 Open Access Article
Open Access ArticleAg-triggered Co4+ active sites enable OH* nucleophilic attack for efficient electrocatalytic oxidation of alcohols to acids
Yu-Wei Du,
Jian-Yu Zhang,
Hao-Jun Luo,
You Zhang,
Xi-Ting Zhang,
Ting Ouyang* and
Zhao-Qing Liu *
*
School of Chemistry and Chemical Engineering, Institute of Clean Energy and Materials, Key Laboratory for Clean Energy and Materials, Huangpu Hydrogen Innovation Center, Guangzhou University, Guangzhou 510006, P. R. China. E-mail: ouyt@gzhu.edu.cn; lzqgzhu@gzhu.edu.cn
First published on 26th December 2025
Abstract
The selective oxidation of primary alcohols to carboxylic acids is a widely employed transformation in organic chemistry. However, the intrinsic mechanism by which R(Ph)–OH (R is an alkyl or phenyl) in alcohols undergoes dehydrogenation to form oxygen-rich acids remains elusive. Here, we demonstrate an efficient cooperative interface structure (Ag/Co(OH)2) as an electrocatalyst. Ag/Co(OH)2 significantly lowers the energy barrier of the rate-determining step 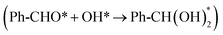 to 0.78 eV and enhances the chemisorption energy of the Ph-CHO* intermediate to −0.89 eV, both superior to Co(OH)2 (ΔG = 1.22 eV, Gads = −0.72 eV), and the faradaic efficiency for carboxylic acids reached 99.6% under mild conditions. Kinetic studies reveal the formation of Co4+–O species on the composite surface, promoting the capture of OH*. Furthermore, Ag/Co(OH)2 also demonstrates excellent faradaic efficiencies (ranging from 41.48% to 88.73%) in the oxidation of methanol, furfuryl alcohol, ethylene glycol, and 5-hydroxymethylfurfural to their corresponding carboxylic acids. This work provides a new idea for designing efficient and stable catalysts for electrocatalytic carboxylic acid synthesis.
to 0.78 eV and enhances the chemisorption energy of the Ph-CHO* intermediate to −0.89 eV, both superior to Co(OH)2 (ΔG = 1.22 eV, Gads = −0.72 eV), and the faradaic efficiency for carboxylic acids reached 99.6% under mild conditions. Kinetic studies reveal the formation of Co4+–O species on the composite surface, promoting the capture of OH*. Furthermore, Ag/Co(OH)2 also demonstrates excellent faradaic efficiencies (ranging from 41.48% to 88.73%) in the oxidation of methanol, furfuryl alcohol, ethylene glycol, and 5-hydroxymethylfurfural to their corresponding carboxylic acids. This work provides a new idea for designing efficient and stable catalysts for electrocatalytic carboxylic acid synthesis.
Introduction
As the problems of fossil fuel depletion and environmental degradation intensify, green hydrogen energy has received much attention due to its sustainability and low environmental impact.1,2 Driven by renewable electricity, water electrolysis is expected to be an ideal long-term low-carbon method for hydrogen production.3,4 However, the overpotential of the anodic oxygen evolution reaction (OER) is relatively high, which limits the hydrogen production efficiency of the overall water electrolysis process. Therefore, there is an urgent need to develop alternative reaction systems with lower overpotentials.5–8The catalytic oxidation of primary alcohols or primary aldehydes to carboxylic acids is one of the most important chemical transformations and is widely used in fine-chemical production.9 Currently, industrial oxidation processes still face the challenge of thermal management under high-temperature and high-pressure conditions. If the reaction heat cannot be effectively removed, it is likely to cause local overheating, leading to catalyst deactivation, reduced selectivity, and even safety issues such as a “runaway reaction”. Traditional Cr/Mn-based compounds and bromide catalysts are both toxic and costly to recover and dispose of, seriously hindering the development of green chemical processes. From the perspectives of thermodynamics and kinetics, electro-oxidation reactions involving primary alcohols or primary aldehydes can be carried out at a potential lower than that of the OER.10,11 Therefore, converting alcohol electrocatalysis into carboxylic acids under mild conditions provides a new approach to replacing the high overpotential electrolysis water OER. This method can also be combined with renewable energy sources, simultaneously producing high-value chemicals at the anode, thereby achieving efficient conversion of electrical energy into chemical energy.12–15 Electrocatalytic alcohol oxidation reactions involve multiple electrochemical steps (e.g. proton-coupled electron transfer (PCET)) and chemical steps (e.g. C–C cleavage).16,17 Through different reaction pathways, various products can be obtained. Furthermore, the electronic structure and steric hindrance of different alcohol molecules (such as aromatic alcohols and aliphatic alcohols) also affect the catalytic rate.18 Accordingly, developing efficient catalysts to regulate the oxidation processes of various alcohols and obtain the desired high-value chemicals remains a significant challenge.
