- K. Chen, Y. Shen, Y. Zhang, Y. Lin and C.-W. Nan, High Capacity and Cyclic Performance in a Three-Dimensional Composite Electrode Filled with Inorganic Solid Electrolyte, J. Power Sources, 2014, 249, 306–310 Search PubMed.
- Y. Fan, O. I. Malyi, H. Wang, X. Cheng, X. Fu, J. Wang, H. Ke, H. Xia, Y. Shen, Z. Bai, S. Chen, H. Shao, X. Chen, Y. Tang and X. Bao, Surface-Confined Disordered Hydrogen Bonds Enable Efficient Lithium Transport in All-Solid-State PEO-Based Lithium Battery, Angew. Chem., Int. Ed., 2025, 64, e202421777 CrossRef CAS PubMed.
- J. Li, J. Long, H. Du, J. Wang, W. Zhao, H. Gong, W. Zou, F. Wang, J. Shi, Y. Zhang, Z. Bai, O. I. Malyi and Y. Tang, Breaking Diffusion Limit in Ester-Flame-Proof Na-Ion Electrolytes Through Solvent Coordination Chemistry, Angew. Chem., Int. Ed., 2025, 64, e202512950 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |