Open Access Article
Open Access ArticlePhotocatalytic CO2 reduction using a diazabenzacenaphthenium photosensitizer and a Mn catalyst
Kei Kamogawa *a,
Shintaro Okumura
*a,
Shintaro Okumura *b and
Osamu Ishitani
*b and
Osamu Ishitani *a
*a
aDepartment of Chemistry, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8526, Japan. E-mail: kamokei@hiroshima-u.ac.jp; iosamu@hiroshima-u.ac.jp
bDepartment of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto 615-8510, Japan. E-mail: okumura.shintaro.6e@kyoto-u.ac.jp
First published on 2nd January 2026
Abstract
Redox photosensitizers are key components in photoredox catalysis, mediating photoinduced electron transfer from an electron donor to a catalyst or substrate. In systems proceeding through reductive quenching, the efficiency of forming the one-electron-reduced photosensitizer critically determines the overall quantum yield. Here, we report a comprehensive photophysical investigation of 4,5,9,10-tetraethyl-1,2-dihydrobenz[de]imidazo[1,2,3-ij]-1,8-naphthridinium cation (N-BAP+), originally developed as a redox photosensitizer for oxidative quenching in photocatalytic organic synthesis. The photochemical reduction of N-BAP+ by 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH) was also analyzed, focusing on the contributions of the singlet and triplet excited states of N-BAP+. Spectroscopic studies demonstrate that N-BAP+ undergoes efficient reductive quenching with BIH, generating the one-electron-reduced species N-BAP˙. Based on these findings, we applied N-BAP+ to photocatalytic CO2 reduction using a Mn–complex catalyst and BIH as a reductant, resulting in an efficient and durable photocatalytic CO2 reduction system. Kinetic analysis revealed that the reductive quenching of the triplet excited state was the essential pathway governing the activity of this photocatalytic CO2 reduction. These findings suggest that numerous candidates, with or without TADF properties, could serve as organic redox photosensitizers free of heavy metal ions. For optimal performance, such molecules should achieve as high a triplet excited-state formation yield as possible while keeping the singlet excited-state lifetime as short as possible.
Introduction
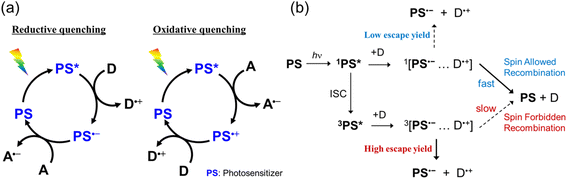
Redox photosensitizers are frequently used in various photocatalytic reactions, especially in the fields of artificial photosynthesis1–5 and organic synthesis.6–9 The photosensitizer absorbs light to transfer an electron from an electron donor to an acceptor as shown in Scheme 1a. Since this redox photosensitized process initiates various photocatalytic reactions, the selection of a photosensitizer has a critical effect on the overall efficiency of photocatalytic reactions.Recently, in addition to transition metal complexes, organic dyes have been actively investigated as photosensitizers. For example, several organic TADF (thermally activated delayed fluorescence) dyes are suitable as photosensitizers because they exhibit strong UV-vis absorption, appropriate electrochemical properties, and long-lived triplet excited states with high yields.10–13 Many organic photosensitizers differ significantly from conventional heavy-metal-based photosensitizers. For example, the singlet metal-to-ligand charge transfer (1MLCT) excited state of [Ru(diimine)3]2+ is rapidly converted to the triplet (3MLCT) excited state within approximately 100 fs via intersystem crossing (ISC), and only the 3MLCT excited state contributes to photocatalytic reactions.14 In contrast, since the ISC rates from the singlet to the triplet excited states of organic molecules are mostly much slower, both singlet and triplet excited states can be quenched by an electron donor and/or acceptor. For instance, the rate constants of ISC of 2,4,5,6-tetrakis(diphenylamino)-isophthalonitrile (4DPAIPN) and 2,4,6-tri(diphenylamino)-5-fluoroisophthalonitrile (3DPAFIPN), which have been employed as efficient TADF organic photosensitizers for photocatalytic CO2 reduction, are 2.9 × 108 s−1 and 1.5 × 108 s−1, respectively.15 These rates are slow enough to compete with dynamic quenching of their singlet excited states in solution. Recently, our group reported that reductive quenching of the triplet excited state of these TADF organic photosensitizers by 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH) affords a triplet geminate radical ion pair, which experiences a slower backward electron transfer affording the ground state than the corresponding singlet geminate radical ion pair.15 The escape yield from the triplet geminate radical ion pair is approximately 10 times higher than that from the singlet state. The significant difference is attributed to the spin selection rule on the charge recombination processes: the spin-forbidden nature of the charge recombination within the triplet geminate radical pair results in slower recombination and higher escape yield (Scheme 1b).16 Therefore, in photoredox reactions employing organic photosensitizers, it is essential to investigate in detail the reactivities of both the singlet and triplet excited states.
There are several reports on efficient photocatalytic CO2 reduction reactions employing organic photosensitizers that are not TADF dyes, such as anthraquinone derivatives,17–19 phenoxazine derivatives,20,21 acriflavine derivatives,22,23 and triazatriangulenium salts.24–26 However, in these organic photosensitizers, the relative contributions of the singlet and triplet excited states to the reactions, including the influence of the spin multiplicity of the excited states on the quantum yield of the photocatalytic reaction, have not been quantitatively evaluated. Transient absorption spectroscopy should be an effective method for probing the photoreactivity of triplet excited states in such photosensitizers that lack both TADF and room-temperature phosphorescence.
4,5,9,10-Tetraethyl-1,2-dihydrobenz[de]imidazo[1,2,3-ij]-1,8-naphthridinium cation (N-BAP+ in Chart 1) also acts as a photosensitizer without the TADF properties owing to relatively large energy gap between the singlet and triplet excited states (ΔEST = 290 meV). Its excited states 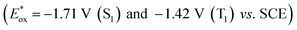 and one-electron reduced species (Ep,red = −1.89 V) exhibit strong reducing power. Owing to this reducing ability and intense visible-light absorption, N-BAP+ functions as a redox photosensitizer for visible-light-driven four-electron reduction of esters to generate a carbinol anion intermediate.27 Although the high durability and strong reducing power of N-BAP+ suggest its potential applicability to photocatalytic reduction of CO2, such systems have not been reported yet, and the photophysical properties of N-BAP+, particularly the photoreactivity of its triplet excited state, have not been thoroughly investigated.
and one-electron reduced species (Ep,red = −1.89 V) exhibit strong reducing power. Owing to this reducing ability and intense visible-light absorption, N-BAP+ functions as a redox photosensitizer for visible-light-driven four-electron reduction of esters to generate a carbinol anion intermediate.27 Although the high durability and strong reducing power of N-BAP+ suggest its potential applicability to photocatalytic reduction of CO2, such systems have not been reported yet, and the photophysical properties of N-BAP+, particularly the photoreactivity of its triplet excited state, have not been thoroughly investigated.
Herein, we report detailed photophysical properties of N-BAP+, including the rates associated with ISC and the yield of its triplet excited state. The reductive quenching processes of the singlet and triplet excited states of N-BAP+ by BIH were also thoroughly investigated. Because N-BAP+ does not exhibit delayed fluorescence or room-temperature phosphorescence, the dynamics of these photophysical and photochemical processes were investigated in detail using transient absorption spectroscopy, in contrast to previous studies on TADF organic photosensitizers that relied on emission-decay analysis. Based on these findings, we successfully developed a new photocatalytic system for CO2 reduction employing N-BAP+ as a redox photosensitizer, fac-[MnI(6-mesityl-4,4′-dimethyl-2,2′-bipyridine)(CO)3(OC(O)OCH2CF3)] (MnMes in Chart 1) as a catalyst, and BIH as an electron donor, in which N-BAP+ exhibited remarkable durability and effectively functioned as a redox photosensitizer for the selective reduction of CO2 to CO. Furthermore, we constructed a kinetic model for the reductive quenching of the singlet and triplet excited states in non-TADF molecules and examined how the spin multiplicity of the excited state influences the photocatalytic activity for CO2 reduction.
Results and discussion
Photophysical properties of N-BAP+
N-BAP+ exhibited a strong absorption band at λmax = 441 nm (ε = 9200 M−1 cm−1) with a vibronic structure in a DMSO solution containing 3.78 M trifluoroethanol (TFE) (Fig. 1a). This mixed solvent was used for the photocatalytic CO2 reduction described later. Fluorescence was observed at λmax = 452 nm at room temperature. The fluorescence lifetime (τf) and quantum yield (Φf) were determined to be 5.6 ns and 33%, respectively, under Ar (Fig. 1b and Table 1). The fluorescence lifetime was marginally reduced under air (5.3 ns), indicating minor quenching by O2. Although N-BAP+ exhibits phosphorescence at 77 K,27 phosphorescence was not detected at room temperature.To gain deeper insight into the excited-state dynamics of N-BAP+, particularly the triplet excited state, ns-transient absorption (TA) measurements were performed. Fig. 2a shows the TA spectra of N-BAP+ in an Ar-purged DMSO–TFE mixed solution following pulsed excitation at 355 nm. The spectra were globally analyzed using a two-component sequential model (Fig. 2b and c).28,29 The evolution-associated spectra (EAS) shown in Fig. 2b represent the spectral profiles of transient species associated with each kinetic component. The first component (EAS1) exhibited intense absorption at λmax ≈ 660 nm with a shoulder near 610 nm. This state was converted to the second component (EAS2) with a time constant of 5.7 ns. Because this time constant matches the fluorescence lifetime (Fig. 1b), EAS1 is attributed to the singlet excited state (1*N-BAP+). The second component displayed broad absorption at λmax ≈ 620 nm and a long lifetime (>10 µs). Under aerobic conditions, the decay of this component was significantly accelerated, while the decay of the first component was only marginally affected, suggesting that EAS2 corresponds to the triplet excited state (3*N-BAP+) (Fig. S1). Furthermore, the calculated T–T absorption spectrum of 3*N-BAP+ closely matches that of EAS2 (Fig. S2). Based on these results, EAS2 is assigned to 3*N-BAP+. Therefore, a fraction of the photochemically generated 1*N-BAP+ is converted to 3*N-BAP+ via ISC even at room temperature.
The quantum yield of ISC (ΦISC) was determined to be 57% using a relative actinometry method (see SI for details);30–32 ΦISC was calculated by comparing the triplet-excited-state yield of N-BAP+ with that of a reference solution containing [Ru(bpy)3]Cl2, whose ISC quantum yield (ΦISC,Ru) is close to unity,14,33 with both solutions excited by 355 nm laser light of identical intensity (eqn (1)).
