Open Access Article
Open Access ArticleGuest-induced emission enhancement in the permanent porous conjugated carboracycle crystal
Kazuhiro
Yuhara
 a,
Sota
Takemori
a,
Takumi
Yanagihara
a,
Sota
Takemori
a,
Takumi
Yanagihara
 a,
Shunsuke
Ohtani
a,
Shunsuke
Ohtani
 b,
Tomoki
Ogoshi
b,
Tomoki
Ogoshi
 bc and
Kazuo
Tanaka
bc and
Kazuo
Tanaka
 *ad
*ad
aDepartment of Polymer Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615–8510, Japan. E-mail: tanaka@poly.synchem.kyoto-u.ac.jp
bDepartment of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615–8510, Japan
cWPI Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, Kakuma-machi, Kanazawa, Ishikawa 920–1192, Japan
dDepartment of Technology and Ecology, Graduate School of Global Environmental Studies, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615–8510, Japan
First published on 15th December 2025
Abstract
Macrocyclization can provide space for realizing host–guest-mediated molecular sensing based on the regulation of excited-state structural change. Here, we report guest-induced luminescent changes in crystalline o-carborane-containing conjugated macrocycles, which develop an intrinsically porous crystal with 1D channels. The crystal can capture C4-length vaporous guests and subsequently exhibit guest-induced emission enhancement through the suppression of excited-state structural relaxation. Notably, the crystal showed successful reversible guest encapsulation without losing its crystalline porosity, as well as heat resistance up to 500 °C.
Introduction
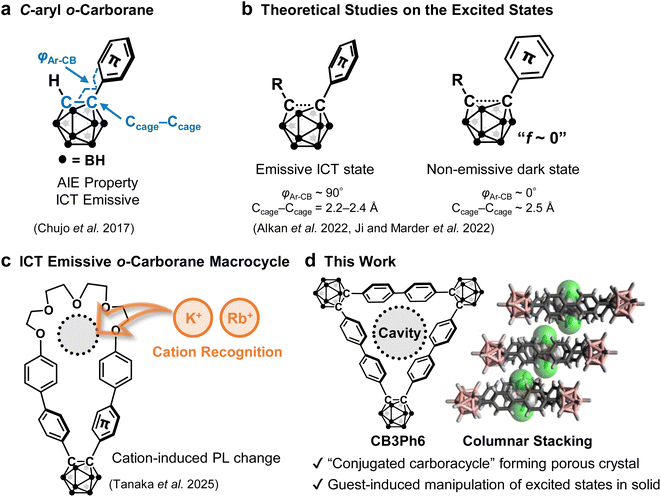
Solid-state emissive molecules with stimuli-responsiveness have attracted tremendous attention as a platform for next-generation optoelectronics.1 However, the environmental sensitivity of conventional organic luminophores is often spoiled due to aggregation-caused quenching (ACQ). One of the potential strategies for overcoming the problem of ACQ is the employment of aggregation-induced emission (AIE)-active molecules (AIEgens), which exhibit intense emission only in solid states and their intrinsic environment-sensitive optical properties.2 Previous reports have suggested that the solid-state luminescence properties in AIE should be related to the degree of structural relaxation in the excited state.3,4 When surrounding molecules tightly restrict the molecular motion of the chromophores in the excited state, the photochemical channels to non-radiative deactivation processes should be closed, resulting in significant emission enhancement.5 Based on this mechanism, various AIEgens have been extensively studied in terms of their structure–property relationships and applied as imaging probes.6 Currently, further applications in next-generation smart devices are being explored, and solid-state emissive materials including AIEgens are expected to have more stimuli-responsiveness, i.e. luminochromic properties, in response to external stimuli or environmental changes.7 As a next research step, the design of external stimuli to control the solid-state luminescence would be of importance. The restriction of molecular motion by aggregation often hinders responses to the surrounding environment, thus decreasing their stimuli-responsiveness and impeding the controllability of their luminescence properties.8 One promising design strategy to enhance sensitivity toward environmental changes is the introduction of spaces within the molecule. For example, macrocycle-containing AIEgens can provide an internal cavity that can accommodate molecular motion, and the host–guest properties of macrocycles can be a trigger for controlling their solid-state optical properties.9 To date, many studies have reported AIEgen-based macrocycles,10 whereas only a few reports have addressed solid-state host–guest properties and their effect on luminescence.A boron and carbon cluster, o-carborane, is known to act as a key unit for constructing functional AIEgens.11C-Aryl o-carborane derivatives typically show intramolecular charge transfer (ICT) emission originating from orbital conjugation between the aryl moiety and the cluster unit (Fig. 1a).12 The excited-state structures in the ICT states were theoretically studied using the following two structural parameters: the lengths of the carbon–carbon bond in the cluster (Ccage–Ccage bond) and the dihedral angles between aryl units and the Ccage–Ccage bond (φAr–CB). The ICT state can be formed when the π-plane of the aryl moiety orients perpendicularly to the Ccage–Ccage bond (φAr–CB ∼ 90°), accompanied with significant elongation of the bond from 1.6–1.9 Å in the ground state to 2.2–2.4 Å in the excited state (Fig. 1b left).13 Furthermore, recent studies suggest that when the aryl moiety aligns parallel to the Ccage–Ccage bond (φAr–CB ∼ 0°) accompanied with its further elongation to ∼2.5 Å (Fig. 1b right), a completely charge-separated state, followed by a non-emissive dark state possessing only slight oscillator strength, should be formed.14,15 These emissive ICT and non-emissive dark states play key competitive roles in photochemistry of o-carboranes. As a related example, we previously found that host–guest interaction can regulate excited-state structural relaxation in o-carborane-based macrocycles containing pseudo-crown ether (Fig. 1c).16 The macrocycles showed emission enhancement upon solution-state host–guest complexation due to the suppression of molecular motion, which quenches emission.
There have been a number of C3-symmetric macrocyclic compounds with precise host–guest chemistry.17,18C,C′-Difunctionalized o-carborane can be implemented at the corner of molecular triangles.19 Herein, we report a macrocycle consisting of cyclo-trimerized diphenyl o-carborane (CB3Ph6, Fig. 1d) as a conjugated carboracycle.20 Interestingly, CB3Ph6 formed a crystal with permanent intrinsic porosity,21 in which guest-accessible 1D channels were developed through columnar stacking of the macrocycles. Moreover, CB3Ph6 showed AIE properties, and its porous crystal showed emission enhancement upon encapsulation of vaporous guests. Theoretical calculations suggest that the filling of the cavities by guests should restrict the excited-state structural relaxation, leading to the closing of the channel to the non-emissive dark states. In addition, the crystal showed reversible guest release and capture without changing its crystalline porosity and no chemical decomposition up to 500 °C. To the best of our knowledge, this is the first example of a porous crystal with guest-encapsulation-triggered luminochromic behaviors and superior thermal stability based on the carboracycle molecule.
Results and discussion
Synthesis and crystallization
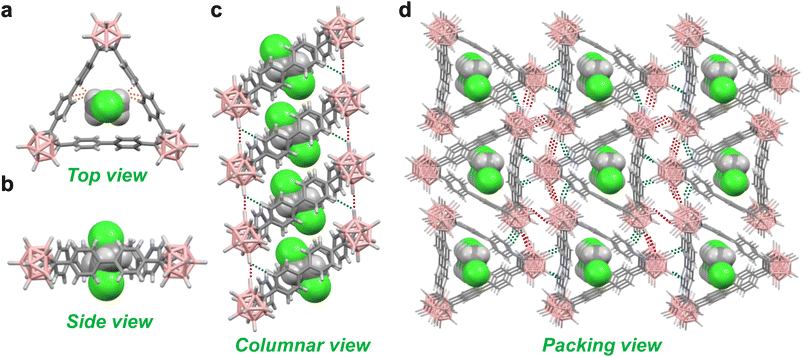
The macrocycle CB3Ph6 was successfully synthesized by Ni(0)-mediated trimerization of 1,2-bis(4-bromophenyl)-o-carborane22 (Schemes S1, S2 and Charts S1–S3, S10). The dibrominated precursor was consumed quantitatively under the reaction conditions. Purification was initially performed by column chromatography on SiO2. After column chromatography, a mixture of the product and other byproducts was still obtained. Recrystallization was then performed with 1,2-dichloroethane (DCE) to afford pure crystalline products. The synthesis of the macrocycle proceeded with a poor isolated yield of 4%, probably due to the formation of a large amount of precipitate, likely an oligomerization or polymerization product. The single-crystal of CB3Ph6 containing DCE (DCE@CB3Ph6) was obtained by vapor diffusion of MeOH into the DCE solution at −30 °C, followed by slow evaporation of the solvent at room temperature. Single-crystal X-ray diffraction (SCXRD) analysis revealed that DCE molecules occupied the cavity of each macrocycle to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex (Fig. 2a, b, S1 and Table S1). The encapsulated DCE molecules had multiple C–H⋯π interactions with the biphenyl sides located between o-carborane corners. The CB3Ph6 molecules aligned in a columnar structure with 1D channels (Fig. 2c). The stacked CB3Ph6 molecules were involved in B–H⋯H–B and B–H⋯H–C contacts. The columns assembled to form a packed structure, with several B–H⋯B, B–H⋯H–B, and B–H⋯H–C contacts existing among them (Fig. 2d).
1 host–guest complex (Fig. 2a, b, S1 and Table S1). The encapsulated DCE molecules had multiple C–H⋯π interactions with the biphenyl sides located between o-carborane corners. The CB3Ph6 molecules aligned in a columnar structure with 1D channels (Fig. 2c). The stacked CB3Ph6 molecules were involved in B–H⋯H–B and B–H⋯H–C contacts. The columns assembled to form a packed structure, with several B–H⋯B, B–H⋯H–B, and B–H⋯H–C contacts existing among them (Fig. 2d).
Adsorption and thermal properties
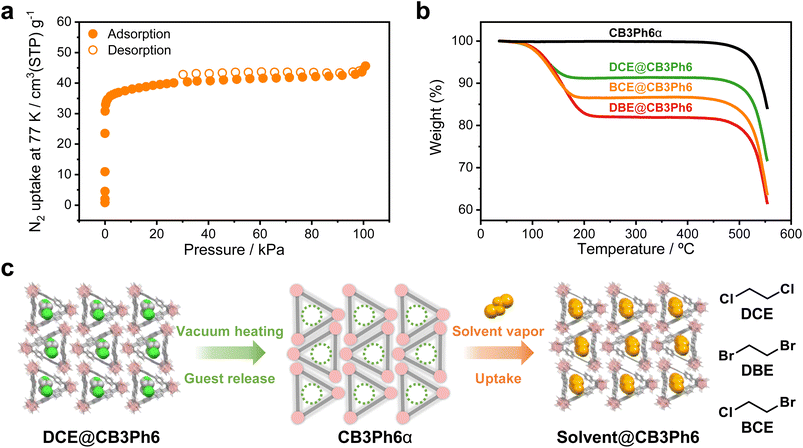
To investigate the effects of solvent inclusion on the physical properties, the guest-free crystal (CB3Ph6α) was prepared by heating DCE@CB3Ph6 at 100 °C under vacuum for 3 h. The removal of DCE was monitored via1H NMR spectroscopy (Chart S4). Unfortunately, a single-crystal sample of CB3Ph6α suitable for SCXRD analysis could not be obtained. The crystal structure of CB3Ph6α was then examined using powder X-ray diffraction (PXRD) analysis (Fig. S3 and S4). Initially, the PXRD peak positions of CB3Ph6α were almost coincident with those of DCE@CB3Ph6, suggesting that they should have similar molecular packing. Moreover, the index assignment of the PXRD patterns revealed that CB3Ph6α has the same space group (C2/c) as DCE@CB3Ph6, with only slight changes in the crystal lattice upon guest removal. These results indicate that the crystalline molecular arrangement of CB3Ph6α can be preserved during the guest-removal process. From these facts, we presumed that CB3Ph6α might form unfilled channels in which guest molecules could be accommodated. To examine the validity of this assumption, the N2 adsorption isotherm of CB3Ph6α was collected (Fig. 3a). It was found that CB3Ph6α had a typical type-I N2 isotherm, indicating the presence of microporosity. The specific surface area was calculated to be 1.5 × 102 m2 g−1 (Fig. S8), which was on the same order of magnitude as a porous crystal based on triangle compounds.18 These results indicate that CB3Ph6α has permanent intrinsic porosity and maintains crystalline molecular alignment even after the removal of guest molecules.18 Furthermore, adsorption isotherms were collected for CO2, methane (CH4), ethane (C2H6), butane (C4H10), and n-hexane (C6H14) (Fig. S9 and Table 1). CB3Ph6α showed the highest adsorption capability toward C4H10, which has multiple C–H moieties and the most similar molecular size to DCE among the tested gases. Therefore, guests with a C4 chain length should show optimal size matching with the cavity. The decrease in the adsorption capacity on C6H14 is likely due to the size incompatibility, leading to the ineffective formation of C–H⋯π contacts between the host and guest molecules. Consequently, the adsorption data suggest that CB3Ph6α can capture vaporous guests of C4-length via C–H⋯π interactions.The guest encapsulation of CB3Ph6α was studied by solid–vapor adsorption experiments. A crystalline sample of CB3Ph6α was exposed to the vapor of 1,2-dibromoethane (DBE) or 1-bromo-2-chloroethane (BCE), which have a similar molecular size to DCE, to prepare guest-loaded crystals (DBE@CB3Ph6 or BCE@CB3Ph6, respectively). 1H NMR spectra indicated that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 encapsulation of the solvent molecules proceeded in both DBE@CB3Ph6 and BCE@CB3Ph6 (Charts S5, S6 and Table S2). The PXRD patterns of DBE@CB3Ph6 and BCE@CB3Ph6 were almost identical to those of DCE@CB3Ph6 and CB3Ph6α (Fig. S4), indicating that the crystal-state arrangement of CB3Ph6 should be hardly changed upon the adsorption of DBE or BCE. The re-uptake of DCE on CB3Ph6α was also monitored by PXRD and 1H NMR measurements (Fig. S5 and Chart S7). The resulting crystals were evaluated using thermogravimetric analysis (TGA) (Fig. 3b and S10). The solvated crystals showed 10–20% weight loss between 100–200 °C, which was assignable to solvent release. The molar ratio of the included solvents to CB3Ph6 was calculated to be 1
1 encapsulation of the solvent molecules proceeded in both DBE@CB3Ph6 and BCE@CB3Ph6 (Charts S5, S6 and Table S2). The PXRD patterns of DBE@CB3Ph6 and BCE@CB3Ph6 were almost identical to those of DCE@CB3Ph6 and CB3Ph6α (Fig. S4), indicating that the crystal-state arrangement of CB3Ph6 should be hardly changed upon the adsorption of DBE or BCE. The re-uptake of DCE on CB3Ph6α was also monitored by PXRD and 1H NMR measurements (Fig. S5 and Chart S7). The resulting crystals were evaluated using thermogravimetric analysis (TGA) (Fig. 3b and S10). The solvated crystals showed 10–20% weight loss between 100–200 °C, which was assignable to solvent release. The molar ratio of the included solvents to CB3Ph6 was calculated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the three solvated crystals, as observed via their 1H NMR spectra (Table S2). The solvent release was also confirmed by endothermic peaks in differential scanning calorimetry thermograms (Fig. S11). In contrast, CB3Ph6α showed hardly any weight loss in the range of 100–200 °C, indicating complete removal of the guest solvents during activation. Reported porous organic compounds typically lose their porosity above approximately 100 °C,23 with a maximum of ∼400 °C at best.24 Notably, the decomposition temperature of the CB3Ph6 crystal is over 500 °C, as calculated from TGA curves (Table S2), which highlights the robustness of the material owing to the inorganic 3D aromaticity of o-carborane scaffolds.25,26 Moreover, in the variable-temperature PXRD measurement, the PXRD pattern of the crystal hardly changed up to at least 400 °C, indicating the superior heat resistance of the porous crystal without decomposition or melting (Fig. S12).
1 in the three solvated crystals, as observed via their 1H NMR spectra (Table S2). The solvent release was also confirmed by endothermic peaks in differential scanning calorimetry thermograms (Fig. S11). In contrast, CB3Ph6α showed hardly any weight loss in the range of 100–200 °C, indicating complete removal of the guest solvents during activation. Reported porous organic compounds typically lose their porosity above approximately 100 °C,23 with a maximum of ∼400 °C at best.24 Notably, the decomposition temperature of the CB3Ph6 crystal is over 500 °C, as calculated from TGA curves (Table S2), which highlights the robustness of the material owing to the inorganic 3D aromaticity of o-carborane scaffolds.25,26 Moreover, in the variable-temperature PXRD measurement, the PXRD pattern of the crystal hardly changed up to at least 400 °C, indicating the superior heat resistance of the porous crystal without decomposition or melting (Fig. S12).
To evaluate the stability of the crystal, the non-covalent interaction (NCI) plot27 was prepared to visualize intra- and inter-column interaction in the 1D CB3Ph6 array formed in the crystal (Fig. S13). The NCI plot indicated that vdW interactions existed between the o-carborane units in the crystal, and other interactions such as B–H⋯π and C–H⋯H–B were also visualized, as indicated by the SCXRD data based on interatomic distances (Fig. S1 and S2). Therefore, at this stage, these data suggest that multiple non-covalent interactions cooperatively contribute to the reinforcement of the porous structure and its thermal stability.
Furthermore, the PXRD patterns confirmed their high crystallinity, as indicated by consistent peaks even after guest removal and refilling (Fig. S4). These data suggest that the rigid microporosity should be hardly affected by the desorption and adsorption processes. This high crystallinity enabled SCXRD analysis on DBE@CB3Ph6 (Table S1 and Fig. S2). As we expected, DBE@CB3Ph6 had the same crystalline packing as DCE@CB3Ph6 except for the replacement of the halogen atoms. Its space group was determined to be C2/c, which is the same as that of DCE@CB3Ph6 and CB3Ph6α. The differences between the lattice constants of the two crystals were less than 1%. From these results, it can be concluded that the removal and re-uptake of the vaporous guests proceed without crystal phase transition, amorphization or chemical decomposition (Fig. 3c). The solid–gas adsorption experiment of CB3Ph6α with C4H10 was also carried out to investigate the guest selectivity. The adsorbed C4H10 molecules were quantified using 1H NMR measurements. After 30 min and 2 days of the adsorption experiment, the guest/host molar ratio was calculated to be 0.49 and 0.13, respectively (Charts S8 and S9). With DCE, DBE, and BCE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest binding was maintained for at least 30 min after the solid–vapor adsorption experiments (Charts S5–S7). These results indicate that CB3Ph6α can capture gaseous C4H10, while its host–guest binding with C4H10 could be weaker than that with halogenated ethane derivatives. Thus, it is presumed that the halogen atoms might render the C–H bonds more electron-deficient, which in turn enhances the C–H⋯π interactions with the biphenyl units.
1 host–guest binding was maintained for at least 30 min after the solid–vapor adsorption experiments (Charts S5–S7). These results indicate that CB3Ph6α can capture gaseous C4H10, while its host–guest binding with C4H10 could be weaker than that with halogenated ethane derivatives. Thus, it is presumed that the halogen atoms might render the C–H bonds more electron-deficient, which in turn enhances the C–H⋯π interactions with the biphenyl units.
Optical properties
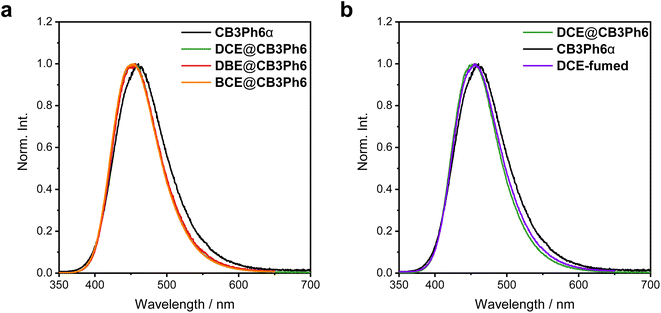
Optical measurements were conducted to understand the effect of macrocyclization and host–guest complexation in the crystalline state on excited-state relaxation processes. CB3Ph6 showed only slight photoluminescence (PL) in the CHCl3 solution (emission quantum yield (ΦPL) < 0.01), while crystalline samples exhibited values over 10 times larger (ΦPL > 0.10) (Tables 2 and S3). Moreover, CB3Ph6 exhibited emission enhancement by aggregation in the THF/H2O mixture, in which THF and H2O behaved as good and poor solvents, respectively (Fig. S14). These results indicate that CB3Ph6 is an AIEgen. The absorption spectra were hardly influenced by solvent polarity, whereas the PL spectra were significantly red-shifted as the polarity increased (Fig. S15), meaning that the emission bands should be obtained from the ICT state in the solution state. As observed in the solution state, the crystalline samples showed structureless PL bands, indicating that the crystals should show ICT emission. These results suggested that in the excited state, macrocyclic CB3Ph6 should have a significant relaxation pathway that is permissible even under structural constraints. Next, the effect of guest encapsulation was investigated for CB3Ph6 crystals (Fig. 4a and Table 2). The correspondence between the PXRD patterns for the experimental and SCXRD simulated data (Fig. S6 and S7) confirmed the phase purity of the solvated crystals. The guest-free crystal, CB3Ph6α, showed a PL band with a maximum wavelength (λPL) of 461 nm and a ΦPL of 0.11. Host–guest complexation in the solvated crystals resulted in a 14–23% increase of ΦPL and a 4–12 nm blue-shift of λPL. The PL spectra of the solvated crystals completely overlapped each other, suggesting that the luminescence properties should be obtained through a similar mechanism despite the uptake of different guests. Reversible guest encapsulation was also confirmed by the correspondence between the PL spectra of CB3Ph6α exposed to DCE vapor (DCE-fumed) and DCE@CB3Ph6 (Fig. 4b) and re-enhancement of the ΦPL value to 0.33 in DCE-fumed. To gather kinetic information, the radiative and non-radiative constants (kr and knr, respectively) were calculated using ΦPL and lifetime data (Table 2 and Fig. S16, S17). It was revealed that the kr values were hardly affected by solvent encapsulation, while the knr values decreased by an order of magnitude. This result suggests that guest molecules should inhibit non-radiative decay processes. It is widely known that cryogenic temperatures can suppress molecular motion that leads to non-radiative emission quenching. For the CB3Ph6 crystals, cooling from room temperature to 77 K induced a blue-shift of the PL spectra by 15–17 nm (Fig. S18) and significant emission enhancement regardless of the presence of guest solvents in the crystal packing (Fig. S19). These cryogenic results corresponded to the phenomena observed upon solvent encapsulation, which further implies that the included guest solvents should block the molecular motions that cause emission quenching.| Crystal | λ PL /nm | Φ PL , | τ PL /ns | τ avePL /ns | χ 2 | k r /ns−1 | k nr /ns−1 |
|---|---|---|---|---|---|---|---|
a Excited at 276 nm.
b Absolute quantum yield determined by the integrating sphere method.
c Excited at 375 nm.
d
τ
avePL =  , where fi: relative amplitude of the ith component (%), τi: luminescent decay lifetime of the ith component(s).
e
k
r = ΦPL/τavePL.
f
k
nr = (1 − ΦPL)/τavePL. , where fi: relative amplitude of the ith component (%), τi: luminescent decay lifetime of the ith component(s).
e
k
r = ΦPL/τavePL.
f
k
nr = (1 − ΦPL)/τavePL.
|
|||||||
| CB3Ph6α | 461 | 0.11 | 2.8 (51%), 0.9 (38%), 0.07 (11%) | 0.5 | 1.22 | 0.22 | 1.8 |
| DCE@CB3Ph6 | 456 | 0.29 | 2.6 (17%), 1.5 (79%), 0.1 (4%) | 1.1 | 1.06 | 0.26 | 0.65 |
| DBE@CB3Ph6 | 457 | 0.25 | 2.6 (19%), 1.2 (76%), 0.1 (4%) | 0.9 | 1.09 | 0.28 | 0.83 |
| BCE@CB3Ph6 | 454 | 0.34 | 2.4 (14%), 1.3 (83%), 0.1 (3%) | 1.1 | 0.99 | 0.28 | 0.63 |
DFT calculations
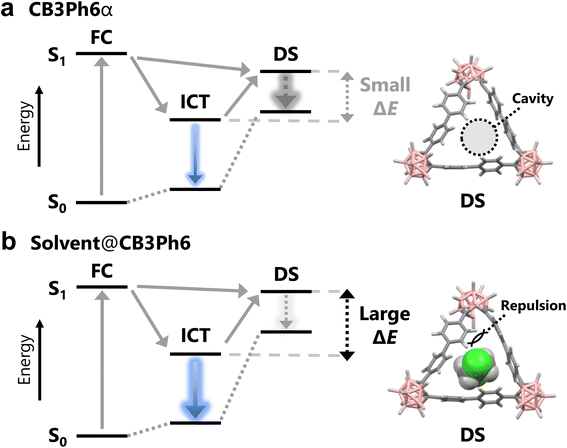
To understand the mechanism behind the guest-induced emission enhancement, theoretical calculations were performed using density functional theory (DFT) and time-dependent DFT (TD-DFT). DFT calculations for the ground state show that the calculated structure is identical to the SCXRD structure (Fig. 2a), and that isolated CB3Ph6 should have a C3-symmetry with an equal length of the Ccage–Ccage bonds in three o-carborane units (1.75 Å) and vertically oriented biphenyl sides to the Ccage–Ccage bonds (Fig. 5 left and S20a). The absorption transition (S0 to S1) involved molecular orbitals (MOs) contributed by biphenyl units (Fig. S21). TD-DFT calculations for the excited state suggest a locally-stable geometry with the Ccage–Ccage bond elongated to 2.36 Å (Fig. 5 middle and S20b). The emission transition (S1 to S0) was calculated as the ICT character between the lowest unoccupied MO (LUMO) and the highest occupied MO (HOMO). The HOMO contains the π* orbitals of two biphenyl units, while the LUMO mainly extends over the o-carborane unit (Fig. 5 middle). These results support that CB3Ph6 exhibits ICT emission contributed by one o-carborane unit and two biphenyl units connected to it.To understand the guest-induced PL change, crystal-state calculations were performed with the quantum mechanics and molecular mechanics (QM/MM) method.28 The QM/MM calculation was modeled using a cut out cluster of the SCXRD packing structures (Fig. S22). The model structure for the guest-free crystal, CB3Ph6α, was prepared based on solvent-removed DCE@CB3Ph6. From the calculations, it was proposed that guest encapsulation of DCE and DBE can induce 4 and 6 nm blue shifts of the PL spectra, respectively. These results are in close agreement with the experimental data, with blue shifts of up to 7 nm being observed (Table S4). In addition, guest encapsulation slightly suppressed Ccage–Ccage bond elongation (Fig. S23a–c). It is implied that structural relaxation in the excited state, followed by the PL blue-shift, might be caused by the limited structural relaxation. Furthermore, in the non-emissive dark excited states, the distributions of the HOMO and LUMO were almost separate (Fig. 5 right). As a result, the corresponding emission transitions are forbidden and show almost no oscillator strength, owing to only slight overlap between the HOMO and LUMO distributions (Table S17). In the dark state, isolated CB3Ph6 had a Ccage–Ccage bond length of 2.49 Å and one biphenyl unit oriented parallel to the elongated bond (Fig. 5 right and S20c). The rotated biphenyl moiety partially filled the internal cavities of CB3Ph6. The crystalline dark states were also found from the calculations using the QM/MM method (Fig. S23d–f). The dark state of CB3Ph6α was destabilized by 0.19 eV as compared to the corresponding ICT state. Significantly, the degree of destabilization was enlarged by guest encapsulation; the energy levels of the dark states of DCE and DBE in the crystal should be 0.38 and 0.44 eV higher than those of their ICT states, respectively (Table S5). In other systems, it has been reported that guest-induced PL changes are related to the suppression of structural changes in the excited state.29 In this case, the experimentally observed guest-induced emission enhancement of CB3Ph6 was associated with the decreased non-radiative decay constants, possibly due to the suppression of excited-state structural relaxation. Assuming ultrafast decay in the dark state, these results suggest the possibility that, in the absence of guest-loading, the benzene rings can rotate smoothly in the cavity, which accelerates relaxation to the non-emissive dark state, while guest filling could partially disturb the formation of dark states via steric repulsion. This should then facilitate the trapping of more excitons in the emissive ICT states. Finally, emission enhancements could be induced by guest encapsulation (Fig. 6).
Conclusion
In conclusion, we have shown that CB3Ph6, a novel AIE-active o-carborane-based macrocycle, can form a permanent intrinsic porous crystal with 1D channels. Solid–vapor adsorption indicated that vaporous halogenated ethane derivatives can thread into the channel to construct a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex. The crystal can be processed with reversible guest removal and encapsulation without losing its porosity and crystallinity. Vapor inclusion induced a blue-shift and the enhancement of PL efficiency. Theoretical calculation data suggest that filling of the cavity by vaporous guests could restrict excited-state structural relaxation in the crystal state. Furthermore, the porous crystal has excellent thermal stability up to 500 °C. One possible application of our material is the separation and detection of alkanes and alkynes based on whether multiple effective C–H⋯π interactions can be formed. Our findings might contribute to the expansion of the design and application of smart materials based on porous molecular crystals utilizing AIEgens.
1 host–guest complex. The crystal can be processed with reversible guest removal and encapsulation without losing its porosity and crystallinity. Vapor inclusion induced a blue-shift and the enhancement of PL efficiency. Theoretical calculation data suggest that filling of the cavity by vaporous guests could restrict excited-state structural relaxation in the crystal state. Furthermore, the porous crystal has excellent thermal stability up to 500 °C. One possible application of our material is the separation and detection of alkanes and alkynes based on whether multiple effective C–H⋯π interactions can be formed. Our findings might contribute to the expansion of the design and application of smart materials based on porous molecular crystals utilizing AIEgens.
Author contributions
K. Y., S. T., T. Y., and S. O. performed experiments and conducted data analysis. K. Y. conducted investigation and wrote the original draft. K. Y. and K. T. conceptualized the research framework. T. O. and K. T. provided resources and supervised the project. All authors discussed the results and contributed to reviewing and editing the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc08753h.CCDC 2495493 (DCE@CB3Ph6) and 2495494 (DBE@CB3Ph6) contain the supplementary crystallographic data for this paper.30a,b
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (B) (for K. T., JSPS KAKENHI Grant Number, 24K01570) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2024-00406152).References
- M. K. Bera, P. Pal and S. Malik, Solid-state emissive organic chromophores: design, strategy and building blocks, J. Mater. Chem. C, 2020, 8, 788–802 RSC.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Aggregation-induced emission: the whole is more brilliant than the parts, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- Y. Cai, L. Du, K. Samedov, X. Gu, F. Qi, H. H. Y. Sung, B. O. Patrick, Z. Yan, X. Jiang, H. Zhang, J. W. Y. Lam, I. D. Williams, D. Lee Phillips, A. Qin and B. Z. Tang, Deciphering the working mechanism of aggregation-induced emission of tetraphenylethylene derivatives by ultrafast spectroscopy, Chem. Sci., 2018, 9, 4662–4670 RSC.
- Y. Fujimoto, Y. Mochiduki, H. Sotome, R. Shimada, H. Okajima, Y. Toda, A. Sakamoto, H. Miyasaka and F. Ito, Excited State Dynamics of Geometrical Evolution of α-Substituted Dibenzoylmethanatoboron Difluoride Complex with Aggregation-Induced Emission Property, J. Am. Chem. Soc., 2024, 146, 32529–32538 CrossRef CAS.
- Z. Jianyu, Z. Haoke, L. W. Jacky Y and T. Ben Zhong, Restriction of Intramolecular Motion (RIM): Investigating AIE Mechanism from Experimental and Theoretical Studies, Chem. Res. Chin. Univ., 2021, 37, 1–15 CrossRef.
- Y. Duo, L. Han, Y. Yang, Z. Wang, L. Wang, J. Chen, Z. Xiang, J. Yoon, G. Luo and B. Z. Tang, Aggregation-Induced Emission Luminogen: Role in Biopsy for Precision Medicine, Chem. Rev., 2024, 124, 11242–11347 CrossRef CAS PubMed.
- Y. Huang, L. Ning, X. Zhang, Q. Zhou, Q. Gong and Q. Zhang, Stimuli-fluorochromic smart organic materials, Chem. Soc. Rev., 2024, 53, 1090–1166 RSC.
- K. Imato and Y. Ooyama, Stimuli-responsive smart polymers based on functional dyes, Polym. J., 2024, 56, 1093–1109 CrossRef CAS.
- J. R. Wu, G. Wu, D. Li and Y. W. Yang, Macrocycle-Based Crystalline Supramolecular Assemblies Built with Intermolecular Charge-Transfer Interactions, Angew. Chem., Int. Ed., 2023, 62, e202218142 CrossRef CAS.
- A. Liu and Y. W. Yang, Aggregation-induced emission in synthetic macrocycle-based supramolecular systems, Chem. Commun., 2025, 61, 13827–13840 RSC.
- J. Ochi, K. Tanaka and Y. Chujo, Recent Progress in the Development of Solid-State Luminescent o-Carboranes with Stimuli Responsivity, Angew. Chem., Int. Ed., 2020, 59, 9841–9855 CrossRef CAS.
- L. A. Boyd, W. Clegg, R. C. B. Copley, M. G. Davidson, M. A. Fox, T. G. Hibbert, J. A. K. Howard, A. Mackinnon, R. J. Peace and K. Wade, Exo-π-bonding to an ortho-carborane hypercarbon atom: systematic icosahedral cage distortions reflected in the structures of the fluoro-, hydroxy- and amino-carboranes, 1-X-2-Ph-1,2-C2B10H10 (X = F, OH or NH2) and related anions, Dalton Trans., 2004, 2786–2799 RSC.
- H. Naito, K. Nishino, Y. Morisaki, K. Tanaka and Y. Chujo, Solid-State Emission of the Anthracene-o-Carborane Dyad from the Twisted-Intramolecular Charge Transfer in the Crystalline State, Angew. Chem., Int. Ed., 2017, 56, 254–259 CrossRef CAS PubMed.
- D. Tahaoğ Lu, H. Usta and F. Alkan, Revisiting the Role of Charge Transfer in the Emission Properties of Carborane–Fluorophore Systems: A TDDFT Investigation, J. Phys. Chem. A, 2022, 126, 4199–4210 CrossRef.
- L. Ji, S. Riese, A. Schmiedel, M. Holzapfel, M. Fest, J. Nitsch, B. F. E. Curchod, A. Friedrich, L. Wu, H. H. Al Mamari, S. Hammer, J. Pflaum, M. A. Fox, D. J. Tozer, M. Finze, C. Lambert and T. B. Marder, Thermodynamic equilibrium between locally excited and charge-transfer states through thermally activated charge transfer in 1-(pyren-2′-yl)-o-carborane, Chem. Sci., 2022, 13, 5205–5219 RSC.
- M. Tokutomi, K. Yuhara and K. Tanaka, Macrocyclic C,C′-diaryl-o-carborane derivatives containing pseudo-crown ether: emission enhancement upon cation recognition, Phys. Chem. Chem. Phys., 2025, 27, 15845–15849 RSC.
- Y. Wang, H. Wu and J. F. Stoddart, Molecular Triangles: A New Class of Macrocycles, Acc. Chem. Res., 2021, 54, 2027–2039 CrossRef CAS.
- A. Chaix, G. Mouchaham, A. Shkurenko, P. Hoang, B. Moosa, P. M. Bhatt, K. Adil, K. N. Salama, M. Eddaoudi and N. M. Khashab, Trianglamine-Based Supramolecular Organic Framework with Permanent Intrinsic Porosity and Tunable Selectivity, J. Am. Chem. Soc., 2018, 140, 14571–14575 CrossRef CAS PubMed.
- Q. Qu, M. Fu, C. Lin, Y. Geng, Y. Li and Y. Yuan, Synthesis, properties and application of o-carborane-based π-conjugated macrocycles, Org. Chem. Front., 2023, 10, 3293–3299 RSC.
- W. Jiang, I. T. Chizhevsky, M. D. Mortimer, W. Chen, C. B. Knobler, S. E. Johnson, F. A. Gomez and M. F. Hawthorne, Carboracycles: Macrocyclic Compounds Composed of Carborane Icosahedra Linked by Organic Bridging Groups, Inorg. Chem., 1996, 35, 5417–5426 CrossRef CAS.
- M. Mastalerz, Permanent porous materials from discrete organic molecules-towards ultra-high surface areas, Chem.–Eur. J., 2012, 18, 10082–10091 CrossRef CAS PubMed.
- S. Cheng, L. Zong, K. Yuan, J. Han, X. Jian and J. Wang, Synthesis and thermal properties of an acetylenic monomer containing boron and silicon, RSC Adv., 2016, 6, 88403–88410 RSC.
- H. Yamagishi, Functions and fundamentals of porous molecular crystals sustained by labile bonds, Chem. Commun., 2022, 58, 11887–11897 RSC.
- C. G. Bezzu, L. A. Burt, C. J. McMonagle, S. A. Moggach, B. M. Kariuki, D. R. Allan, M. Warren and N. B. McKeown, Highly stable fullerene-based porous molecular crystals with open metal sites, Nat. Mater., 2019, 18, 740–745 CrossRef CAS PubMed.
- A. Muñoz-Castro, Aromatic trails: persistence and interplay between linked spherical aromatic dicarboranes in dimer to hexamer linear arrays, Phys. Chem. Chem. Phys., 2025, 27, 5249–5255 Search PubMed.
- P. L. Rodríguez-Kessler and A. Muñoz-Castro, Macrocyclic meta-carborane hexamer. Evaluation of aromatic characteristics as a cluster-based analog to phenyl-bridged macrocyclic structures, Phys. Chem. Chem. Phys., 2025, 27, 6744–6750 Search PubMed.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, Revealing noncovalent interactions, J. Am. Chem. Soc., 2010, 132, 6498–6506 Search PubMed.
- Q. Wu, T. Zhang, Q. Peng, D. Wang and Z. Shuai, Aggregation induced blue-shifted emission-the molecular picture from a QM/MM study, Phys. Chem. Chem. Phys., 2014, 16, 5545–5552 RSC.
- M. Rojas-Poblete, P. L. Rodríguez-Kessler, R. Guajardo Maturana and A. Muñoz-Castro, Coinage-metal pillarplexes hosts. Insights into host-guest interaction nature and luminescence quenching effects, Phys. Chem. Chem. Phys., 2021, 23, 15917–15924 Search PubMed.
- (a) CCDC 2495493: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2prrs0; (b) CCDC 2495494: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2prrt1.
| This journal is © The Royal Society of Chemistry 2026 |