Open Access Article
Open Access ArticleEnantioselective cyclization of bromoenynes: mechanistic understanding of gold(I)-catalyzed alkoxycyclizations
Andrea Cataffo†
 ab,
Eduardo García-Padilla†ab,
Imma Escofeta,
Nicolás Finciasa,
Anna Arnanzab,
Giuseppe Zuccarelloab,
Guilong Tianab,
Luyu Caiab,
Fereshteh Khorasanidarehdora,
Feliu Maseras
ab,
Eduardo García-Padilla†ab,
Imma Escofeta,
Nicolás Finciasa,
Anna Arnanzab,
Giuseppe Zuccarelloab,
Guilong Tianab,
Luyu Caiab,
Fereshteh Khorasanidarehdora,
Feliu Maseras a and
Antonio M. Echavarren
a and
Antonio M. Echavarren *ab
*ab
aInstitute of Chemical Research of Catalonia (ICIQ, CERCA), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
bDepartament de Química Analítica i Química Orgànica, Universitat Rovira i Virgili, C/ Marcel·lí Domingo s/n, 43007 Tarragona, Spain
First published on 5th January 2026
Abstract
The first enantioselective gold(I)-catalyzed alkoxycyclization of bromo-1,6-enynes has been achieved using a modified JohnPhos ligand bearing a distal C2-chiral 2,5-diarylpyrrolidine unit. Using an achiral catalyst of the same family, the enantioselective cascade cyclization of bromo-1,5-enynes to form polycyclic scaffolds was also accomplished for the first time. Interestingly, performing the cyclization of bromo-1,6-enynes in the absence of an alcohol nucleophile led to the formation of nearly racemic cycloisomerization products. Control experiments and DFT calculations support a mechanism involving an in-cycle racemization process mediated by a 1,2-hydrogen shift, shedding new light on the mechanism of gold(I)-catalyzed alkoxycyclizations.
Introduction
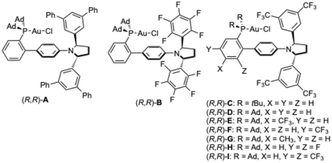
Highly polarized haloalkynes constitute valuable building blocks in organic synthesis due to their dual reactivity under transition metal catalysis: the π-system allows functionalization with nucleophiles and the halogen moiety opens the possibility for further diversification by coupling reactions, oxidative cyclometallation, or vinylidene formation (Scheme 1a).1 The latter pathway has also been proposed with haloalkynes in the context of gold(I)-catalyzed cyclizations with carbon-based nucleophiles.2–4 On the other hand, the group of Zhang reported that chloroalkynes undergo gold(I)-catalyzed intermolecular [2 + 2] cycloadditions with alkenes to afford cyclobutenes.5 Yang and Hashmi reported the hydroarylation of chloroalkynes with phenols6 and Haberhauer studied the chloroalkynylation of alkenes in detail.7 Several examples of gold(I)-catalyzed cyclizations with carbon nucleophiles involving iodo-8 and bromoalkynes9 have also been described. The group of Magauer reported the gold(I)-catalyzed reaction of bromo-1,5-enynes with phenols, which undergoes a 1,2-H shift prior to the intermolecular trapping of the cycloisomerized intermediate by the phenol (Scheme 1b).10 Our group reported cascade cyclizations of 1-susbstituted bromo-1,5-enynes, including tri- and tetraenynes, to form complex polycyclic structures.11 We also recently described the gold(I)-catalyzed cross coupling-type reaction of bromoalkynes with allylsilanes through cyclic bromonium species, which evolves to the product through a 1,2-aryl shift (Scheme 1c).9dEnantioselective cyclizations of halo-1,n-enynes have not yet been reported. In fact, only a few examples of enantioselective reactions of bromo- and chloroarylalkynes with cyclopentene have been described.9e Here, we report the enantioselective cyclization of bromo-1,5- and 1,6-enynes employing modified JohnPhos ligands with a distal C2-chiral 2,5-diarylpyrrolidine.12
When the first alkoxycyclization of 1,6-enynes with Pt(II) was described by the group of Genêt in 2004 (ref. 13) and with gold(I) by our group one year later,14 the alcohol was used as a reaction solvent and only later it was noticed that its nature and quantity could play a role in the selectivity of the transformation.15 Later, our group observed that using the alcohol as a solvent in 1,6-enyne alkoxycyclizations gave an enhanced stereospecificity compared to when using 5 equiv of nucleophile, which was attributed to the bond rotation of the carbocationic intermediate formed after the cyclization step.16
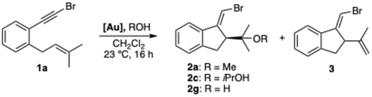
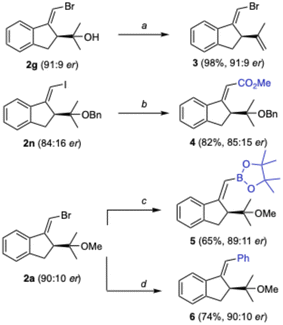
In our study of the alkoxy- and hydroxycyclization of bromo-1,6-enynes such as 1, we obtained adducts 2a–g with good enantioselectivities (Scheme 1d). However, using the same chiral gold(I) catalysts in the absence of a nucleophile, the product of formal cycloisomerization 3 was obtained in a nearly racemic form, which is unexpected considering that both products are presumably formed from the same intermediate after the enantiodiscriminating step. This led us to perform an in-depth mechanistic study of the gold(I)-catalyzed alkoxycyclization, one of the representative transformations in gold(I) catalysis.17
Results and discussion
Alkoxycyclization of bromo-1,6-enynes
We first studied the enantioselective gold(I)-catalyzed methoxycyclization of bromo-1,6-enyne 1a to form 2a by a 5-exo-dig process (Table 1). Among the different catalysts A–I bearing JohnPhos type ligands with a distal C2-chiral 2,5-diarylpyrrolidine,12 the sterically hindered terphenyl catalyst (R,R)-A performed the best affording 2a in 80% yield and 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 er at 23 °C (Table 1, entry 1). The catalyst loading could be lowered from 5% to 3% having no effect in yield and enantioselectivity (Table 1, entry 8). Running the reaction in α,α,α-trifluorotoluene with AgSbF6 and lowering the quantity of MeOH to 10 equiv led to an er of 83
19 er at 23 °C (Table 1, entry 1). The catalyst loading could be lowered from 5% to 3% having no effect in yield and enantioselectivity (Table 1, entry 8). Running the reaction in α,α,α-trifluorotoluene with AgSbF6 and lowering the quantity of MeOH to 10 equiv led to an er of 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 (Table 1, entry 10).18 Lowering the temperature to −20 °C led to 2a in 83% yield and 85
17 (Table 1, entry 10).18 Lowering the temperature to −20 °C led to 2a in 83% yield and 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 er (Table 1, entry 11). To lower the temperature to −60 °C, the solvent was changed to CH2Cl2, leading to 2a in 99% isolated yield and 90
15 er (Table 1, entry 11). To lower the temperature to −60 °C, the solvent was changed to CH2Cl2, leading to 2a in 99% isolated yield and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 er after 4 days (Table 1, entry 12).
10 er after 4 days (Table 1, entry 12).
| Entry | [Au] (mol%) | Solvent | MeOH equiv | T (°C) | Yield (%) | er |
|---|---|---|---|---|---|---|
| a Reaction time = 4 days. | ||||||
| 1 | (R,R)-A (5%) | 1,2-DCE | 30 | 23 | 80 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 2 | (R,R)-C (5%) | 1,2-DCE | 30 | 23 | 74 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
| 3 | (R,R)-D (5%) | 1,2-DCE | 30 | 23 | 59 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 4 | (R,R)-F (5%) | 1,2-DCE | 30 | 23 | 62 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| 5 | (R,R)-G (5%) | 1,2-DCE | 30 | 23 | 78 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
| 6 | (R,R)-H (5%) | 1,2-DCE | 30 | 23 | 81 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
| 7 | (R,R)-I (5%) | 1,2-DCE | 30 | 23 | 84 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| 8 | (R,R)-A (3%) | 1,2-DCE | 30 | 23 | 80 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 9 | (R,R)-A (3%) | PhCF3 | 30 | 23 | 80 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 10 | (R,R)-A (3%) | PhCF3 | 10 | 23 | 80 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 11 | (R,R)-A (3%) | PhCF3 | 10 | −20 | 83 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 12a | (R,R)-A (3%) | CH2Cl2 | 10 | −60 | 99 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
 |
||||||
The enantioselective alkoxycyclization was applied to a variety of bromo-1,6-enynes (Scheme 2). Substrate 1a was cyclized in the presence of different alcohols affording in most cases good to excellent conversion and er ranging from 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 to 90
12 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (2a–e). The assigned S absolute configurations for the major enantiomers are based on the X-ray structure of 2a.19 Less nucleophilic trifluoroethanol as a nucleophile afforded 2f in good yield, although with lower enantioselectivity. Water could be used as a nucleophile too, affording alcohol 2g in 99% yield and 91
10 (2a–e). The assigned S absolute configurations for the major enantiomers are based on the X-ray structure of 2a.19 Less nucleophilic trifluoroethanol as a nucleophile afforded 2f in good yield, although with lower enantioselectivity. Water could be used as a nucleophile too, affording alcohol 2g in 99% yield and 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er. Methoxycyclization products with electron-donating (2h) and electron-withdrawing groups (2i) on the aryl were also obtained from 1,6-enynes 1b–c. When substituting the prenyl with a geranyl chain, 2j was obtained with moderate yield but a high 94
9 er. Methoxycyclization products with electron-donating (2h) and electron-withdrawing groups (2i) on the aryl were also obtained from 1,6-enynes 1b–c. When substituting the prenyl with a geranyl chain, 2j was obtained with moderate yield but a high 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er. Substituting one of the two methyls of the prenyl chain with a phenyl afforded 2k in excellent yield and 82
6 er. Substituting one of the two methyls of the prenyl chain with a phenyl afforded 2k in excellent yield and 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 er. The reaction of chloro- (1f) and iodo-1,6-enynes (1g) analogues of 1a led to 2l–n. Products of methoxycyclization 2o and 2p were obtained from 1,6-enynes 1e and 1i with different tethers.
18 er. The reaction of chloro- (1f) and iodo-1,6-enynes (1g) analogues of 1a led to 2l–n. Products of methoxycyclization 2o and 2p were obtained from 1,6-enynes 1e and 1i with different tethers.
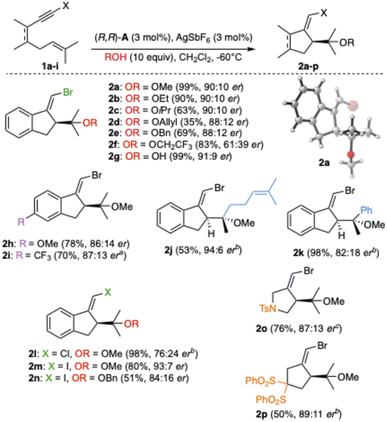 | ||
| Scheme 2 Alkoxycyclization of bromo-1,6-enynes. Reaction times are specified in the SI. aThe reaction was run using 5 mol% catalyst loading. bT = −40 °C. cT = 23 °C. | ||
The reactivity of the bromoalkene motif was then further exploited, affording different enantio-enriched structures. Product 3, which corresponds to the formal cycloisomerization of 1a, was obtained almost quantitatively from enantiomerically enriched 2g without loss in er by dehydration with Burgess reagent (Scheme 3). Methoxycarbonylation of 2n catalyzed by palladium afforded ester 4 in good yield. Similarly, adduct 2a was converted into 5 and 6 by Pd-catalyzed borylation and Suzuki coupling, respectively.
Compound 6 corresponds to the product of a formal 5-exo-dig cyclization of phenyl-1,6-enyne 7a. However, substrates of that type undergo 6-endo-dig instead of 5-exo-dig cyclization using gold(I) catalysis to form 1,2-dihydronaphthalene 8 (Scheme 4).12a,20,21 Products of 5-exo-dig cyclization similar to 6, but with the opposite Z-configuration at the alkene, are obtained by a photoredox-assisted arylative cyclization procedure developed by our group.22
 | ||
| Scheme 4 Bromo- (1a) and aryl-1,6-enynes (7a)12a react with the Re-prochiral face of the alkene with (R,R)-catalysts A and D. | ||
Regarding the alkene face selectivity in the enantioselective cyclization, we have found that the enantioselective cyclization of aryl-1,6-enynes of type 7 with gold(I) catalysts bearing JohnPhos type ligands with a distal C2-2,5-diarylpyrrolidine is mainly directed by the interaction of 1,2-substituted benzene tethering the alkyne and the prenyl chain, whereas the aryl at C-1 of the alkyne plays only a secondary role.12a In full accord with this prediction, the new cyclization of bromo-1,6-enynes such as 1a proceeds with the same face selectivity with respect to the alkene using a similar chiral gold(I) catalyst ((R,R)-A and (R,R)-D) (Scheme 4).
Cascade cyclization of bromo-1,5-enynes
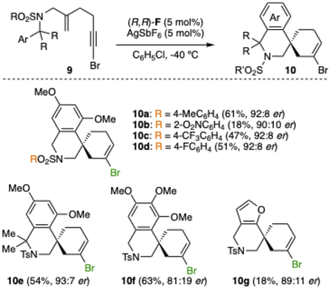
Considering the relatively few reports of asymmetric gold(I)-catalyzed polyenyne cyclizations23,24 and the low enantioselectivities that we have observed before for some of these transformations using gold(I) catalysts with commercially available ligands,25 we envisioned that the same family of chiral 2,5-diarylpyrrolidine-JohnPhos chiral catalysts could be effective for the enantioselective cascade cyclizations of bromo-1,5-enynes such as 9a to form spiro derivatives 10a (Table 2). The initial result using (R,R)-D and AgSbF6 in CH2Cl2 (Table 2, entry 1) was improved using chlorobenzene as the solvent leading to alkenylbromide 10a with promising 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 er (Table 2, entry 2).18 Performing the reaction at −40 °C gave 10a in 91
18 er (Table 2, entry 2).18 Performing the reaction at −40 °C gave 10a in 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er but with a substantial amount of unreacted enyne 9a (Table 2, entry 3). While other members of the family of chiral gold(I) complexes gave only traces of product 10a with low er (Table 3, entries 4–7), catalyst (R,R)-F, bearing a strongly electron-withdrawing CF3 substituent meta to the coordinating phosphine, gave 10a in 86% yield and 92
9 er but with a substantial amount of unreacted enyne 9a (Table 2, entry 3). While other members of the family of chiral gold(I) complexes gave only traces of product 10a with low er (Table 3, entries 4–7), catalyst (R,R)-F, bearing a strongly electron-withdrawing CF3 substituent meta to the coordinating phosphine, gave 10a in 86% yield and 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er at −40 °C, although at this temperature the reaction required 7 days (Table 2, entry 8).
8 er at −40 °C, although at this temperature the reaction required 7 days (Table 2, entry 8).
| Entry | [Au] | Solvent | T (°C) | aConv. (%) | ere |
|---|---|---|---|---|---|
| a Yields determined by 1H NMR using 1,3,5-tribromobenzene as the internal standard.b 2 h reaction time.c 7 days of reaction time.d Traces of 10a.e Conversion calculated based on the recovered starting material after 7 days of reaction time. | |||||
| 1 | (R,R)-D | CH2Cl2 | 24 | 100b | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 2 | (R,R)-D | C6H5Cl | 24 | 100b | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 3 | (R,R)-D | C6H5Cl | −40 | 20c | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 4 | (R,R)-A | C6H5Cl | −40 | —d | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 5 | (R,R)-B | C6H5Cl | −40 | —d | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| 6 | (R,R)-C | C6H5Cl | −40 | —d | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 7 | (R,R)-E | C6H5Cl | −40 | —d | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 8 | (R,R)-F | C6H5Cl | −40 | 86e | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
Different sulfonamides 9 were cyclized to form 10a–d in satisfactory enantiomeric ratios (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 92
10 to 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er), although low yield was obtained in the case of 9b with an o-nitro group at the aryl of the sulfonamide (Scheme 5). Spiro compound 10e, containing a gem-dimethyl substitution at the α-position to the nitrogen, was isolated in 54% yield and 93
8 er), although low yield was obtained in the case of 9b with an o-nitro group at the aryl of the sulfonamide (Scheme 5). Spiro compound 10e, containing a gem-dimethyl substitution at the α-position to the nitrogen, was isolated in 54% yield and 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 er. A drop in enantioselectivity (81
7 er. A drop in enantioselectivity (81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 er) was observed in the formation of spiro compound 10f with a 3,4,5-OMe substituted aromatic ring. Finally, furan-containing bromo-1,5-enyne 9g was converted into product 10g with good 89
19 er) was observed in the formation of spiro compound 10f with a 3,4,5-OMe substituted aromatic ring. Finally, furan-containing bromo-1,5-enyne 9g was converted into product 10g with good 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er albeit in modest 18% yield.
11 er albeit in modest 18% yield.
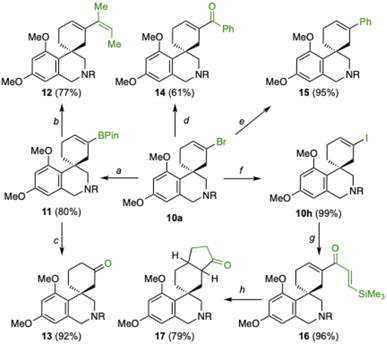
The alkenyl bromide moiety in the final compounds provides a versatile handle for further derivatization (Scheme 6). Thus, borylation of 10a with B2Pin2 and Pd(dppf)Cl2 gave 11 in 80% yield (Scheme 6). Suzuki coupling of 11 with (Z)-2-bromobut-2-ene gave 12 in 77% yield, while the oxidation of 11 with NaBO3 gave known enantioenriched ketone (R)-13 (ref. 24) in 92% yield and 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er. This allowed to determine the absolute configuration of spirocyclic compounds 10a–f as R by correlation (compound 10g has the S-configuration).
9 er. This allowed to determine the absolute configuration of spirocyclic compounds 10a–f as R by correlation (compound 10g has the S-configuration).
Alkenyl bromide 10a was converted into ketone 14 (61%) by Br/Li exchange, followed by a reaction with N-methoxy-N-methylbenzamide and into 15 by Suzuki coupling with PhB(OH)2 (95% yield) (Scheme 6). Quantitative conversion of 10a into iodide 10h with NaI and CuI was followed by carbonylative Stille coupling to afford 16 (96%), which underwent Nazarov cyclization with FeCl3 to afford 17 in 79% yield as a 5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers, which were assigned to the two possible cis-fused octahydro-1H-inden-1-ones.
1 mixture of diastereomers, which were assigned to the two possible cis-fused octahydro-1H-inden-1-ones.
Analysis of the enantioselectivity
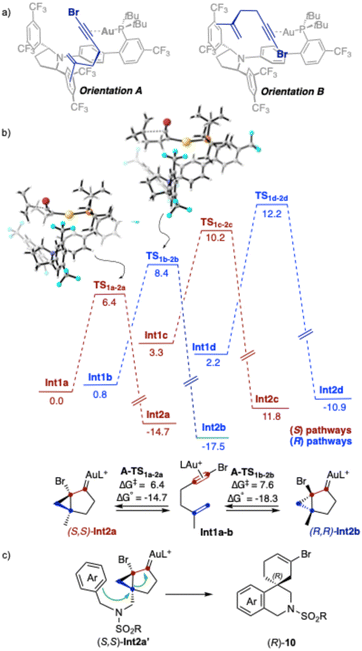
With the aim to better rationalize the enantioselective formation of spiro derivatives (R)-10 by cascade cyclization of bromo-1,5-enynes 1 catalyzed by 2,5-diarylpyrrolidine-JohnPhos chiral catalysts (Table 1), we performed DFT calculations with 6-bromo-2-methylhex-1-en-5-yne as the model substrate and (R,R)-F′ as the gold(I) catalyst, which is similar but simpler than (R,R)-F (di-tert-butylphosphine instead of di-adamantylphosphine). BP86-D3 has been chosen as a functional due to its efficiency being proved in other studies of enantioselective gold(I) catalysis12,26 and in our recent benchmark of DFT functionals using similar systems.27Our calculations centered on the nucleophilic attack of the alkene at the [LAu(η2-alkyne)]+ complex to form a cyclopropylgold(I) carbene intermediate, as the enantiodetermining step. We calculated four possible minima (Int1a–d) resulting from two binding orientations (A and B) of the substrate coordinated to gold(I) through the alkyne, and the reaction of the two enantiotopic faces of the alkene (R and S pathways) (Scheme 7a). The preferred binding orientation is A, with Int1a (0.0 kcal mol−1) and Int1b (0.8 kcal mol−1) being more stable than Int1c (3.3 kcal mol−1) and Int1d (2.2 kcal mol−1). In Int1c and Int1d, the two aryl groups of the pyrrolidine ring adopt a pseudoaxial/pseudoaxial conformation, whereas in Int1a and Int1b the pyrrolidine groups adopt a conformation with pseudoaxial/pseudoequatorial aryl groups. The most stable intermediate is Int1a, and the activation energy to reach TS1a–2a was also found to be lower than other possible pathways by at least ΔΔG‡R–S = 2 kcal mol−1. Therefore, this model predicts that (S,S)-Int2a would be preferentially formed (Scheme 7b). This agrees with our experimental results, since the final cyclization of (S,S)-Int2a′ would proceed to form the (R)-configured spiro derivatives 10 (Scheme 7c).
Experimental mechanistic studies
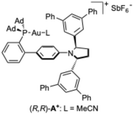
Detailed studies on the mechanism of gold(I)-catalyzed alkoxycyclizations have not yet been performed. Usually, gold(I)-catalyzed alkoxycyclizations of 1,6-enynes are conducted in the presence of alcohol in large excess or as solvent,17,20,28 In this work, we found that the outcome of the reaction is highly dependent on the amount and nucleophilicity of the alcohols used as nucleophiles.First, we performed control experiments on the cyclization of bromo-1,6-enyne 1a in the presence of MeOH (Table 3). As expected, running the reaction in the absence of a silver(I) salt as a chloride scavenger led to no conversion (Table 3, entry 1). However, in the absence of gold(I), 15% of the methoxycyclization product 2a was observed by 1H NMR, which shows that silver(I) is moderately active for this transformation (Table 3, entry 2). A reaction performed in the absence of MeOH in non-anhydrous CH2Cl2 gave a 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the hydroxycyclization product 2g and cycloisomerization product 3 (Table 3, entry 3). Surprisingly, while adduct 2g was isolated in 82
1 ratio of the hydroxycyclization product 2g and cycloisomerization product 3 (Table 3, entry 3). Surprisingly, while adduct 2g was isolated in 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 er, 3 was nearly racemic (51
18 er, 3 was nearly racemic (51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 er). Reaction with (R,R)-A+, a cationic version of (R,R)-A with MeCN as the ligand,18 in the absence of MeOH, led to racemic 3 in low yield (Table 3, entry 4). The reaction with 1 equiv of MeOH with (R,R)-A+ gave 2a with 80
49 er). Reaction with (R,R)-A+, a cationic version of (R,R)-A with MeCN as the ligand,18 in the absence of MeOH, led to racemic 3 in low yield (Table 3, entry 4). The reaction with 1 equiv of MeOH with (R,R)-A+ gave 2a with 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 er, along with 3 (54
20 er, along with 3 (54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 er) (Table 3, entry 5). The efficiency of catalyst (R,R)-A+ decreased in the presence of 10 equiv of MeOH, giving a mixture of 2a (80
46 er) (Table 3, entry 5). The efficiency of catalyst (R,R)-A+ decreased in the presence of 10 equiv of MeOH, giving a mixture of 2a (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 er) and 3 (60
20 er) and 3 (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 er) in lower yield (Table 3, entry 6). (R)-DTBM-Segphos(AuCl)2 (L), which has been previously used in gold(I)-catalyzed alkoxycyclzations,28a led to lower yields in this reaction under anhydrous conditions (Table 3, entries 7 and 8). The yield of 3 increased in the presence of iPrOH with catalyst (R,R)-A+ (Table 3, entries 9 and 10). Finally, a reaction in the presence of even less nucleophilic hexafluoroisopropanol (HFIP) gave 3 as the only product in 59% yield with nearly the same enantioselectivity found for the alkoxycyclization products 2a,c,g (76
40 er) in lower yield (Table 3, entry 6). (R)-DTBM-Segphos(AuCl)2 (L), which has been previously used in gold(I)-catalyzed alkoxycyclzations,28a led to lower yields in this reaction under anhydrous conditions (Table 3, entries 7 and 8). The yield of 3 increased in the presence of iPrOH with catalyst (R,R)-A+ (Table 3, entries 9 and 10). Finally, a reaction in the presence of even less nucleophilic hexafluoroisopropanol (HFIP) gave 3 as the only product in 59% yield with nearly the same enantioselectivity found for the alkoxycyclization products 2a,c,g (76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 er) (Table 3, entry 11). As a solvent, HFIP has received attention for its beneficial effects on reaction rates in gold(I)-catalysis.29
24 er) (Table 3, entry 11). As a solvent, HFIP has received attention for its beneficial effects on reaction rates in gold(I)-catalysis.29
| Entry | [Au]a | [Ag] | Alcohol | Productsb |
|---|---|---|---|---|
| a 3 mol%.b Yields determined by 1H NMR using 1,1,2,2-tetrachloroethane or trichloroethylene as internal standards. The er was measured on the pure isolated products.c Reaction in PhCF3.d 5 mol%.e Rigorous anhydrous conditions.f (R)-L = (R)-DTBM-Segphos(AuCl)2. | ||||
| 1 | (R,R)-A | — | MeOH (10 equiv) | — |
| 2 | — | AgSbF6d | MeOH (10 equiv) | 2a (15%) |
| 3c | (R,R)-A | AgSbF6d | — | 2g (45%, 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 er) + 3 (32%, 51 18 er) + 3 (32%, 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 er) 49 er) |
| 4 | (R,R)-A+ | — | — | 3 (8%, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 er) 50 er) |
| 5e | (R,R)-A+ | — | MeOH (1 equiv) | 2a (92%, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 er) + 3 (9%, 54 20 er) + 3 (9%, 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 er) 46 er) |
| 6e | (R,R)-A+ | — | MeOH (10 equiv) | 2a (65%, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 er) + 3 (3%, 60 20 er) + 3 (3%, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 er) 40 er) |
| 7e | (R)-L f | AgSbF6d | — | 3 (12%, 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 er) 39 er) |
| 8e | f(R)-L | AgSbF6d | MeOH (1 equiv) | 2a (20%, 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 er) + 3 (37%, 70 29 er) + 3 (37%, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 er) 30 er) |
| 9e | (R,R)-A+ | — | iPrOH (1 equiv) | 2c (55%, 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 er) + 3 (28%, 70 19 er) + 3 (28%, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 er) 30 er) |
| 10e | (R,R)-A+ | — | iPrOH (10 equiv) | 2c (76%, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 er) + 3 (22%, 75 20 er) + 3 (22%, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 er) 25 er) |
| 11e | (R,R)-A+ | AgSbF6d | HFIP (10 equiv) | 3 (59%, 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 er) 24 er) |
 |
||||
We repeated the reaction with (R,R)-A+ in the absence of MeOH (Table 1, entry 4) using deuterated 1a–d1 as the substrate (Scheme 8).18 In this experiment, we obtained 3–d1 in poor yield without any scrambling or change in isotopic ratio. Interestingly, instead of obtaining a racemic product, 3–d1 was isolated in 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 er.
42 er.
Neither enantioenriched 2a nor 3, obtained by water elimination from 2a (Scheme 3), underwent racemization under the conditions of the gold(I)-catalyzed cyclization.18 Also, the possibility that racemic 3 would be formed by a secondary proton-catalyzed pathway was excluded, since 1a did not afford 2a nor 3 when treated with MeSO3H (10 mol%) in CH2Cl2 at 23 °C for 16 h in the absence or presence of MeOH.18
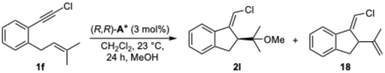
When chloroenyne 1f was subjected to the standard reaction conditions using (R,R)-A+ as a catalyst and varying the quantity of nucleophile from 0 to 10 equiv, the cycloisomerization product 18 was isolated with a lower er than that of the alkoxycyclization product 2l (Table 4), which was expected, given the similar nature of the two haloalkyne substrates. Again, using more equivalents of nucleophile did not affect the er of the alkoxycyclization product 2l, while it gave a lower yield (Table 4, entry 3).
| Entry | MeOH equiv | 2l | 18 |
|---|---|---|---|
| a Anhydrous conditions. Yields determined by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard. The er was measured on the pure isolated products. | |||
| 1 | 0 | — | 16%, 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 er 45 er |
| 2 | 1 | 74%, 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 er 26 er |
8%, 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 er 47 er |
| 3 | 10 | 47%, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 er 25 er |
10%, 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 er 40 er |
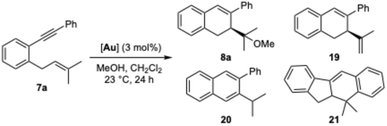
Among other substrates,18 electronically different phenylenyne 7a was examined (Table 5). Reaction with (R,R)-A+ as the catalyst in the absence of MeOH gave a ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of naphthalene 20 and 21, a product of formal intramolecular [4 + 2] cycloaddition30 (Table 5, entry 1). In the presence of 1 equiv of MeOH, alkoxycyclization product 8a was formed in good yield and high 98
1 mixture of naphthalene 20 and 21, a product of formal intramolecular [4 + 2] cycloaddition30 (Table 5, entry 1). In the presence of 1 equiv of MeOH, alkoxycyclization product 8a was formed in good yield and high 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er (Table 5, entry 2). Unfortunately, cycloisomerization product 19 was formed only in a small amount, which was not enough to measure its er. We repeated the experiments using catalyst (R,R)-F, which was previously used in our group for cyclizing this kind of phenyl-1,6-enynes.12a In the absence of nucleophile, again 20 and 21 were the only identified products (Table 5, entry 3). However, when increasing the quantity of MeOH to 1 or 5 equiv, 8a and 19 were isolated with identical er (Table 5, entries 4–5). The formation of naphthalene 20 suggests that an irreversible 1,2-H shift might be favored (see computational mechanistic discussion below).
2 er (Table 5, entry 2). Unfortunately, cycloisomerization product 19 was formed only in a small amount, which was not enough to measure its er. We repeated the experiments using catalyst (R,R)-F, which was previously used in our group for cyclizing this kind of phenyl-1,6-enynes.12a In the absence of nucleophile, again 20 and 21 were the only identified products (Table 5, entry 3). However, when increasing the quantity of MeOH to 1 or 5 equiv, 8a and 19 were isolated with identical er (Table 5, entries 4–5). The formation of naphthalene 20 suggests that an irreversible 1,2-H shift might be favored (see computational mechanistic discussion below).
| Entry | [Au] | [Ag] | MeOH equiv | 8a | 19 | 20 (%) | 21 (%) |
|---|---|---|---|---|---|---|---|
| a Reactions were performed under anhydrous conditions using glovebox solvents. Yields were determined by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard. The er was measured on the pure isolated products. | |||||||
| 1 | (R,R)-A+ | — | — | — | — | 37 | 35 |
| 2 | (R,R)-A+ | — | 1 | 76%, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er 2 er |
2% | — | — |
| 3 | (R,R)-F | AgSbF6 | — | — | — | 56 | 14 |
| 4 | (R,R)-F | AgSbF6 | 1 | 3%, 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er 11 er |
42%, 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er 11 er |
— | — |
| 5 | (R,R)-F | AgSbF6 | 5 | 39%, 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er 11 er |
20%, 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er 11 er |
— | — |
Mechanistic DFT calculations
In previous mechanistic studies,28c,31 steps following the formation of the initial cationic intermediates were considered unimportant, both kinetically and for determining selectivity, as well as bringing additional computational challenges. To understand the processes that can lead to the racemization of formal cycloisomerized products obtained from 1, we carried out detailed DFT calculations on the full mechanism.32 The calculations were performed with Gaussian09,33 with the B3LYP34 functional including Grimme's D3 dispersion correction,35 modelling the solvent (CH2Cl2) with implicit solvation using the PCM polarizable continuum model.36 Gold was modelled with the Stuttgart-Dresden basis set and effective core potential,37 bromine with LANL2TZ(f)38 and all other non-metal atoms with Pople basis set39 6-311+G(d,p). This level of theory had been previously found to perform well in DFT calculations of gold(I)-catalyzed transformations.40 The counteranion was excluded from our computational model. We are aware that previous work had described a non-trivial involvement of hexafluoroantimonate,41 but we suspect that the effect of the ion pairs in that case was exaggerated by the performance of geometry optimizations in vacuum. When we tried to reproduce them with our PCM approach, the short contacts disappeared.As the preformed products 2a and 3 do not undergo racemization under the reaction conditions, all observed differences in enantiomeric ratio must arise during the reaction. To address this question, we searched the possible pathways that could interconvert the two chiral products. The enantioselectivity itself would not have to be determined, so the modelled ligand on gold was simplified to trimethylphosphine.
We first reproduced the exo-dig selectivity of the haloenyne cyclizations from intermediate A1 as the starting point through TSA2 to form intermediate A2 (Scheme 9). The alternative transition state leading to 6-endo-dig products (not shown) has a difference in energy higher than 5 kcal mol−1.18
 | ||
| Scheme 9 5-exo-dig Cyclization of bromo-1,6-enyne-Au(I) complex A1 followed by MeOH-mediated nucleophilic attack/elimination. Free energy in kcal mol−1. | ||
The correct model for the alcohol as nucleophile or base was hitherto unknown. Thus, it was unclear if simple monomeric MeOH was accurate enough, or whether considering higher MeOH clusters in CH2Cl2 would give more consistent results. The implications are significant in the pKa, kinetic effects, as well as in the reference energy for free solvated MeOH and in the deprotonation or nucleophilic attack transition states. After calculating several neutral and protonated clusters of MeOH in CH2Cl2, we found the neutral monomer and a protonated tetramer to be the most stable among those studied.18 A protonated MeOH dimer was found to be comparable in energy and, hence, dimers were used as a model in alcohol-mediated deprotonations, as well as a proton sink and as the nucleophilic species. However, it is important to note that, since a wide range of MeOH-containing species are accessible, several almost isoenergetic pathways presumably coexist.
Our calculations show an equilibrium between the distorted cyclopropylcarbene intermediate A2, which is formed directly in the cyclization, and a cyclic bromonium species A2b of similar energy formed from A2 via TSA2b. Intermediate A2 can undergo MeOH addition to form A4b or E2 elimination (TSA6) to form A6. Unsurprisingly, all attempts to find the transition state to deprotonate alkoxycyclization intermediate A4b and related species failed, hinting at a close-to-barrierless process. A2b can only undergo elimination through TSA6b, which is more favorable than deprotonation at the stereogenic center that would form an achiral diene (A7).18
A reversible 1,2-H shift42,43 through TSA2b-8 was found as an explanation for the racemization (Scheme 9). The hydride shift originates from bromonium A2b through an open-form vinyl gold(I) hidden intermediate HIntA2b′,40,44 which was located by analysis of the IRC.18 This non-stationary geometry is common to both TSA2b and TSA2b-8 and corresponds to a homoallylic gold(I) cationic species known to be minima for many cyclizations of enynes.40 At the low MeOH concentrations at which TSA2b-8 is accessed and entropically favored, other elimination or addition pathways from intermediate A8 were considered less likely.18
We also calculated the 1,2-hydride shift racemization barrier for 1a–d1 (Scheme 8) by modeling TSA2b-8-D.18 The ΔG‡, at 16.1 kcal mol−1, is 0.8 kcal mol−1 higher for TSA2b-8-D than with TSA2b-8, as expected from the different rate in the migration of deuterium and consistent with the lower racemization observed experimentally (58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 er for 3–d1 vs. 50
42 er for 3–d1 vs. 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 er for 3).
50 er for 3).
These calculations explain the disparity in enantiomeric ratios of products 2 and 3 (Table 3). Thus, initially, alkoxycyclization happens at a much faster rate than 1,2-H shift. The effect of MeOH concentration on the rate of elimination to give 3 is smaller because the transition state does not depend on the presence of higher-order MeOH oligomers. Therefore, at lower relative concentrations of nucleophile, elimination and the 1,2-H shift become competitive, leading to the racemization.
This is also consistent with the experiments in the presence of HFIP (Table 3, entry 11), which affords enantioenriched elimination products by outcompeting the 1,2-H shift with a good proton shuttle, presumably lowering the energy of TSA6b. Furthermore, the use of isopropanol (Table 3, entries 9 and 10), which favors elimination due to its relatively higher basicity and steric bulk, afforded product 2c in higher enantiomeric ratios. This observed pattern was confirmed through additional computational studies.18
We then explored possible 1,2-hydrogen shifts in the reaction of phenyl-substituted enyne 7a, which undergoes 6-endo-dig cyclization. In this case, the enantiomeric excess of formal cycloisomerization product 19 was not affected by the MeOH concentration (Table 5, entries 4 and 5). The 1,2-H shift pathway should therefore be inaccessible or not result in racemization. Computationally, B1 shows a small preference for 6-endo-dig attack via TSB2 and TSB2b (Scheme 10). This preference is larger experimentally, perhaps due to the stereoelectronic effects of the ligand. In addition to other pathways,18 there is presumably an interconversion pathway between B2 and B2b, which was not searched. As opposed to haloenynes, 1,2-H shift through TSB8 is irreversible, as to return from B8 would require 32 kcal mol−1. The barrier of 1,2-H shift TSB8 is 17.8 kcal mol−1, comparable to elimination and, presumably, alkoxycyclization. Indeed, experimentally, 2-phenyl-3-isopropylnaphthalene 20, which would be formed from B8 after deprotonation and protodemetallation, was found to be the major product (Table 5, entries 1 and 3). This brings forth more evidence for the mediation of 1,2-H shifts in gold(I)-catalyzed cyclizations.
 | ||
| Scheme 10 6-endo-dig Cyclization of phenyl-1,6-enyne-Au(I) complex B1 followed by MeOH-mediated nucleophilic attack or elimination. 1,2-H shift is irreversible. Free energy in kcal mol−1. | ||
The observation of 1,2-H shift in our systems is consistent with the hypothesis advanced by Magauer and co-workers that 1,2-H shift operates in the cyclization of bromo-1,5-enynes (Scheme 1b).10 The groups of Marco-Contelles3b and Xia3c reported computational work supporting 1,2-H shifts in gold(I)-catalyzed hydroarylation of haloalkynes. Our group also reported the formation of side products which could only be produced by 1,2-H shift in the context of the gold(I)-catalyzed intramolecular [4 + 2] cycloaddition of arylalkynes.30 Finally, Sanz and co-workers also proposed the involvement of 1,2-H shift in the gold(I)-catalyzed formation of dihydrobenzo[a]fluorenes.45
Conclusions
We have developed the first enantioselective gold(I)-catalyzed cyclizations of bromo-1,5-enyne and bromo-1,6-enynes. The formation of spiro derivatives by cyclization of bromo-1,5-enynes represents the first example of enantioselective cyclization of 1,5-enynes catalyzed by 2,5-diarylpyrrolidine-JohnPhos chiral ligands, and, in fact, the first involving a substrate lacking an arylalkyne group.12Our computational study demonstrates that, like in the cyclization of aryl-1,6-enynes with gold(I) catalysts with JohnPhos type ligands with a distal C2-2,5-diarylpyrrolidine,12a the enantioselectivity in the new cyclization of bromo-1,6-enynes is mainly directed by the interaction of the 1,2-substituted benzene tethering the alkyne and the prenyl chain with the chiral catalyst. However, bromo-1,6-enynes give rise to five-membered rings, whereas six-membered rings are obtained from aryl-1,6-enynes.
Both experimental and computational mechanistic studies on the cyclization of halo-1,6-enynes reveal that a reversible 1,2-H shift is in operation, leading to nearly racemic cycloisomerization products, which is unprecedented. At lower concentrations of MeOH, elimination is favored because it requires fewer molecules of MeOH, compared to alkoxycyclization. Simultaneously, the 1,2-H shift becomes competitive resulting in the observed racemization. At a higher concentration of MeOH, alkoxycyclization happens at a much faster rate than 1,2-H shift or elimination leading to enantioenriched compounds. Aryl-substituted enynes show instead an irreversible 1,2-H shift and, consequently, no racemization is observed. This is consistent with the formation of naphthalenes in the absence of a nucleophile. All these findings shed new light on the complex mechanisms of gold(I)-catalyzed alkoxycyclizations.
Author contributions
A. C. developed the main part of the experimental work, together with N. F., A. A., L. C., and F. K. Computational work was carried out by A. C. and E. G.-P., with the advice of F. M. Theoretical analysis of the enantioselectivity was performed by I. E. G. Z. performed the initial bromo-1,5-enyne cascade cyclizations with the help of G. T. The manuscript was written by A. C, E. G.-P., G. Z., and A. M. E. with input from the other authors. A. M. E. supervised this work.Conflicts of interest
There are no conflicts to declare.Data availability
A dataset collection of computational results is available in the ioChem-BD repository and can be accessed at https://doi.org/10.19061/iochem-bd-1-307.32CCDC 2337764 (2a) contains the supplementary crystallographic data for this paper.19
Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5sc09023g.
Acknowledgements
We thank the MICIU (PID2022-136623NB-I00/MICIU/AEI/10.13039/501100011033/FEDER, UE and Severo Ochoa Excellence Accreditation CEX2024-001469-S funded by MCIU/AEI/10.13039/501100011033, the European Research Council (Advanced Grant 835080), the AGAUR (2021 SGR 01256) and the CERCA Program/Generalitat de Catalunya for the financial support. Luyu Cai thanks the Chinese Research Council for funding of a predoctoral fellowship (202306540012).References
- W. Wu and H. Jiang, Acc. Chem. Res., 2014, 47(8), 2483–2504 CrossRef CAS.
- (a) V. Cadierno, Eur. J. Inorg. Chem., 2020, 886–898 CrossRef; (b) D. Campeau, D. F. León Rayo, A. Mansour, K. Muratov and F. Gagosz, Chem. Rev., 2021, 121, 8756–8867 CrossRef CAS PubMed; (c) P. Fernández-Canelas, P. Barrio and J. M. González, Tetrahedron Lett., 2022, 99, 153857 Search PubMed; (d) M. Kreuzahler and G. Haberhauer, Chem. – Eur. J., 2022, 28, e202103046 CrossRef CAS.
- (a) V. Mamane, P. Hannen and A. Fürstner, Chem. – Eur. J., 2004, 10, 4556–4575 CrossRef CAS; (b) E. Soriano and J. Marco-Contelles, Organometallics, 2006, 25, 4542–4553 CrossRef CAS; (c) G. Huang, B. Cheng, L. Xu, Y. Li and Y. Xia, Chem. – Eur. J., 2012, 18, 5401–5415 CrossRef CAS PubMed; (d) P. Morán-Poladura, E. Rubio and J. M. González, Angew. Chem., Int. Ed., 2015, 54, 3052–3055 CrossRef PubMed.
- F. Gagosz, Synthesis, 2019, 51, 1087–1099 CrossRef CAS.
- Y.-B. Bai, Z. Luo, Y. Wang, J.- M Gao and L. Zhang, J. Am. Chem. Soc., 2018, 140, 5860–5865 CrossRef CAS.
- (a) C. Liu, Y. Xue, L. Ding, H. Zhang and F. Yang, Eur. J. Org Chem., 2018, 6537–6540 CrossRef CAS; (b) T. Adak, J. Schulmeister, M. C. Dietl, M. Rudolph, F. Rominger and A. S. K. Hashmi, Eur. J. Org Chem., 2019, 3867–3876 CrossRef CAS.
- (a) M. Kreuzahler, A. Daniels, C. Wölper and G. Haberhauer, J. Am. Chem. Soc., 2019, 141(3), 1337–1348 CrossRef CAS; (b) M. Kreuzahler and G. Haberhauer, J. Org. Chem., 2019, 84(12), 8210–8224 Search PubMed; (c) M. Kreuzahler and G. Haberhauer, Angew. Chem., Int. Ed., 2020, 59, 9433–9437 CrossRef CAS; (d) M. Kreuzahler and G. Haberhauer, Angew. Chem., Int. Ed., 2020, 59, 17739–17749 CrossRef CAS; (e) N. Semleit, M. Kreuzahler and G. Haberhauer, Eur. J. Org Chem., 2020, 6629–6634 CrossRef CAS; (f) H. Siera, N. Semleit, M. Kreuzahler, C. Wölper and G. Haberhauer, Synthesis, 2021, 53(08), 1457–1470 Search PubMed.
- (a) S. T. Staben, J. J. Kennedy-Smith and F. D. Toste, Angew. Chem., Int. Ed., 2004, 43, 5350–5352 Search PubMed; (b) T. Shibata, Y. Ueno and K. Kanda, Synlett, 2006, 411–414 Search PubMed; (c) D. B. Walker, J. Howgego and A. P. Davis, Synthesis, 2010, 21, 3686–3692 Search PubMed; (d) P. Morán-Poladura, S. Suárez-Pantiga, M. Piedrafita, E. Rubio and J. M. González, J. Organomet. Chem., 2011, 696, 12–15 Search PubMed; (e) T. Nakae, R. Ohnishi, Y. Kitahata, T. Soukawa, H. Sato, S. Mori, T. Okujima, H. Uno and H. Sakaguchi, Tetrahedron Lett., 2012, 53(13), 1617–1619 Search PubMed; (f) P. Morán-Poladura, E. Rubio and J. M. González, Beilstein J. Org. Chem., 2013, 9, 2120–2128 CrossRef PubMed; (g) P. Nösel, T. Lauterbach, M. Rudolph, F. Rominger and A. S. K. Hashmi, Chem. – Eur. J., 2013, 19, 8634–8641 CrossRef; (h) P. Nösel, V. Müller, S. Mader, S. Moghimi, M. Rudolph, I. Braun, F. Rominger and A. S. K. Hashmi, Adv. Synth. Catal., 2015, 357, 500–506 Search PubMed; (i) S. Mader, L. Molinari, M. Rudolph, F. Rominger and A. S. K. Hashmi, Chem. – Eur. J., 2015, 21, 3910–3913 CrossRef CAS PubMed; (j) T. Johannsen, C. Golz and M. Alcarazo, Angew. Chem., Int. Ed., 2020, 59, 22779–22784 Search PubMed.
- (a) C. Lim, J.-E. Kang, J.-E. Lee and S. Shin, Org. Lett., 2007, 9, 3539–3542 Search PubMed; (b) T. Matsuda, S. Kadowaki, Y. Yamaguchi and M. Murakami, Chem. Commun., 2008, 2744–2746 RSC; (c) G. Bellavance and L. Barriault, Angew. Chem., Int. Ed., 2014, 53, 6701–6704 CrossRef CAS; (d) M. E. de Orbe, M. Zanini, O. Quinonero and A. M. Echavarren, ACS Catal., 2019, 9, 7817–7822 CrossRef CAS; (e) P. D. García-Fernández, C. Izquierdo, J. Iglesias-Sigüenza, E. Díez, R. Fernández and J. M. Lassaletta, Chem. – Eur. J., 2020, 26, 629–633 CrossRef; (f) P. D. García-Fernández, J. Iglesias-Sigüenza, P. S. Rivero-Jerez, E. Díez, E. Gómez-Bengoa, R. Fernández and J. M. Lassaletta, J. Am. Chem. Soc., 2020, 142, 16082–16089 CrossRef PubMed; (g) X.-Y. Qin, F.-T. Meng, M. Wang, S.-J. Tu, W.-J. Hao, J. Wang and B. Jiang, ACS Catal., 2021, 11, 6951–6959 CrossRef CAS; (h) C. Wei, J. Wu, L. Zhang and Z. Xia, Org. Lett., 2022, 24, 4689–4693 CrossRef CAS PubMed; (i) R. Miguélez, N. Semleit, C. Rodríguez-Arias, P. Mykhailiuk, J. M. González, G. Haberhauer and P. Barrio, Angew. Chem., Int. Ed., 2023, 62, e202305296 CrossRef; (j) R. Miguélez, O. Arto, C. Rodríguez-Arias, Á. del Blanco, P. Barrio and J. M. González, Eur. J. Org Chem., 2024, 27, e202301274 CrossRef; (k) R. Miguélez, O. Arto, N. Semleit, G. Haberhauer and P. Barrio, Adv. Synth. Catal., 2024, 366, 780–789 Search PubMed; (l) C. Rodríguez-Arias, R. Miguélez, Y. Holota, P. K. Mykhailiuk and P. Barrio, Org. Lett., 2025, 27, 10342–10347 CrossRef; (m) O. Arto, R. Miguélez, H. Siera, J. Schulte, I. Merino, G. Haberhauer and P. Barrio, Org. Lett., 2025, 27, 11071–11076 CrossRef PubMed.
- K. Speck, K. Karaghiosoff and T. Magauer, Org. Lett., 2015, 17, 1982–1985 CrossRef CAS.
- Z. Rong and A. M. Echavarren, Org. Biomol. Chem., 2017, 15, 2163–2167 Search PubMed.
- (a) G. Zuccarello, J. G. Mayans, I. Escofet, D. Scharnagel, M. S. Kirilova, A. H. Pérez-Jimeno, P. Calleja, J. R. Boothe and A. M. Echavarren, J. Am. Chem. Soc., 2019, 141, 11858–11863 CrossRef CAS; (b) G. Zuccarello, L. J. Nannini, A. Arroyo-Bondía, N. Fincias, I. Arranz, A. H. Pérez-Jimeno, M. Peeters, I. Martín-Torres, A. Sadurní, V. García-Vázquez, Y. Wang, S. M. Kirillova, M. Montesinos-Magraner, U. Caniparoli, G. D. Núñez, F. Maseras, M. Besora, I. Escofet and A. M. Echavarren, JACS Au, 2023, 3(6), 1742–1754 CrossRef CAS PubMed.
- L. Charruault, V. Michelet, R. Taras, S. Gladiali and J.-P. Genêt, Chem. Commun., 2004, 850–851 RSC.
- M. P. Muñoz, J. Adrio, J. C. Carretero and A. M. Echavarren, Organometallics, 2005, 24, 1293–1300 Search PubMed.
- (a) A. Martínez, P. García-García, M. A. Fernández-Rodríguez, F. Rodríguez and R. Sanz, Angew. Chem., 2010, 122, 4737–4741 CrossRef; (b) C. Tugny, N. del Rio, M. Koohgard, N. Vanthuyne, D. Lesage, K. Bijouard, P. Zhang, J. Meijide Suárez, S. Roland, E. Derat, O. Bistri- Aslanoff, M. Sollogoub, L. Fensterbank and V. Mouriès-Mansuya, ACS Catal., 2020, 10(11), 5964–5972 CrossRef CAS; (c) I. Martín-Torres, G. Ogalla, Y.-M. Yang, A. Rinaldi and A. M. Echavarren, Angew. Chem., Int. Ed., 2021, 60, 9339–9344 (Angew. Chem., 2021, 133, 9425–9430) Search PubMed; (d) A. Franchino, À. Martí and A. M. Echavarren, J. Am. Chem. Soc., 2022, 144, 3497–3509 CrossRef CAS.
- (a) E. Jiménez-Núñez, C. K. Claverie, C. Bour, D. J. Cárdenas and A. M. Echavarren, Angew. Chem., Int. Ed., 2008, 47, 7892–7895 CrossRef; (b) E. García-Padilla, F. Maseras and A. M. Echavarren, ACS Org. Inorg. Au, 2023, 3(5), 312–320 CrossRef.
- E. Jiménez-Núñez and A. M. Echavarren, Chem. Rev., 2008, 108, 3326–3350 CrossRef PubMed.
- See the supplementary information for additional details.
- CCDC 2337764: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2jgmrd.
- A. Franchino, À. Martí and A. M. Echavarren, J. Am. Chem. Soc., 2022, 144, 3497–3509 CrossRef CAS PubMed.
- A. M. Sanjuán, A. Martínez, P. García-García, M. A. Fernández-Rodríguez and R. Sanz, Beilstein J. Org. Chem., 2013, 9, 2242–2249 CrossRef PubMed.
- J. G. Mayans, J.-S. Suppo and A. M. Echavarren, Org. Lett., 2020, 22, 3045–3049 Search PubMed.
- (a) S. G. Sethofer, T. Mayer and F. D. Toste, J. Am. Chem. Soc., 2010, 132(24), 8276–8277 CrossRef CAS; (b) T.-L. Zheng, C.-Y. Huo, W. Bao, X.-T. Xu, W.-H. Dai, F. Cheng, D.-S. Duan, L.-L. Yang, X.-M. Zhang, D.-Y. Zhu and S.-H. Wang, Org. Lett., 2023, 25(41), 7476–7480 CrossRef CAS PubMed.
- A. Cataffo, M. Peña-López, R. Pedrazzani and A. M. Echavarren, Angew. Chem., Int. Ed., 2023, 62, e202312874 (Angew. Chem., 2023, 135, e202312874) CrossRef CAS PubMed.
- Z. Rong, PhD thesis, Institute of Chemical Research of Catalonia (ICIQ), Universitat Rovira i Virgili (URV), 2017, https://www.tdx.cat/handle/10803/440512#page=1 Search PubMed.
- (a) R. Kang, H. Chen, S. Shaik and J. Yao, J. Chem. Theory Comput., 2011, 7, 4002–4011 CrossRef CAS PubMed; (b) G. Ciancaleoni, S. Rampino, D. Zuccaccia, F. Tarantelli, P. Belanzoni and L. Belpassi, J. Chem. Theory Comput., 2014, 10, 1021–1034 CrossRef CAS; (c) C. García-Morales, B. Ranieri, I. Escofet, C. Obradors, L. López-Suárez, A. I. Konovalov and A. M. Echavarren, J. Am. Chem. Soc., 2017, 139, 13628–13631 CrossRef.
- I. Escofet, H. Armengol-Relats, H. Bruss, M. Besora and A. M. Echavarren, Chem. – Eur. J., 2020, 26, 15738–15745 CrossRef CAS PubMed.
- (a) M. P. Muñoz, J. Adrio, J. C. Carretero and A. M. Echavarren, Organometallics, 2005, 24, 1293–1300 CrossRef; (b) A. Martínez, P. García-García, M. A. Fernández-Rodríguez, F. Rodríguez and R. Sanz, Angew. Chem., Int. Ed., 2010, 49, 4633–4637 CrossRef; (c) C. Tugny, N. del Rio, M. Koohgard, N. Vanthuyne, D. Lesage, K. Bijouard, P. Zhang, J. Meijide Suárez, S. Roland, E. Derat, O. Bistri-Aslanoff, M. Sollogoub, L. Fensterbank and V. Mouriès-Mansuya, ACS Catal., 2020, 10(11), 5964–5972 CrossRef CAS; (d) I. Martín-Torres, G. Ogalla, Y.-M. Yang, A. Rinaldi and A. M. Echavarren, Angew. Chem., Int. Ed., 2021, 60, 9339–9344 CrossRef PubMed.
- (a) X. Zeng, S. Liu, G. B. Hammond and B. Xu, ACS Catal., 2018, 8(2), 904–909 CrossRef CAS; (b) N. V. Tzouras, A. Gobbo, N. B. Pozsoni, S. G. Chalkidis, S. Bhandary, K. Van Hecke, G. C. Vougioukalakis and S. P. Nolan, Chem. Commun., 2022, 58, 8516 RSC; (c) N. V. Tzouras, L. P. Zorba, E. Kaplanai, N. Tsoureas, D. J. Nelson, S. P. Nolan and G. C. Vougioukalakis, ACS Catal., 2023, 13, 8845–8860 CrossRef CAS.
- (a) C. Nieto-Oberhuber, S. López and A. M. Echavarren, J. Am. Chem. Soc., 2005, 127, 6178–6179 Search PubMed; (b) C. Nieto-Oberhuber, P. Pérez-Galán, E. Herrero-Gómez, T. Lauterbach, C. Rodríguez, S. López, C. Bour, A. Rosellón, D. J. Cárdenas and A. M. Echavarren, J. Am. Chem. Soc., 2008, 130, 269–279 CrossRef CAS PubMed.
- (a) C. Virumbrales, S. Suárez-Pantiga, M. Marín-Luna, C. Silva López and R. Sanz, Chem. – Eur. J., 2020, 26, 8443–8451 CrossRef CAS PubMed; (b) À. Martí, G. Ogalla and A. M. Echavarren, ACS Catal., 2023, 13, 10217–10223 CrossRef.
- M. Álvarez-Moreno, C. de Graaf, N. Lopez, F. Maseras, J. M. Poblet and C. Bo, J. Chem. Inf. Model., 2015, 55, 95–103, A dataset collection of computational results is available in the ioChem-BD repository and can be accessed at DOI:10.19061/iochem-bd-1-307.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B. 1, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 Search PubMed; (b) A. D. Becke, J. Chem. Phys., 1993, 98, 1372–1377 CrossRef CAS; (c) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B:Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- S. Grimme, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 211–228 CAS.
- E. Cancès, B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 107, 3032–3041 CrossRef.
- D. Andrae, U. Häußermann, M. Dolg, H. Stoll and H. Preuß, Theor. Chim. Acta, 1990, 77, 123–141 CrossRef CAS.
- W. R. Wadt and P. J. Hay, J. Chem. Phys., 1985, 82, 284–298 CrossRef CAS.
- W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS.
- E. García-Padilla, I. Escofet, F. Maseras and A. M. Echavarren, ChemPlusChem, 2024, 89, e202300502 Search PubMed.
- L. Zhou, Y. Zhang, R. Fang and L. Yang, ACS Omega, 2018, 3, 9339–9347 Search PubMed.
- H. R. Hudson, A. J. Koplick and D. J. Poulton, Tetrahedron Lett., 1975, 17, 1449–1452 Search PubMed.
- S. S. Shapirot and D. Dennis, Biochemistry, 1965, 4, 2283–2288 CrossRef.
- (a) E. Kraka and D. Cremer, Acc. Chem. Res., 2010, 43, 591–601 CrossRef CAS PubMed; (b) D. Roca-López, V. Polo, T. Tejero and P. Merino, J. Org. Chem., 2015, 80, 4076–4083 CrossRef PubMed.
- (a) P. García-García, M. A. Rashid, A. M. Sanjuán, M. A. Fernández-Rodríguez and R. Sanz, Org. Lett., 2012, 14(18), 4778–4781 CrossRef; (b) A. M. Sanjuán, M. A. Rashid, P. García-García, A. Martínez- Cuezva, M. A. Fernández-Rodríguez, F. Rodríguez and R. Sanz, Chem. – Eur. J., 2015, 21, 3042–3052 CrossRef.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |


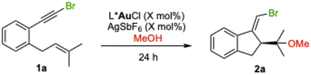

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)