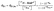 | (1) |
| τf−1 = kr + knr + kISC | (2) |
| Φf = krτf | (3) |
| ΦISC = kISCτf | (4) |
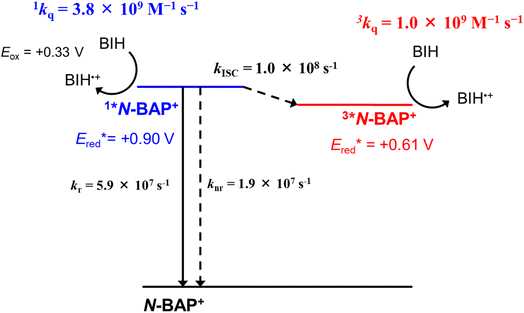
As described above, photoexcitation of N-BAP+ generates both 1*N-BAP+ and 3*N-BAP+. Although their lifetimes differ, both are sufficiently long to enable bimolecular reactions via diffusion in solution and they exhibit substantial photo-oxidation power 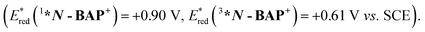 27 Therefore, the reductive quenching processes of 1*N-BAP+ and 3*N-BAP+ were examined using 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH), which is a commonly employed reductant in various photocatalytic reactions (Eox(BIH) = +0.33 V).34–37
27 Therefore, the reductive quenching processes of 1*N-BAP+ and 3*N-BAP+ were examined using 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH), which is a commonly employed reductant in various photocatalytic reactions (Eox(BIH) = +0.33 V).34–37
Reductive quenching of the excited N-BAP+ by BIH
Fluorescence from 1*N-BAP+ was quenched upon addition of BIH in a DMSO solution containing TFE (3.78 M) (Fig. S3). From the slope of the linear Stern–Volmer plot (KSV = 20.9 M−1) and the fluorescence lifetime (τf = 5.6 ns) under Ar, the quenching rate constant for 1*N-BAP+ was determined to be 1kq = 3.8 × 109 M−1 s−1. Because BIH has a much higher singlet excitation energy (E00 = 3.79 eV) than 1*N-BAP+ (2.79 eV),27,38 energy transfer can be ruled out as a possible quenching mechanism. Thus, we conclude that 1*N-BAP+ is reductively quenched by BIH.The quenching process of 3*N-BAP+ by BIH was investigated using TA measurements because N-BAP+ does not exhibit phosphorescence and delayed fluorescence at room temperature. Fig. 3a shows the typical TA spectra of an Ar-purged DMSO solution containing N-BAP+ (0.4 mM), TFE (3.78 M), and BIH (1 mM) following pulsed excitation at 355 nm. In this experiment, the absorption of BIH at 355 nm was negligible owing to its low concentration, indicating that N-BAP+ was selectively excited by the pump pulse (Fig. S4). The reductive quenching of 1*N-BAP+ by BIH was also negligible due to the low BIH concentration (only 2% of 1*N-BAP+ is quenched in the presence of 1 mM of BIH; see eqn (6) described below). At 38 ns after pulsed excitation, when ISC is complete, the absorption band assigned to 3*N-BAP+ was observed. The spectra were globally analyzed using a three-component sequential model (Fig. 3b and c).
The first component (EAS1), assigned to 3*N-BAP+, decayed rapidly with a time constant τ1 = 1.0 µs, forming the second component (EAS2), which exhibited a weak and broad absorption band at λmax ≈ 470 nm. τ1 depends linearly on the BIH concentration between 0.5 mM and 2 mM, with a bimolecular rate constant of 1.0 × 109 M−1 s−1 (Fig. S5), indicating that 3*N-BAP+ is dynamically quenched by BIH. Because the triplet excitation energy of BIH (3.04 eV) is much higher than that of 3*N-BAP+ (2.50 eV),27,38 we conclude that 3*N-BAP+ is reductively quenched by BIH with the rate constant 3kq = 1.0 × 109 M−1 s−1, forming the one-electron-reduced species N-BAP˙ and the one-electron-oxidized species BIH˙+. The second component subsequently decayed, giving rise to the long-lived third component (EAS3) with a time constant τ2 = 7.7 µs. The third component exhibited a broad absorption band at λmax ≈ 650 nm, similar to the absorption spectrum of BI˙, which is a deprotonated species of BIH˙+ (Fig. 3d).39,40 Therefore, EAS2 is attributed to the combined absorption of BIH˙+ and N-BAP˙, and EAS3 to those of BI˙ and N-BAP˙ (Fig. 3). Although BI˙ has relatively strong reduction power (Ep,ox = −1.68 V vs. SCE),41 it is insufficient to reduce N-BAP+ in the ground state (Ep,red = −1.89 V vs. SCE), leading to BI˙ accumulation during TA measurements. A much slower decay of EAS3 (>100 µs) was observed, likely due to decomposition and/or bond formation among accumulated radical species. It is noteworthy that these decomposition processes should not proceed in the photocatalytic reactions described below because both N-BAP˙ and BI˙ supply an electron to the catalyst (MnMes) and the intermediate(s) derived from MnMes owing to the strong reduction powers of these radical species.
Scheme 2 summarizes the rate constants for all processes following the excitation of N-BAP+ in the presence of BIH. These experiments and kinetic analyses clearly demonstrate that both 1*N-BAP+ and 3*N-BAP+ can be reductively quenched by BIH, with large but distinct rate constants. Based on these results and the suitable redox potential of MnMes (Ep,red = −1.12 V vs. SCE),42 we employed N-BAP+ as a redox photosensitizer for photocatalytic CO2 reduction in combination with BIH and MnMes.
Photocatalytic CO2 reduction using N-BAP+ and MnMes
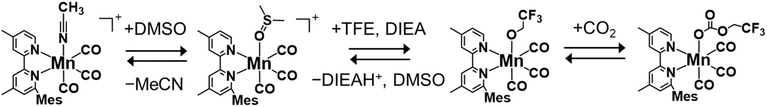
The solution containing MnMes was prepared according to a reported method:42 the corresponding MeCN complex, [Mn(6-mesityl-4,4′-dimethyl-2,2′-bipyridine)(CO)3(MeCN)](PF6), was dissolved in DMSO, followed by the addition of TFE and diisopropylethylamine (DIEA). TFE serves as both a CO2-capturing reagent and a proton source in the CO2 reduction cycle of the Mn complex. DIEA is essential for in situ generation of MnMes by coordinating deprotonated TFE to the Mn(I) center, facilitating CO2 insertion into the Mn–O bond to form the carbonate ester complex MnMes (Scheme 3). This CO2 capturing reaction increases the local concentration of CO2 near the Mn center, enabling the efficient photocatalytic CO2 reduction to CO even under low CO2 concentration.As a typical photocatalytic CO2 reduction reaction, a CO2-saturated DMSO solution containing N-BAP+ (0.1 mM), MnMes (0.05 mM), BIH (0.1 M), TFE (3.78 M), and DIEA (1 vol%, 58 mM) was irradiated with LED light (λmax = 430 nm). MnMes also exhibits UV-vis absorption in the excitation wavelength region (Fig. S6). To suppress the inner-filter effect and photodecomposition of MnMes during the photocatalytic reaction, we used two equivalents of the photosensitizer relative to the catalyst. Fig. 4 shows the results of the photocatalytic reaction. After 72 h of irradiation, 142 ± 15 µmol of CO was selectively produced, along with small amounts of H2 (0.2 ± 0.1 µmol) and HCOO− (0.45 ± 0.06 µmol). The turnover number (TON) for CO production reached 1420 based on MnMes and 710 based on N-BAP+. The absorption spectra of the reaction solutions remained nearly unchanged during the initial 8 h of the photocatalytic reaction, but underwent a substantial change after 24 h of irradiation, which correlates with the decline in the CO formation rate (Fig. S7). This suggests that the decomposition of N-BAP+ is a contributing factor to the reduced photocatalytic activity, as evidenced by the significant decrease in its characteristic vibronic absorption at the final stage of the reaction (≥48 h irradiation).
Table 2 summarizes the control experiments. In the absence of N-BAP+, MnMes, CO2, excitation light, or BIH, no or only trace amounts of CO2 reduction products were detected, confirming that all components are essential for the photocatalytic CO2 reduction reaction. The small amount of CO detected in these control experiments (entry 2, 4 and 6) likely originated from the decomposition of MnMes, which can release up to three equivalents of CO. Although the source of the trace amounts of H2 detected in the absence of either MnMes or CO2 remains unclear, this observation clearly indicates that reduced N-BAP+ cannot directly transfer an electron to CO2 and that CO formation requires the presence of CO2. The small amounts of HCOO− and H2 observed in the absence of BIH may result from photochemical decomposition of components in the reaction solution (entry 6). Changing the counter anion of N-BAP+ from Cl− to PF6− had no significant effect on the photocatalytic activity, with a comparable amount of CO (128 µmol) being selectively produced and only minor amounts of H2 (0.03 µmol) and HCOO− (0.47 µmol) formed after 72 h of irradiation.
| Entrya | Excluded component | Products/µmol (TON)a | ||
|---|---|---|---|---|
| CO | HCOO− | H2 | ||
| a (Entry 1) as shown in Fig. 4, the photocatalytic solution was irradiated with LED light (λmax = 430 nm) for 72 h. (Entries 2–6) either MnMes (0.05 mM), N-BAP+ (0.1 mM), BIH (0.1 M), CO2, or light were excluded from the photocatalytic reactions.b The photocatalytic reaction was performed under Ar. | ||||
| 1 | — | 142 ± 15 (1420 ± 150) | 0.45 ± 0.06 (4.5 ± 0.6) | 0.2 ± 0.1 (2 ± 1) |
| 2 | N-BAP+ | 0.41 (4.1) | N.D. | <0.1 |
| 3 | MnMes | N.D. | N.D. | 0.33 (3.3) |
| 4b | CO2 | 0.21 (2.1) | N.D. | 0.35 (3.5) |
| 5 | Light | N.D. | N.D. | N.D. |
| 6 | BIH | 0.34 (3.4) | 0.29 (2.9) | 0.17 (1.7) |
Furthermore, the reaction was performed under a 13CO2 atmosphere to verify the carbon source of the produced CO. The peak at m/z = 29, attributed to 13CO, was dominant (Fig. S8a), whereas the peak at m/z = 28 was mainly observed in the corresponding experiment using ordinary CO2 (Fig. S8b). Thus, CO2 was verified to be the carbon source of the produced CO. These results demonstrate that N-BAP+ functions as a highly durable redox photosensitizer for photocatalytic CO2 reduction using MnMes as a catalyst.
To evaluate the reaction quantum yields for CO formation (ΦCO; eqn (5)), DMSO solutions containing MnMes (0.05 mM), N-BAP+ (0.1 mM), BIH, TFE (3.78 M), and DIEA (58 mM) were irradiated with a monochromatic light at 430 nm (5.0 × 10−9 einstein per s). In the presence of 0.1 M BIH, ΦCO was 13.4%. Notably, ΦCO increased as the BIH concentration decreased (Fig. 5), reaching a maximum of 30.9% at 0.01 M BIH. To the best of our knowledge, this is the first report demonstrating that the quantum yield of a photocatalytic CO2 reduction reaction using a non-TADF organic photosensitizer depends on the concentration of the reductant.