Co(OH)2, as a highly promising anode catalyst, is rich in surface hydroxyl groups that can dehydrogenate from Co2+–OH to form Co3+–O species, acting as a proton mediator to efficiently drive the PCET process during alcohol oxidation.19–22 Furthermore, the Co3+-oxo species over CoOOH formed after the reconstruction of Co(OH)2 play a key role in the benzyl alcohol oxidation reaction (AOR, using BA as the substrate) process.23–25 However, Co(OH)2 has a limited range for regulating the adsorption strengths of the intermediates, which can easily lead to the desorption or over-oxidation of the reacting intermediates. In this process, the adsorption behavior of the reacting molecules on the catalyst directly affects the electro-oxidation efficiency. Notably, constructing a coupled active-metal-transition-metal compound support is an effective strategy to enhance the electrocatalytic activity.26,27 The construction of such heterogeneous interfaces enhances the electrical conductivity28 and optimizes the local coordination environments of the active sites and regulates the adsorption behavior,29–31 thus significantly improving the catalytic performance. Recently, nano-Ru-modified Ni(OH)2 enhanced the adsorption capacity for phenol by regulating the electronic structure of Ru sites, thereby achieving its efficient conversion into p-quinone.32 By utilizing the thermal and electronic plasmonic effects of Ag, Ag-modified CoV-LDH@G with Mott–Schottky heterojunctions showed significantly improved OER performance.33 In addition, Pd-Cu2O nanoparticles, with the enhanced adsorption of CO intermediates, achieved a significant improvement in the methanol oxidation reaction (MOR) activity.34 Certainly, it is crucial to enhance the catalytic performance for the catalytic oxidation of alcohols by rationally modulating the adsorption energies of key reaction intermediates on the catalyst surface.
In this work, we demonstrate a simple and feasible electrocatalytic strategy for the oxidation of primary alcohols over a silver-modified cobalt hydroxide (Ag/Co(OH)2) electrocatalyst. The AOR was selected as a model reaction to evaluate the catalytic performance. A series of electrochemical tests in alkaline solution revealed that Ag/Co(OH)2 exhibits high activity for the AOR, with high selectivity for the generation of benzoic acid (BAC) products (Fig. 1a and b). Experimental characterization and theoretical calculations show that silver modification could effectively modulate the surface charge transfer kinetics of the catalyst and induce the formation of high-valence active sites (Co4+–O). More importantly, Ag/Co(OH)2 exhibits superior performance over Co(OH)2 in terms of both the activation barrier of the rate-limiting step of the oxidation reaction (the combination of Ph-CHO* and OH*; 0.78 eV vs. 1.22 eV) and the adsorption energy of Ph-CHO at the interface (−0.89 eV vs. −0.72 eV), thereby achieving an faradaic efficiency (FE) of 99.6% for BAC under mild conditions (1.45 V vs. RHE). Impressively, the unique cooperative interface mechanism of Ag/Co(OH)2 can adapt to the electronic structures and spatial configurations of different alcohol molecules, also affording universal oxidation activity for other primary alcohols (methanol, furfuryl alcohol, ethylene glycol, and 5-hydroxymethylfurfural: the corresponding FEs for the acids can reach 41.48% to 88.73%).
Results and discussion
Catalyst synthesis and characterization
Ag/Co(OH)2 was synthesized on nickel foam (NF) via electrodeposition and spontaneous redox methods. Scanning electron microscopy (SEM) images show that Co(OH)2 is uniformly plated on NF with a dense nanosheet morphology. With the introduction of Ag, the sample is transformed into a complex structure of nanoparticles and ultrathin nanosheets stacked on top of each other (Fig. S1). High-resolution transmission electron microscopy (HRTEM) imaging exhibits the presence of both Ag (111) and Co(OH)2 (100) stripes corresponding to Ag/Co(OH)2 (Fig. S2). Also, XRD spectra show crystallographic peaks consistent with Ag and Co(OH)2 (PDF# 04-0783 and PDF# 51-1731) (Fig. S3), indicating the successful synthesis of Ag/Co(OH)2. The quantitative determination of Ag and Co in the catalyst was performed using inductively coupled plasma optical emission spectrometry (ICP-OES, Table S1). The results indicate that the content of Ag in the material is relatively low (5.19 at%). Energy-dispersive spectroscopy (EDS) further confirms that the elements Co and O are uniformly distributed, while Ag appears as blocky aggregates on top of Co (Fig. S4). The surface composition and electronic states of Co(OH)2 before and after Ag modification were examined by X-ray photoelectron spectroscopy (XPS). Compared to Co(OH)2, Ag modification promotes the formation of Co3+ and oxygen vacancies (Fig. 1c and S5).The X-ray absorption near-edge structure (XANES) spectra of Co are also displayed (Fig. 1d and S6a). The absorption edges of the white line peaks correlate with the 1s → 4p electronic transitions, reflecting the valence state information of the catalysts. Ag/Co(OH)2 and Co(OH)2 show white line peaks that are higher than CoO and lower than Co3O4 (wherein Co has an average oxidation state of +2.67), which implies that the average oxidation state of the Co atoms in Ag/Co(OH)2 and Co(OH)2 is between +2 and +3. Also, the higher intensity of the white line peak for Ag/Co(OH)2 confirms that the introduction of Ag increases the oxidation state of Co, which is consistent with the results from XPS. The Fourier transform extended X-ray absorption fine structure (FT-EXAFS) spectra show two distinct peaks at 1.6 Å and 2.8 Å, corresponding to the coordination structures of Co–O and Co–Co (Fig. 1e and S6b). The corresponding EXAFS fitting images in K and R space are illustrated (Fig. S7 and S8), and detailed numerical values are presented (Table S2). EXAFS analysis using wavelet transform (WT) provides the same evidence (Fig. 1f and S9).35 It is noteworthy that the coordination number of Co–O decreases from 6.2 to 5.9 after Ag introduction, suggesting the formation of oxygen vacancies, which matches the results of O 1s XPS analysis (Fig. S5a). In addition, Ag was also subjected to K-edge XANES measurements. The results indicate that the valence state of Ag lies between 0 and +1, and the main coordination structure is Ag–Ag (Fig. 1g–i, S10–S12, and Table S3).
Performance evaluation of the alcohol oxidation reaction
BA was selected as a model substrate for alcohol oxidation, and the performance of the catalysts in the AOR was evaluated using cobalt-based material as the anode and a platinum sheet electrode as the cathode. Linear scanning voltammetry (LSV) analysis was first performed. As shown in Fig. 2a, Ag/Co(OH)2 exhibits the earliest onset potential and a higher current density. The oxidation peak at 1.21 V vs. RHE was identified as the Co2+/Co3+ oxidation peak, which was related to the oxidation of Co(OH)2 to CoOOH. For Co(OH)2, the oxidation peak appeared at 1.25 V vs. RHE, suggesting that Ag modification promotes the electro-oxidation of Co2+–OH to Co3+–OH. Cyclic voltammetry (CV) curves exhibit the same results (Fig. 2b and S13).Within the potential range of 1.2–1.6 V vs. RHE, the AOR current density of Ag/Co(OH)2 is higher than that of Co(OH)2 (Fig. 2c), further confirming that the promoting effect of Ag modification enhances the electrocatalytic activity. In addition, the Tafel slopes over a potential range are displayed in Fig. 2d. The Tafel value of Ag/Co(OH)2 (325 mV dec−1) is lower than that of Co(OH)2 (501 mV dec−1). These results, together with electrochemical impedance spectroscopy (EIS) measurements at the open-circuit potential, indicate that the introduction of Ag could accelerate the reaction kinetics (Fig. S14). In general, the double-layer capacitance (Cdl) is directly related to the electrochemically active surface area (ECSA), with a larger Cdl value indicating a larger ECSA. The Cdl values of Ag/Co(OH)2 are significantly higher than those of Co(OH)2 (Fig. S15), indicating that the introduction of silver effectively enhanced the ECSA of the material, thereby improving its catalytic performance. Moreover, the Cdl values of both catalysts measured in 1.0 M KOH are higher than their corresponding values in 1.0 M KOH + 0.1 M BA. This might be due to the adsorption of BA on the catalyst surface at the measurement potential, partially covering active sites available for double-layer charging. The zeta potential reflects the potential at the slipping plane between a particle and a solution. This is affected by pH, as a change in pH alters the protonation state of the functional groups on a particle's surface.36,37 The pH at which the zeta potential is zero is the isoelectric point (IEP). As seen in Fig. 2e, the IEP of Ag/Co(OH)2 (∼pH 10) is higher than that of Co(OH)2 (∼pH 9), maintaining a more positive and stable zeta potential in the pH > 10 range. Notably, the zeta potential of Co(OH)2 exhibits a non-monotonic change (positive → negative → positive) with an increase in pH, potentially due to surface remodelling under strongly alkaline conditions, whereas Ag/Co(OH)2 shows a monotonically positive shift. This suggests that introducing Ag enhances the stability of the hydroxyl groups on the surface of Co(OH)2 in alkaline environments and increases the positive electronegativity of the surface by optimizing the interfacial charge, thus promoting the adsorption of OH−.
The electrocatalytic activities of Co(OH)2 and Ag/Co(OH)2 in the AOR were investigated using 1.0 M KOH + 0.1 M BA as the electrolyte, and the post-oxidation products were detected by gas chromatography-mass spectrometry (GC-MS). The electrolyte after 12 h of reaction at 1.4 V vs. RHE was collected and analysed (Fig. S16). Clearly, the Co(OH)2-catalyzed product has the presence of both benzaldehyde (BAD) and BAC, while the Ag/Co(OH)2 product is dominated by BAC, indicating that modification with Ag might affect the desorption of intermediate products and thus achieve the highly selective oxidation of BA to BAC. The amount of Ag modification of the catalyst was optimized using LSV and GC-MS data (Fig. S17 and S18). When the concentration of Ag+ is 13.3 mM, the catalyst shows the best AOR performance at high potential with faster product formation (larger GC-MS peak area). In order to further quantify the reactants and products, standard curves for BA, BAD and BAC were made (Fig. S19). The yields and FEs of BAC in the voltage range of 1.4–1.6 V vs. RHE are shown in Fig. 2f and g. The yield increases with an increase in potential; however, the FE decreases with an increase in potential; a possible reason is that at higher potentials, the OER competes with the AOR and the charge is dispersed. Therefore, it is necessary to select a suitable potential to minimize the effects of OER competition. 1.45 V vs. RHE is the optimal potential, where the yield of BAC reaches 77.7% and the FE is 99.6%. After 12 h of the AOR reaction, the structure of the Ag/Co(OH)2 catalyst was characterized (Fig. S20–S22). The XRD results show that the intensity of the characteristic diffraction peaks of Ag metal weakened, indicating that Ag undergoes a chemical-state or dispersion-state transformation under the electrocatalysis conditions. EDS mapping and XPS analysis confirm that the Ag element does not leach out, and it remains uniformly distributed. The intensity of the oxygen vacancy-related peak in the O 1s XPS spectrum increases significantly after the reaction, indicating a higher concentration of surface oxygen defects. These oxygen vacancies and the highly dispersed Ag species form a more efficient catalytic interface, thereby supporting the performance of Ag/Co(OH)2 in maintaining a high FE during the long-term reaction. The Ag/Co(OH)2 catalyst exhibits a lower onset potential compared to other reported AOR catalysts (Fig. 2h and Table S4).11,13,38–49
Mechanistic elucidation
To further investigate the adsorption behavior of BA on the catalyst, open-circuit potential testing was performed. As seen in Fig. 3a, the potential drop of Ag/Co(OH)2 (0.30 V) is higher than that of Co(OH)2 (0.22 V) upon the addition of 0.1 M BA at 200 s, indicating that the BA better adsorbs on Ag/Co(OH)2. This helps to optimize the initial reaction steps and facilitate the subsequent PCET process. To further verify that modification with Ag could modulate the product selectivity of the AOR, the catalytic performance of the catalysts in 1.0 M KOH + 0.1 M BAD was also investigated. Ag/Co(OH)2 exhibits higher oxidizing activity toward BAD compared with Co(OH)2, which promotes further oxidation of the intermediate product (Fig. 3b and c). Next, the oxidation processes occurring at the catalyst interface during the OER and AOR were monitored and analyzed using in situ EIS. For Co(OH)2, the phase angle gradually decreases with increasing voltage (0.8–1.1 V vs. RHE) in the high frequency region, which is associated with a phase transition process from Co(OH)2 to CoOOH inside the catalyst. When the voltage exceeds 1.4 V vs. RHE, the phase angle in the low-frequency region gradually decreases, corresponding to the progression of the OER. Upon the addition of BA, the phase transition in the low-frequency region was advanced to 1.3 V vs. RHE (Fig. 3d and S23), consistent with the LSV results in Fig. 2a. As seen in Fig. 3e, Ag/Co(OH)2 displays a smaller phase angle at all potentials, which indicates faster reaction kinetics, i.e., faster charge transfer, at its surface. Moreover, during the AOR, Co(OH)2 shows a passivation signal at a high potential of 1.6 V vs. RHE, which did not occur at the Ag/Co(OH)2 interface. This might be due to the slower AOR kinetics at the Co(OH)2 interface compared to Ag/Co(OH)2. At high oxidation potentials, such kinetic limitation promotes the accumulation of OH+ oxide species, eventually leading to the formation of a CoOOH passivation layer.In order to investigate the interfacial structure evolution of the catalysts during the reaction process, in situ Raman spectroscopy tests were performed (Fig. 3f–i and S24). During the OER with the Ag/Co(OH)2 catalyst, two characteristic peaks at 484 cm−1 and 602 cm−1 are observed at the OCP, which may be attributed to the Eg and A1g modes of CoOOH, respectively.10,35 With an increase in the applied potential, a new peak appears at 425 cm−1, and the signal could be clearly assigned to the characteristic vibrations of CoO2 or CoO2-like species,17,20 indicating that the catalyst surface undergoes a transition from a Co3+ to a Co4+ oxidation state. Notably, when BA is introduced into the electrolyte, the intensity of the Co4+-related signal at 425 cm−1 gradually decreases with increasing potential, suggesting that the oxidation of BA consumes Co4+ active species, leading to their reduction to a lower valence state. This result is further corroborated by the attenuation of the intensity of the characteristic peak of the benzene ring at 1000 cm−1,50 confirming the depletion of BA molecules in the reaction. In contrast, the Raman spectra of Co(OH)2 catalysts in the same potential interval (OER and AOR systems) do not show significant changes, and their peak positions and intensities remain stable, indicating that the material does not undergo any obvious valence or phase structure transitions under the reaction conditions. These results highlight the key regulatory role of Ag modification on the electronic structure and catalytic activity of Co(OH)2. The adsorption behavior of the catalyst during the AOR process was investigated by in situ Fourier transform infrared spectroscopy (FTIR) at a constant potential of 1.45 V vs. RHE (Fig. 3j and k). The characteristic peak at 1697 cm−1 is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of BAD.51–53 After Ag modification, a new peak, gradually intensifying over time, at 1540 cm−1 is observed, which could be attributed to the asymmetric stretching vibration of the –C(O)OH group in BAC.54 This phenomenon confirms that Ag/Co(OH)2 could further oxidize the Ph-CHO intermediate to BAC.
O stretching vibration of BAD.51–53 After Ag modification, a new peak, gradually intensifying over time, at 1540 cm−1 is observed, which could be attributed to the asymmetric stretching vibration of the –C(O)OH group in BAC.54 This phenomenon confirms that Ag/Co(OH)2 could further oxidize the Ph-CHO intermediate to BAC.
Density functional theory calculations
In order to deeply investigate the reaction mechanism and C–H bond activation mechanism of the AOR over Ag/Co(OH)2, density functional theory (DFT) calculations were performed. First, Co(OH)2 (100) pure surface and Ag-modified Co(OH)2 (100) surface models were constructed for simulating the interfacial effects in the AOR reaction (Fig. S25). Density of states (DOS) analysis reveals that compared to pure Co(OH)2, the d-electrons of Co in Ag/Co(OH)2 are more concentrated near the Fermi level, suggesting a higher valence electron density, improved conductivity, and enhanced electron transfer capabilities (Fig. 4a and b).55,56 Moreover, the d-electron orbitals of Co in Ag/Co(OH)2 exhibit greater dispersion, which means that it has a wider bonding range and the electrons could participate in the reaction more easily. Additionally, the calculated results show that the adsorption energies (Eads) of both BA and BAD on the Ag/Co(OH)2 surface are significantly higher than those on the pure Co(OH)2 surface (Fig. 4c and Tables S5, S6), and this result is in agreement with the data from the open-circuit potential and LSV tests (Fig. 3a–c). This suggests that Ag/Co(OH)2 has a stronger adsorption affinity for BAD, leading to the lower desorption of BAD, which is more favorable for the subsequent reaction.In order to gain insight into the effect of the introduction of Ag on catalyst performance, we investigated in detail the reaction mechanism of the conversion from BA to BAC and the corresponding energy changes. The reaction starts with the co-adsorption of Ph-CH2OH and OH* in the two models, which produces Ph-CHO* after two dehydrogenation steps. Subsequently, Ph-CHO* hydrates in water to form 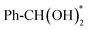 , which then undergoes dehydrogenation to produce Ph-COOH. In the reaction pathway, the potential-determining step (PDS) is the hydration process, with energy barriers of 1.223 eV and 0.782 eV at the Co(OH)2 and Ag/Co(OH)2 sites, respectively (Fig. 4d, e, S26, and Tables S7, S8). The introduction of Ag significantly reduces the PDS energy barrier, thus promoting the formation of key intermediates.
, which then undergoes dehydrogenation to produce Ph-COOH. In the reaction pathway, the potential-determining step (PDS) is the hydration process, with energy barriers of 1.223 eV and 0.782 eV at the Co(OH)2 and Ag/Co(OH)2 sites, respectively (Fig. 4d, e, S26, and Tables S7, S8). The introduction of Ag significantly reduces the PDS energy barrier, thus promoting the formation of key intermediates.
Substrate scope expansion
Having established that Ag can enhance the adsorption of Ph-CHO* intermediates and promote highly selective acid production from alcohols, we further demonstrated the oxidative versatility of the catalyst using other monohydric alcohols (methanol (MeOH), furfuryl alcohol (FFA), ethylene glycol (EG), and 5-hydroxymethylfurfuryl aldehyde (HMF)) (Fig. 5). The catalytic applicability is initially assessed by LSV, followed by the definitive verification of carboxylic acid formation via 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and high-performance liquid chromatography (HPLC) (Fig. S27–S30). Notably, no characteristic peaks corresponding to *CHO intermediates were detected, suggesting that potential aldehyde intermediates were rapidly oxidized under the catalytic conditions. Based on the corresponding standard curves (Fig. S31–S34), quantitative calculations were performed for the conversion rates of alcohols, the yields of acids, and their purity. The average values obtained from three parallel experiments are given (Fig. 5 and S35), and the standard deviations are listed (Table S9). At 1.45 V vs. RHE, Ag/Co(OH)2 exhibits excellent catalytic performance in the oxidation reactions of several alcohols: MeOH conversion reaches 56.06% with a formic acid (FA) yield of 21.77% (FE: 41.48%); FFA shows 97.30% conversion yielding 67.11% furoic acid (FUA; FE: 75.03%); EG achieves 53.97% conversion, producing both glycolic acid (GA; yield: 6.58%, FE: 12.30%) and FA (yield: 41.18%, FE: 55.43%); while HMF exhibits near-quantitative conversion (∼100%) with a 2,5-furandicarboxylic acid yield of 83.30% (FDCA; FE: 88.73%). These results show that the designed Ag-modified Co(OH)2 catalysts exhibit excellent versatility for the synthesis of other carboxylic acids.Conclusions
In summary, we present a highly active and low-cost Ag-modified Co(OH)2 catalyst for the efficient electrocatalytic oxidation of alcohols to carboxylic acids. By enhancing conductivity and forming high-valence Co active sites (Co4+–O), the AOR oxidation current of Ag/Co(OH)2 significantly increases, resulting in rapid AOR kinetics over a wide potential range and achieving a BAC FE of 99.6% at 1.45 V vs. RHE. In situ electrochemical experimental results and DFT calculations indicate that Ag modification facilitates electron transfer from alcohol to the catalyst and strengthens the adsorption and activation of the key intermediate (Ph-CHO*). The rate-determining step for Ag/Co(OH)2 is the transformation from Ph-CHO* + OH* to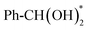 , which has an energy barrier of 0.782 eV. This barrier is significantly lower than that on Co(OH)2 (1.223 eV), indicating the high selectivity toward BAC achieved with the Ag/Co(OH)2 catalyst. Furthermore, Ag/Co(OH)2 can also catalyze the conversion of MeOH, FFA, EG and HMF to the corresponding carboxylic acids, with FEs ranging from 41.48% to 88.73%, demonstrating the universality of this catalyst strategy for regulating the adsorption energies of intermediates.
, which has an energy barrier of 0.782 eV. This barrier is significantly lower than that on Co(OH)2 (1.223 eV), indicating the high selectivity toward BAC achieved with the Ag/Co(OH)2 catalyst. Furthermore, Ag/Co(OH)2 can also catalyze the conversion of MeOH, FFA, EG and HMF to the corresponding carboxylic acids, with FEs ranging from 41.48% to 88.73%, demonstrating the universality of this catalyst strategy for regulating the adsorption energies of intermediates.
Author contributions
Z. Liu and T. Ou Yang: conceived and supervised the research, funding acquisition, writing – review & editing. Y. Du: conducted the experiments, analyzed the data, and wrote the manuscript. J. Zhang, H. Luo and Y. Zhang: assisted with the material synthesis and characterization. X. Zhang: supported supervising and reviewing the manuscript. All authors contributed to the interpretation of the results.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the findings of this work are available within the article and its supplementary information (SI). Supplementary information: details of Experimental section including materials and electrochemical measurement; zeta potential measurements; standard curves of product analysis, calculated faradaic efficiencies; in situ FT-IR; in situ Raman; XAFS measurement; calculation method. See DOI: https://doi.org/10.1039/d5sc09305h.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 22379033, U24A20541 and 22278094), Guangdong Basic and Applied Basic Research Foundation (No. 2025B1515020046), Guangzhou Science and Technology Program (No. 2025A03J0011), Basic and Applied Basic Research Program of Guangzhou (No. 2024A03J0236), and Science and Technology Research Project of Guangzhou (No. 202201020214). We would like to thank Analysis and Test Center of Guangzhou University for their technical support.Notes and references
- N. Johnson, M. Liebreich, D. M. Kammen, P. Ekins, R. McKenna and I. Staffell, Nat. Rev. Clean Technol., 2025, 1, 351–371 CrossRef.
- S. W. Boettcher, Chem. Rev., 2024, 124, 13095–13098 CrossRef CAS.
- X. Zhang, C. Cao, T. Ling, C. Ye, J. Lu and J. Shan, Adv. Energy Mater., 2024, 14, 2402633 CrossRef CAS.
- R. Wan, T. Yuan, L. Wang, B. Li, M. Liu and B. Zhao, Nat. Catal., 2024, 7, 1288–1304 CrossRef CAS.
- J. Li and H. Duan, Chem, 2024, 10, 3008–3039 CAS.
- X. Huang, O. Akdim, M. Douthwaite, K. Wang, L. Zhao, R. J. Lewis, S. Pattisson, I. T. Daniel, P. J. Miedziak, G. Shaw, D. J. Morgan, S. M. Althahban, T. E. Davies, Q. He, F. Wang, J. Fu, D. Bethell, S. McIntosh, C. J. Kiely and G. J. Hutchings, Nature, 2022, 603, 271–275 Search PubMed.
- Z. Yang, L. Chen, Y. Yin, C. Wei, Z. Xue and T. Mu, Energy Environ. Sci., 2024, 17, 8801–8809 RSC.
- L. Chen, Z. Yang, Q. Hu, C. Yan, Y. Yao, Y. Bao, Z. Pei, T. Mu and Z. Xue, Angew. Chem., Int. Ed., 2025, 137, e202511868 CrossRef.
- J. Zhang, Y. Shen, Z. Wu, X. Zhang, J. Kang, Y. Wu, S. Zhang, S. Chen, G. Wang, H. Zhang, H. Yin and H. Zhao, Angew. Chem., Int. Ed., 2025, 64, e202423109 CrossRef CAS PubMed.
- Y. Lin, Y. Chen, H. Ren, Y. Sun, J. Chen, M. Wu and Z. Li, Adv. Funct. Mater., 2024, 34, 2404594 CrossRef CAS.
- H. Huang, C. Yu, X. Han, H. Huang, Q. Wei, W. Guo, Z. Wang and J. Qiu, Energy Environ. Sci., 2020, 13, 4990–4999 RSC.
- Z. Wang, J. Li, Q. Zhang, C. Wu, H. Meng, Y. Tang, A. Zou, Y. Zhang, R. Ma, X. Lv, Z. Yu, S. Xi, J. Xue, X. Wang and J. Wu, Angew. Chem., Int. Ed., 2024, 63, e202411517 CrossRef CAS.
- S. Li, S. Wang, Y. Wang, J. He, K. Li, J. B. Gerken, S. S. Stahl, X. Zhong and J. Wang, Nat. Commun., 2025, 16, 266 CrossRef CAS.
- J. N. Hausmann, P. V. Menezes, G. Vijaykumar, K. Laun, T. Diemant, I. Zebger, T. Jacob, M. Driess and P. W. Menezes, Adv. Energy Mater., 2022, 12, 2202098 CrossRef CAS.
- S. Han, L. Sun, D. Fan and B. Liu, Nat. Commun., 2025, 16, 3426 CrossRef CAS.
- Y. Yan, J. Zhong, R. Wang, S. Yan and Z. Zou, J. Am. Chem. Soc., 2024, 146, 4814–4821 CrossRef CAS.
- L. Wu, Q. Wu, Y. Han, D. Zhang, R. Zhang, N. Song, Y. Fang, H. Liu, M. Wang, J. Chen, A. Du, K. Huang and X. Yao, J. Am. Chem. Soc., 2025, 147, 18033–18043 CrossRef CAS.
- Z. Li, Y. Yan, S.-M. Xu, H. Zhou, M. Xu, L. Ma, M. Shao, X. Kong, B. Wang, L. Zheng and H. Duan, Nat. Commun., 2022, 13, 147 CrossRef CAS PubMed.
- L. Zhang, Y. Liu, L. Li, T. Wu, Q. Wu, J. Z. Y. Seow, X. Lin, S. Sun, L. Tannesia, K. Tang, D. Shao, S. Xi, X. Guo and Z. J. Xu, Energy Environ. Sci., 2025, 18, 5622–5631 RSC.
- X. Huang, Y. Guo, Y. Zou and J. Jiang, Appl. Catal., B, 2022, 309, 121247 CrossRef CAS.
- C. Jing, T. Yuan, L. Li, J. Li, Z. Qian, J. Zhou, Y. Wang, S. Xi, N. Zhang, H.-J. Lin, C.-T. Chen, Z. Hu, D.-W. Li, L. Zhang and J.-Q. Wang, ACS Catal., 2022, 12, 10276–10284 CrossRef CAS.
- K. Xiang, D. Wu, X. Deng, M. Li, S. Chen, P. Hao, X. Guo, J.-L. Luo and X.-Z. Fu, Adv. Funct. Mater., 2020, 30, 1909610 CrossRef CAS.
- Y. Q. Zhu, H. Zhou, J. Dong, S. M. Xu, M. Xu, L. Zheng, Q. Xu, L. Ma, Z. Li, M. Shao and H. Duan, Angew. Chem., Int. Ed., 2023, 62, e202219048 CrossRef CAS.
- S. Liu, S. Dou, J. Meng, Y. Liu, Y. Liu and H. Yu, Appl. Catal., B, 2023, 331, 122709 Search PubMed.
- Z. He, J. Hwang, Z. Gong, M. Zhou, N. Zhang, X. Kang, J. W. Han and Y. Chen, Nat. Commun., 2022, 13, 3777 Search PubMed.
- R. Chen, S. Chen, L. Wang and D. Wang, Adv. Mater., 2024, 36, 2304713 Search PubMed.
- H. Wang, Z. Gao, B. Sun, S. Mu, F. Dang, X. Guo, D. Ma and C. Shi, Chem Catal., 2023, 3, 100768 CAS.
- S. Liu, J. Lu, X. Yu, H. Pang, Q. Zhang and H. S. Park, eScience, 2025, 100378 CrossRef.
- C. Lee, K. Shin, C. Jung, P.-P. Choi, G. Henkelman and H. M. Lee, ACS Catal., 2020, 10, 562–569 CrossRef CAS.
- S. Liu, B. Tian, X. Xu, X. Wang, P. Ran, Y. Sun, J. Wu, A. Qiu, F. Wang, L. Tang, J. Ma and M. Ding, ACS Catal., 2024, 14, 9476–9486 CrossRef CAS.
- A.-Z. Li, X. Wang, S. Li, B.-J. Yuan, X. Wang, R.-P. Li, L. Zhang, B.-J. Li and H. Duan, J. Am. Chem. Soc., 2025, 147, 10493–10503 CrossRef CAS.
- F. Liu, W. Chen, T. Wang, J. Zhang, D. Yang, Y. Dai, G. Liu, J. Zhou, S. Wang and X. Guan, Angew. Chem., Int. Ed., 2025, 64, e202415438 CrossRef CAS.
- X. Lu, Z. Ma, Y. Chang, S. Wang, X. Li, D. Xu, J. Bao and Y. Liu, Adv. Mater., 2024, 36, 2313057 CrossRef CAS PubMed.
- X. Zhang, L. Hui, F. He and Y. Li, J. Am. Chem. Soc., 2025, 147, 436–445 CrossRef CAS.
- A. Moysiadou, S. Lee, C.-S. Hsu, H. M. Chen and X. Hu, J. Am. Chem. Soc., 2020, 142, 11901–11914 CrossRef CAS PubMed.
- Q. Yan, C. Lian, K. Huang, L. Liang, H. Yu, P. Yin, J. Zhang and M. Xing, Angew. Chem., Int. Ed., 2021, 60, 17155–17163 CrossRef CAS PubMed.
- S.-L. Xu, W. Wang, Y. Song, R. Tang, Z.-H. Hu, X. Zhou and H.-Q. Yu, Water Res., 2025, 270, 122851 CrossRef CAS PubMed.
- L. Chen, C. Yu, X. Song, J. Dong, Y. Han, H. Huang, X. Zhu, Y. Xie and J. Qiu, Small, 2024, 20, 2306410 Search PubMed.
- Y. Han, C. Yu, H. Huang, Q. Wei, J. Dong, L. Chen and J. Qiu, SmartMat, 2024, 5, e1206 CrossRef CAS.
- S. Chongdar, A. Ghosh, R. Bal and A. Bhaumik, J. Mater. Chem. A, 2024, 12, 233–246 RSC.
- L. Ming, X.-Y. Wu, S.-S. Wang, W. Wu and C.-Z. Lu, Green Chem., 2021, 23, 7825–7830 RSC.
- Y. Zhao, Y. Yan, Y. Wang, E. M. Björk, S. Wang, T. Li, Y. Lu, D. Wang, P. Schaaf, X. Wang and G. Guo, Mater. Today Energy, 2025, 48, 101780 CrossRef CAS.
- J. Wan, X. Mu, Y. Jin, J. Zhu, Y. Xiong, T. Li and R. Li, Green Chem., 2022, 24, 4870–4876 RSC.
- L. Wei, M. D. Hossain, M. J. Boyd, J. Aviles-Acosta, M. E. Kreider, A. C. Nielander, M. B. Stevens, T. F. Jaramillo, M. Bajdich and C. Hahn, ACS Catal., 2023, 13, 4272–4282 CrossRef CAS.
- J. Zhong, Y. Shen, P. Zhu, S. Yao and C. An, Nano Res., 2023, 16, 202–208 CrossRef CAS.
- X. Wen, K. Chen, Y. Su, K. Xiong, P. Fan, J. Wu, C. Liu, Q. Qu and L. Li, Chem. Eng. J., 2024, 493, 152510 CrossRef CAS.
- R. Li, P. Kuang, S. Wageh, A. A. Al-Ghamdi, H. Tang and J. Yu, Chem. Eng. J., 2023, 453, 139797 CrossRef CAS.
- S. Ye, Z. Chen, G. Zhang, W. Chen, C. Peng, X. Yang, L. Zheng, Y. Li, X. Ren, H. Cao, D. Xue, J. Qiu, Q. Zhang and J. Liu, Energy Environ. Sci., 2022, 15, 760–770 Search PubMed.
- S. Li, H. Tian, W. Luo, H. Wu, W. Sun, X. Cui and J. Shi, Small, 2025, 21, 2408507 CrossRef.
- J. Y. Loh, F. M. Yap, T. J. Siang, X. Zeng and W.-J. Ong, Small, 2024, 21, 2409331 CrossRef.
- Z. Zhang, B.-L. Leng, S.-N. Zhang, D. Xu, Q.-Y. Li, X. Lin, J.-S. Chen and X.-H. Li, J. Am. Chem. Soc., 2024, 146, 27179–27185 Search PubMed.
- H. Zhang, Y. Gao, S. Meng, Z. Wang, P. Wang, Z. Wang, C. Qiu, S. Chen, B. Weng and Y.-M. Zheng, Adv. Sci., 2024, 11, 2400099 CrossRef CAS.
- J. Yu, P. Zhang, L. Li, K. Li, G. Zhang, J. Liu, T. Wang, Z.-J. Zhao and J. Gong, Nat. Commun., 2022, 13, 7909 CrossRef CAS.
- R. Liu, W. Tu, A. Pei, W.-H. Huang, Y. Jia, P. Wang, D. Liu, Q. Wu, Q. Qin, W. Zhou, L. Zhou, K. Yan, Y. Zhao and G. Chen, J. Am. Chem. Soc., 2025, 147, 10339–10348 CrossRef CAS PubMed.
- J. J. Masana, J. Xiao, H. Zhang, X. Lu, M. Qiu and Y. Yu, Appl. Catal., B, 2023, 323, 122199 CrossRef CAS.
- H. Li, K. Gan, R. Li, H. Huang, J. Niu, Z. Chen, J. Zhou, Y. Yu, J. Qiu and X. He, Adv. Funct. Mater., 2023, 33, 2208622 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |