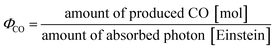 | (5) |
Reactivities of 1*N-BAP+ and 3*N-BAP+ in the photocatalytic CO2 reduction reactions
Since the reductive quenching of 1*N-BAP+ by BIH can compete with the ISC process (Scheme 2), the BIH concentration influences both the formation yield of 3*N-BAP+ and the quantum yields of reductive quenching for both 1*N-BAP+ (1Φq) and 3*N-BAP+ (3Φq). As shown in Fig. 5, ΦCO also varies with BIH concentration. In this section, we discuss in detail the relationships among 1Φq, 3Φq, and ΦCO based on their dependence on BIH concentration.1Φq and 3Φq can be expressed as functions of [BIH] as follows.
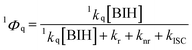 | (6) |
 | (7) |
 | ||
| Fig. 6 Quantum yield for CO formation (ΦCO), and calculated quantum yields for the reductive quenching of 1*N-BAP+ (1Φq) and 3*N-BAP+ (3Φq) as functions of BIH concentration. The black dashed line represents the fit based on eqn (8). | ||
Since CO is a two-electron reduction product of CO2 and BIH acts as a two-electron donor in the photosensitized reactions (Fig. 3d),36 the relationship among 1Φq, 3Φq, and ΦCO is expressed in eqn (8).15
| ΦCO = f × 1/2 × (2 × (1Φq × 1ηesc + 3Φq × 3ηesc)) = f × (1Φq × 1ηesc + 3Φq × 3ηesc) | (8) |
Based on the above results and discussions, along with our recent reports on photocatalytic CO2 reduction using the TADF molecules as redox photosensitizers,15,42 we can summarize the photocatalytic CO2 reduction mechanism in the system employing N-BAP+ as the photosensitizer, as shown in Scheme 4. Although 1*N-BAP+ can be reductively quenched by BIH (particularly at higher concentrations), the yield of free N-BAP˙ formation is very low because of rapid charge recombination within the singlet geminate radical pair 1[N-BAP˙⋯BIH˙+]. At lower BIH concentrations, the ISC process becomes more favorable, leading to a higher concentration of 3*N-BAP+. Because reductive quenching of 3*N-BAP+ proceeds efficiently even in the cases using relatively low concentration of BIH owing to its long lifetime and sufficient oxidation power, and because the escape yield of the triplet geminate radical pair 3[N-BAP˙⋯BIH˙+] (3ηesc) is much higher than that of the singlet pair (1ηesc), the dominant formation pathway for free N-BAP˙ originates from [3N-BAP˙⋯BIH˙+] (Scheme 4a). The resulting N-BAP˙ then initiates photocatalytic reduction of CO2 in the Mn complex via single-electron transfer to MnMes. Oxidative quenching of the excited N-BAP+ by MnMes should be a minor process because its concentration (0.05 mM) is much lower than that of BIH (>10 mM) in the photocatalytic reaction solution. Rapid deprotonation of BIH˙+ leads to efficient formation of BI˙, which serves as an additional electron donor in the photocatalytic reaction. However, because the reduction potential of BI˙ is less negative than that of N-BAP+, BI˙ cannot reduce N-BAP+; instead, it transfers an electron directly to MnMes and/or to intermediates derived from MnMes. Although dimerization of one-electron-reduced Mn(I) species and photochemical decomposition of the produced Mn(0) dimer typically lowers the performance of many Mn(I)–complex systems, steric hindrance from the mesityl group at the 6-position of 4,4′-dimethyl-2,2′-bipyridine completely suppresses such dimerization.42 This structural feature is another reason for the high durability of the photocatalytic system reported herein. The intermediate(s) originating from the one-electron-reduced MnMes subsequently accept(s) an additional electron from BI˙ and/or N-BAP˙ to form [Mn(6-mesityl-4,4′-dimethyl-2,2′-bipyridine)(CO)3(CO)2] (MnMes–CO2), which is a key intermediate for CO production by Mn catalysts (Scheme 4b).42–44 Through subsequent multistep reactions, MnMes–CO2 releases CO and regenerates the original carbonate ester complex, MnMes, through the CO2 capturing reaction. In this process, another CO2 molecule might serve as an acceptor for “O2−”, leading to the formation of HCO3− in an amount equivalent to the CO produced.
From this work, the key properties required for next-generation organic photosensitizers capable of achieving high quantum yields have become evident. The first requirement is a rapid ISC to shorten the lifetime of the singlet excited state because the singlet radical pair produced via reductive quenching of the singlet excited state by a reductant cannot efficiently yield the free one-electron-reduced photosensitizer owing to the rapid charge recombination. Although N-BAP+ exhibits a relatively fast ISC rate (1.0 × 108 s−1), it is still insufficient to kinetically suppress the reductive quenching of the singlet excited state. Further enhancement of the spin–orbit coupling should be required to accelerate the ISC process, for example by introducing heavy atoms, tuning the energy levels of the singlet excited states and triplet excited states, and increasing the angular momentum change associated with ISC. Another important factor is improving the cage-escape yield in the triplet-quenching process. To increase the cage-escape yield of N-BAP+ from 66% toward nearly 100%, it is essential to elucidate the factors that govern the cage-escape process, which is influenced by multiple parameters such as the electron-transfer distance, the driving force for recombination, the noncovalent interactions between the paired radicals, and the solvent environment. Based on these principles, our research group is currently developing N-BAP+ derivatives with even faster ISC rates and higher 3ηesc.
Conclusion
4,5,9,10-Tetraethyl-1,2-dihydrobenz[de]imidazo[1,2,3-ij]-1,8-naphthridinium cation (N-BAP+) functions as a durable and efficient redox photosensitizer for photocatalytic CO2 reduction. The photocatalytic system, comprising N-BAP+, MnMes, and BIH, achieved a high turnover number of 710 based on the photosensitizer N-BAP+ (1420 based on the catalyst MnMes) and a maximum CO formation quantum yield of 30.9%. Detailed investigation of the photophysical properties of N-BAP+ using transient absorption spectroscopy revealed that both its singlet and triplet excited states undergo reductive quenching by BIH. Kinetic analysis demonstrated a clear correlation between ΦCO and the quenching pathway: ΦCO increases when the quenching pathway of the triplet excited state of N-BAP+ becomes dominant. This enhancement arises from the significantly higher cage-escape yield of the triplet geminate radical pair 3[N-BAP˙⋯BIH˙+] compared to the singlet pair. The triplet geminate radical pair exhibits a cage-escape yield 19 times higher than that of the corresponding singlet pair, owing to the spin-forbidden nature of its charge recombination. The high activity of N-BAP+ as a redox photosensitizer can be attributed to its sufficiently high yield of triplet excited state. We have previously identified this principle in systems employing TADF-type photosensitizers.15 This study confirms that the superiority of the triplet pathway in enhancing cage-escape efficiency is a general mechanism applicable not only to TADF-type photosensitizers but also to a broader range of organic photosensitizers that lack TADF properties. The kinetic analysis established in this study is applicable to general organic photosensitizers that do not show delayed fluorescence and thus provides a guideline for investigating the relationship between excited-state spin multiplicity and photocatalytic efficiency across a wide range of organic photosensitizers.Experimental section
General procedures and materials
DMSO was pre-dried by adding activated 4A molecular sieves and stirring overnight under an Ar atmosphere with CaH2. It was then vacuum-distilled at 75 °C under light shielding using an oil rotary vacuum pump. TFE was dehydrated by refluxing with Mg and I2 for several hours prior to distillation. DIEA was dehydrated by refluxing with KOH before distillation. All solvents were stored in the dark under an Ar atmosphere after distillation. UV-vis absorption spectra were recorded using a Shimadzu UV-3600 Plus spectrophotometer. Emission spectra were measured using a Horiba Fluorolog-3-21 spectrofluorometer. Absolute photoluminescence quantum yields were determined using a Hamamatsu Quantaurus-QY Plus instrument. Emission lifetimes were measured using a Horiba FluoroCube 3000U. 1H and 13C NMR spectra were recorded on a JEOL JNM-ECZ400S/L1 (1H at 400 MHz, 13C at 101 MHz, 19F at 376 MHz) spectrometer. Proton chemical shifts were referenced to the residual proton signals of the solvent at 1.94 ppm (CD3CN). Carbon chemical shifts were referenced to the carbon signals of the solvent at 39.52 ppm (DMSO-d6). All NMR spectra were processed using the Delta software (JEOL). IR measurements were performed on FTIR SHIMADZU Affinity-1S spectrometer fitted with a Pike Technologies MIRacle Single Reflection ATR adapter. High-resolution mass spectra were recorded on Thermo Fisher Scientific Exactive Plus (ESI).N-BAP+, BIH, and MnMes were synthesized according to previously reported methods.27,35,41,42 The counter anion of N-BAP+ was exchanged from Cl− to PF6− as follows: N-BAP+ Cl− (35.6 mg, 0.10 mmol) and KPF6 (22.2 mg, 0.12 mmol, 1.2 equiv.) were placed in a Schlenk tube. The tube was sealed with a rubber septum and filled with Ar by vacuum–refill cycles (three times). CH3CN (4.0 mL) was added to the test tube and the reaction mixture was stirred at 50 °C for 21 hours in the dark. After cooling to room temperature, the resulting mixture was washed with H2O and dried over anhydrous MgSO4. After removal of the solvent under reduced pressure, the resulting solid was washed with hexane and AcOEt, and dried in vacuo to give N-BAP+ PF6− (38.3 mg, 0.085 mmol, 85% yield). 1H NMR (400 MHz, CD3CN): δ = 7.92 (t, J = 8.1 Hz, 1H), 7.48 (d, J = 8.1 Hz, 2H), 4.65 (s, 4H), 2.77 (q, J = 7.5 Hz, 4H), 2.75 (q, J = 7.6 Hz, 4H), 1.25 (t, J = 7.6 Hz, 6H), 1.19 (t, J = 7.5 Hz, 6H). 13C NMR (101 MHz, DMSO-d6): δ = 148.1, 140.3, 136.7, 136.6, 120.0, 116.7, 115.9, 47.8, 21.3, 19.4, 13.3, 13.0. 19F NMR (376 MHz, CD3CN): δ = −73.4 (d, J = 706.5 Hz) (trifluoromethylbenzene was used as internal standard: δ = −63.7). HRMS (ESI+): calcd for C21H27N2, ([M]+) 307.2169, found m/z 307.2163. IR (ATR): 2980 (m), 1607 (m), 1306 (m), 837 (vs.) cm−1. Yellow solid.
Photocatalytic reactions
Photocatalytic durability (evaluated by turnover number, TON) was assessed using a merry-go-round-type photoreactor (Iris-MG, CellSystem). A 2 mL reaction solution was placed in an 11 mL test tube, purged with CO2 for over 20 minutes, and sealed with a septum. The tube was then irradiated with 430 nm LED light inside the reactor.Quantum yield measurements were performed using a photoreaction quantum-yield measurement system (PQY-01, Shimadzu). A 4 mL reaction solution was placed in an 11 mL quartz cuvette (1 cm optical path length), purged with CO2 for over 20 minutes, and sealed with a septum. The solution was irradiated with monochromatic light at 430 nm. The sample temperature was maintained at 25 °C using an EYELA NCB-1210A thermostatic bath. CO and H2 were analyzed by gas chromatography (GC-323, GL Science) using a thermal conductivity detector. Formic acid was analyzed by capillary electrophoresis (Agilent Technologies 7100 L, Otsuka Electronics).
Transient absorption measurements
Transient absorption spectra were recorded using a UNISOKU picoTAS-ns-W2 system, equipped with a passively Q-switched microchip laser (wavelength: 355 nm; pulse width: <350 ps; pulse energy: 17 µJ per pulse) as the pump light, and a high-repetition-rate picosecond supercontinuum light source (pulse width: 50–100 ps; repetition rate: 20 MHz ± 5%) as the probe light.45 To prevent accumulation of transient species during measurement, the sample solution was circulated through a custom-made flow cell (optical path length: 0.2 cm; see Fig. S12). UV-vis absorption spectra of the sample solution were recorded before and after TA measurements to confirm that no decomposition occurred. Global analyses were performed using Glotaran.28Quantum chemical calculation
Density functional theory (DFT) calculations were performed using the Gaussian 16 package.46 Geometry optimizations and frequency calculations were carried out using the UB3LYP functional and the 6-311++G(d,p) basis set, in conjunction with the SMD continuum solvation model for DMSO.47 Time-dependent DFT (TD-DFT) calculations were performed in DMSO using the DFT-optimized geometry and the same functional and basis set.Author contributions
O. I. conceived and designed the research. S. O. synthesized N-BAP+. K. K. synthesized MnMes and BIH, and carried out photocatalytic experiments, spectroscopic measurements, and kinetic analyses. All authors contributed to supervision and manuscript preparation.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc08659k.Acknowledgements
This work was supported by the Super Highway Program from JST and the Iwatani Naoji Foundation. S. O. acknowledges the financial support from JST, ACT-X grant number JPMJAX23D6, and JSPS KAKENHI grant numbers JP24H01873 (Green Catalysis Science) and JP24K17688 (Early-Career Scientists). The authors thank Y. Norisada for his help in preparing N-BAP+PF6−. K. K. acknowledges financial support from Hiroshima Carbon Recycling Young Researchers Support Program. The computation was carried out using the computer resources offered under the category of Comprehensive Projects between Hiroshima University and Kyushu University by Research Institute for Information Technology, Kyushu University.References
- S. Fang, M. Rahaman, J. Bharti, E. Reisner, M. Robert, G. A. Ozin and Y. H. Hu, Nat. Rev. Methods Primers, 2023, 3, 61 CrossRef CAS.
- S. Berardi, S. Drouet, L. Francàs, C. Gimbert-Suriñach, M. Guttentag, C. Richmond, T. Stoll and A. Llobet, Chem. Soc. Rev., 2014, 43, 7501–7519 Search PubMed.
- Y.-J. Yuan, Z.-T. Yu, D.-Q. Chen and Z.-G. Zou, Chem. Soc. Rev., 2017, 46, 603–631 RSC.
- C. Bizzarri, Eur. J. Org Chem., 2022, 2022, e202200185 CrossRef CAS.
- Y. Yamazaki, H. Takeda and O. Ishitani, J. Photochem. Photobiol., C, 2015, 25, 106–137 CrossRef CAS.
- Y. Wu, D. Kim and T. S. Teets, Synlett, 2022, 33, 1154–1179 CrossRef CAS.
- N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS.
- M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898–6926 CrossRef CAS.
- C. K. Prier, D. A. Rankic and D. W. C. Macmillan, Chem. Rev., 2013, 113, 5322–5363 Search PubMed.
- M. A. Bryden and E. Zysman-Colman, Chem. Soc. Rev., 2021, 50, 7587–7680 RSC.
- Y. Wang, X.-W. Gao, J. Li and D. Chao, Chem. Commun., 2020, 56, 12170–12173 Search PubMed.
- E. Bassan, R. Inoue, D. Fabry, F. Calogero, S. Potenti, A. Gualandi, P. G. Cozzi, K. Kamogawa, P. Ceroni, Y. Tamaki and O. Ishitani, Sustain. Energy Fuels, 2023, 7, 3454–3463 Search PubMed.
- E. Speckmeier, T. G. Fischer and K. Zeitler, J. Am. Chem. Soc., 2018, 140, 15353–15365 CrossRef CAS.
- N. H. Damrauer, G. Cerullo, A. Yeh, T. R. Boussie, C. V. Shank and J. K. McCusker, Science, 1997, 275, 54–57 CrossRef CAS PubMed.
- Y. Tamaki, K. Kamogawa, R. Inoue, P. Ceroni and O. Ishitani, J. Am. Chem. Soc., 2025, 147, 33010–33022 CrossRef CAS.
- M. J. Goodwin, J. C. Dickenson, A. Ripak, A. M. Deetz, J. S. McCarthy, G. J. Meyer and L. Troian-Gautier, Chem. Rev., 2024, 124, 7379–7464 CrossRef CAS PubMed.
- Z. Guo, S. Cheng, C. Cometto, E. Anxolabehere-Mallart, S. M. Ng, C. C. Ko, G. Liu, L. Chen, M. Robert and T. C. Lau, J. Am. Chem. Soc., 2016, 138, 9413–9416 CrossRef CAS.
- L. Chen, Y. Qin, G. Chen, M. Li, L. Cai, Y. Qiu, H. Fan, M. Robert and T.-C. Lau, Dalton Trans., 2019, 48, 9596–9602 RSC.
- Q. Lei, H. Yuan, J. Du, M. Ming, S. Yang, Y. Chen, J. Lei and Z. Han, Nat. Commun., 2023, 14, 1087 CrossRef CAS PubMed.
- M. Kientz, G. Lowe, B. G. McCarthy, G. M. Miyake, J. Bonin and M. Robert, ChemPhotoChem, 2022, 6, e202200009 CrossRef CAS.
- H. Rao, C. H. Lim, J. Bonin, G. M. Miyake and M. Robert, J. Am. Chem. Soc., 2018, 140, 17830–17834 CrossRef CAS.
- H. Gao, G. Liu, Y. Zhu, Z. Wen, X. Liu, G. Wang and F. Li, Green Chem. Eng., 2023, 4, 433–438 CrossRef CAS.
- X. Chen, Y. Wei, W. Sun, X. Meng, S. Hao and Y. Gao, Mol. Catal., 2021, 500, 111299 CAS.
- S. Hao, K.-K. Chen, P. Liang, Q. Huang, L. Zhou, Y. Wei and Z. Wei, Nat. Commun., 2025, 16, 6167 CrossRef CAS PubMed.
- P.-Y. Ho, S.-C. Cheng, F. Yu, Y.-Y. Yeung, W.-X. Ni, C.-C. Ko, C.-F. Leung, T.-C. Lau and M. Robert, ACS Catal., 2023, 13, 5979–5985 CrossRef CAS.
- S. Hao, Z. Wang, K.-K. Chen, Y. Wei, L. Zhou and Z. Wei, ChemCatChem, 2025, 17, e00733 CrossRef CAS.
- S. Okumura, S. Hattori, L. Fang and Y. Uozumi, J. Am. Chem. Soc., 2024, 146, 16990–16995 CrossRef CAS PubMed.
- J. J. Snellenburg, S. Laptenok, R. Seger, K. M. Mullen and I. H. M. van Stokkum, J. Stat. SoftwareJ. Stat. Software, 2012, 49, 1–22 Search PubMed.
- I. H. M. van Stokkum, D. S. Larsen and R. van Grondelle, Biochim. Biophys. Acta, Bioenerg., 2004, 1657, 82–104 CrossRef CAS.
- S. Neumann, C. Kerzig and O. S. Wenger, Chem. Sci., 2019, 10, 5624–5633 RSC.
- C. Wang, H. Li, T. H. Bürgin and O. S. Wenger, Nat. Chem., 2024, 16, 1151–1159 CrossRef CAS PubMed.
- P. Müller and K. Brettel, Photochem. Photobiol. Sci., 2012, 11, 632–636 CrossRef PubMed.
- A. W. Adamson and J. N. Demas, J. Am. Chem. Soc., 1971, 93, 1800–1801 CrossRef CAS.
- E. Hasegawa, S. Takizawa, T. Seida, A. Yamaguchi, N. Yamaguchi, N. Chiba, T. Takahashi, H. Ikeda and K. Akiyama, Tetrahedron, 2006, 62, 6581–6588 CrossRef CAS.
- E. Hasegawa, T. Seida, N. Chiba, T. Takahashi and H. Ikeda, J. Org. Chem., 2005, 70, 9632–9635 CrossRef CAS PubMed.
- Y. Tamaki, K. Koike, T. Morimoto and O. Ishitani, J. Catal., 2013, 304, 22–28 CrossRef CAS.
- S. Okumura, T. Imuta, K. Kato, K. Shimosaka, H. Aoki, Y. Aoyagi, K. Hikichi and N. Ishida, Chem. Lett., 2025, 54, upaf135 CrossRef CAS.
- N. Hosokawa, K. Ozawa, K. Koike, Y. Tamaki and O. Ishitani, Chem. Sci., 2025, 16, 4279–4289 RSC.
- K. Ozawa, Y. Tamaki, K. Kamogawa, K. Koike and O. Ishitani, J. Chem. Phys., 2020, 153, 154302 CrossRef CAS.
- K. Kamogawa, Y. Shimoda, K. Miyata, K. Onda, Y. Yamazaki, Y. Tamaki and O. Ishitani, Chem. Sci., 2021, 12, 9682–9693 RSC.
- X. Q. Zhu, M. T. Zhang, A. Yu, C. H. Wang and J. P. Cheng, J. Am. Chem. Soc., 2008, 130, 2501–2516 CrossRef CAS.
- K. Kamogawa, H. Koizumi and O. Ishitani, J. Am. Chem. Soc., 2025, 147, 39284–39297 CrossRef CAS.
- K. T. Ngo, M. McKinnon, B. Mahanti, R. Narayanan, D. C. Grills, M. Z. Ertem and J. Rochford, J. Am. Chem. Soc., 2017, 139, 2604–2618 CrossRef CAS.
- C. Riplinger, M. D. Sampson, A. M. Ritzmann, C. P. Kubiak and E. A. Carter, J. Am. Chem. Soc., 2014, 136, 16285–16298 CrossRef CAS.
- T. Nakagawa, K. Okamoto, H. Hanada and R. Katoh, Opt. Lett., 2016, 41, 1498–1501 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Rev. C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |