Open Access Article
Open Access ArticleThe relevance of Cr defects and photoelectrochemical water oxidation activity of monoclinic PbCrO4 films
Jiahe Lia,
Gaili Ke a,
Minji Yangb,
Guoliang Lva,
Lanyi Caoa,
Wenjun Lia,
Tao Han
a,
Minji Yangb,
Guoliang Lva,
Lanyi Caoa,
Wenjun Lia,
Tao Han a,
Wenrong Wanga,
Yong Zhou
a,
Wenrong Wanga,
Yong Zhou c and
Huichao He
c and
Huichao He *a
*a
aCollege of Materials and New Energy, Chongqing University of Science and Technology, Chongqing 401331, China. E-mail: hehuichao@cqust.edu.cn
bSynthetic Lubricant Oil and Grease Branch, Sinopec Lubricant Co., Ltd, Chongqing, 400039, China
cEcomaterials and Renewable Energy Research Center, School of Physics, University Nanjing, Nanjing 211102, China
First published on 2nd January 2026
Abstract
The presence of Cr defects in monoclinic PbCrO4 is closely related to its solar water oxidation activity, which has been overlooked in the preparation of PbCrO4 films and remains unexplored in research on PbCrO4 photoanodes. Herein, monoclinic PbCrO4 films with few Cr defects (PbCrO4-FVCr) were prepared on FTO substrate by drop-coating Pb2+/Cr3+ precursor solution to reduce the loss of Cr during thermal treatment. Relative to the monoclinic PbCrO4 films with rich Cr defects (PbCrO4-RVCr), higher solar water oxidation activity was achieved using the PbCrO4-FVCr films as photoanodes. At 1.23 V vs. RHE, a higher water oxidation photocurrent of 1.13 mA cm−2 was produced on the PbCrO4-FVCr film photoanodes, which is twice that of the PbCrO4-RVCr film photoanodes (0.55 mA cm−2). Meanwhile, the PbCrO4-FVCr film photoanodes had faster water oxidation kinetics than PbCrO4-RVCr film photoanodes. The water oxidation rate constant (kO2) on the PbCrO4-FVCr film photoanodes was 40.6 s−1, while a lower kO2 of 8.2 s−1 was observed on the PbCrO4-RVCr film photoanodes. Experimental and theoretical analyses jointly revealed that PbCrO4 films with fewer Cr defects have higher solar water oxidation activity for the following reasons: (i) the presence of Cr defects can result in the formation of deep energy levels in PbCrO4, which are unfavorable for carrier transfer in the bulk of PbCrO4 films; (ii) the presence of Cr defects leads to the formation of unsaturated O dangling bonds on PbCrO4, which act as detrimental traps that hinder carrier separation at the surface of PbCrO4 films; (iii) Cr has a half-filled 3d5 orbital to provide active sites for reaction, so the presence of Cr defects weakens the catalytic activity of PbCrO4 for water oxidation, and the dehydrogenation of *OH into *O becomes the rate-limiting step, which requires a high energy barrier of 1.88 eV. The present work provides insights into monoclinic PbCrO4 film photoanodes in terms of preparation conditions, Cr defects, water oxidation activity and reaction mechanism.
1 Introduction
n-Type monoclinic PbCrO4 has an electronic structure similar to that of monoclinic BiVO4,1 and it has been investigated as a photoanode material for photoelectrochemical (PEC) water splitting in recent years.2–7 Based on its advantageous band gap (∼2.2 eV) and valence band position (∼2.2 V vs. RHE),2 monoclinic PbCrO4 can theoretically harvest ∼20% of solar energy to drive water oxidation.8 However, the activity of previously reported PbCrO4 photoanodes is far below the theoretical expectation, due to the presence of high carrier recombination and sluggish water oxidation kinetics. As is well known, the composition and structure of materials jointly determine their properties and related performance. The presence of defects in semiconductors could impact their electronic structure, carrier separation and migration behavior, thereby obviously changing the photocatalytic and PEC activity.9,10 For monoclinic PbCrO4, its Cr6+ is located at the center of a CrO4 tetrahedron, and Pb2+ is surrounded by 8–9 oxygen atoms due to its lone pair electron effect.11,12 From the perspectives of electronic structure, one Cr defect carries three effective negative charges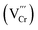 in PbCrO4. In theory, the presence of a high concentration of Cr defects could disrupt the CrO4 tetrahedron network and form unsaturated O dangling bonds as well as defect-level traps, which are disadvantageous to the delocalized separation and transfer of electrons/holes.
in PbCrO4. In theory, the presence of a high concentration of Cr defects could disrupt the CrO4 tetrahedron network and form unsaturated O dangling bonds as well as defect-level traps, which are disadvantageous to the delocalized separation and transfer of electrons/holes.
Significantly, drop-/spin-coating combined with thermal treatment is a common approach for the preparation of monoclinic PbCrO4 film. Typically, Li et al. used a spin-coating/thermal treatment method to prepare PbCrO4 films on fluorine-doped tin oxide-coated glass (FTO), and invented acetylacetone and poly(ethylene glycol) as dual ligands to regulate the nucleation and crystal growth of monoclinic PbCrO4 films.4,6 Cho et al. prepared PbCrO4 films on FTO using a drop-coating/thermal treatment approach, and found that adding citric acid to the precursor solution can stabilize Pb2+ and Cr3+ as well as suppress their segregation to form a smooth and fine monoclinic PbCrO4 film.5,7 Although these pioneering investigations made significant contributions to the preparation of monoclinic PbCrO4 films, the underlying influence of thermal treatment on the composition and structure of the resultant PbCrO4 films has been overlooked. During thermal treatment at high temperature in air, the metal ions in the precursor solution may be lost, along with the pyrolysis of solvents, which could induce the formation of metal defects in the resultant multi-metal oxides. In the preparation of BiVO4 films using a spray pyrolysis method, Lamers et al. found the loss of V during the VO3+/Bi3+ precursor solution annealing above 500 °C in air, and observed that the resultant BiVO4 films contained V defects and low carrier mobility.13 It is worthy of special attention that Cr can be thermally evaporated at lower temperature (T > 500 °C) compared to Pb (T > 600 °C). The thermal evaporation of Cr from stainless steels at T > 500 °C has been extensively observed.14 Thus, it is very possible that Cr defects are formed in monoclinic PbCrO4 films prepared using drop-/spin-coating combined with thermal treatment for synthesis. Several works have demonstrated that semiconductor photoelectrode materials with low-concentration defects usually have high crystallinity and can drive solar water splitting efficiently.15–17 To achieve efficient solar water oxidation on PbCrO4 film photoanodes, suppressing the formation of high concentrations of Cr defects in monoclinic PbCrO4 films during synthesis is crucial.
Herein, monoclinic PbCrO4 films with few Cr defects (PbCrO4-FVCr) were prepared on FTO substrate by drop-coating the Pb2+/Cr3+ precursor solution to reduce the thermal loss of Cr during annealing treatment. Compared with the monoclinic PbCrO4 films with rich Cr defects (PbCrO4-RVCr) for solar water oxidation, a two-fold increased water oxidation photocurrent was produced on the PbCrO4-FVCr film photoanodes (1.13 mA cm−2 at 1.23 V vs. RHE). Meanwhile, faster water oxidation kinetics were achieved on the PbCrO4-RVCr film photoanodes. The PbCrO4-FVCr film photoanodes had a water oxidation rate constant (kO2) of 40.6 s−1, which is ∼5 times the kO2 of the PbCrO4-RVCr film photoanodes (8.2 s−1). Based on experimental and theoretical investigations, PbCrO4 films with fewer Cr defects having higher activity can be ascribed to the following reasons: (i) the presence of Cr defects can result in the formation of a deep energy level in PbCrO4, which is unfavorable to carrier transfer in the bulk of PbCrO4 films; (ii) the presence of Cr defects can cause the formation of unsaturated O dangling bonds on PbCrO4, which are harmful traps for carrier separation on the surface of PbCrO4 films; (iii) Cr has a half-filled 3d5 orbital to provide active sites for reaction, so the presence of Cr defects weakens the catalytic activity of PbCrO4 for water oxidation, and the dehydrogenation of *OH into *O becomes the rate-limiting step, which requires a high energy barrier of 1.88 eV.
2 Experimental section
2.1 Preparation of PbCrO4-FVCr and PbCrO4-RVCr films
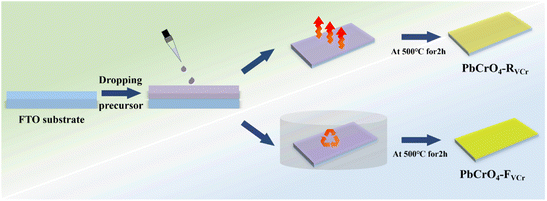
The PbCrO4-FVCr and PbCrO4-RVCr films were prepared by drop-coating combined with thermal treatment (Fig. 1). Typically, Pb(NO3)2 (AR, Aladdin) and Cr(NO3)3 (AR, Aladdin) were dissolved in 5 mL of distilled water to form 0.2 M Pb2+ and 0.2 M Cr3+ precursor solutions, respectively. Then, 100 mg of polyethylene glycol (AR, Aladdin) was added to the Pb2+ precursor solution to stabilize Pb2+, while 70 µL of acetylacetone (Aladdin, 99.8%) was dropped into the Cr3+ precursor solution to stabilize Cr3+. After that, the Pb2+ and Cr3+ precursor solutions were treated by ultrasonication at 60 °C for 30 min. Subsequently, the Pb2+ and Cr3+ precursor solutions were mixed and diluted using ethylene glycol (AR, Kelong) to form a 0.01 M Pb2+/Cr3+ precursor solution. Finally, 200 µL of Pb2+/Cr3+ precursor solution was dropped onto a clean FTO glass (10 × 20 × 2 mm, 15 Ω sq−1), and thermally treated in a muffle furnace (chamber: 20 × 30 × 20 cm) at 500 °C (heating rate: 2.5 °C min−1) for 2 h in air. For the preparation of PbCrO4-FVCr films, a culture dish (diameter: 6.0 cm; height: 1.6 cm) was used to cover the Pb2+/Cr3+ precursor solution during thermal treatment. For comparison, PbCrO4-RVCr films were prepared without using a culture dish cover during thermal treatment of the Pb2+/Cr3+ precursor solution.To check the presence and content difference of O dangling bonds in the as-prepared PbCrO4 films, PbCrO4-FVCr and PbCrO4-RVCr films were immersed in 0.01 M 1-octadecanethiol (C18H38S, 97%, Macklin) ethanol solution for 24 h to passivate their O dangling bonds. Then, the two types of PbCrO4 film were washed with deionized water for photocurrent measurements.
2.2 Characterizations of samples
The crystal structure, morphology and elemental chemical state of the PbCrO4-FVCr and PbCrO4-RVCr films were characterized using an X-ray diffraction (XRD, SmartLab-9 kW, Rigaku), scanning electron microscopy (SEM, Regulus 8100), transmission electron microscopy (TEM, Tecnai G220) and X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi), respectively. The UV-vis absorption spectra for the PbCrO4-FVCr and PbCrO4-RVCr films were measured using a Hitachi spectrophotometer (UH5700). The photoluminescence emission (PL) spectra for the PbCrO4-FVCr and PbCrO4-RVCr films were recorded using an FLS1000 photoluminescence spectrometer. The electron paramagnetic resonance (EPR) spectra for the PbCrO4-FVCr and PbCrO4-RVCr films were recorded using a Bruker EPR spectrometer (EMPplus-10/12). The water contact angle of the PbCrO4-FVCr and PbCrO4-RVCr films was observed using a contact angle meter (SINDIN, SCD-100). The surface photovoltage and transient surface photovoltage spectra for the PbCrO4-FVCr and PbCrO4-RVCr films were detected using surface photovoltage spectroscopy (Beijing China Education Au-light Co., Ltd, CEL-TPV2000). For the PbCrO4-FVCr and PbCrO4-RVCr films before and after stability testing, their Raman spectra were detected using a Raman spectrometer (MultiRAM, Bruker).2.3 Photoelectrochemical measurements
All PEC measurements were conducted on a CHI 760e electrochemical workstation with a three-electrode cell at room temperature. The as-prepared PbCrO4 films were used directly as working electrodes; the counter electrode was a Pt wire (99%), and the reference electrode was an Ag/AgCl electrode. The light source was a 300 W xenon lamp (Beijing China Education Au-light Co., Ltd) equipped with a 1.5G filter to provide 100 mW cm−2 illumination. The PEC measurements were carried out using back-side illumination, namely the light first penetrated the FTO, then the PbCrO4 film. 0.5 M phosphate buffer (PBS, pH 7.0) aqueous solution and 0.1 M potassium hydrogen phthalate (KHP)/0.5 M Na2SO3 mixed aqueous solution were used as the electrolytes. The potential applied on the working electrode was converted into RHE potential using the following equation:| ERHE = EAg/AgCl + 0.0592 pH + 0.197 | (1) |
The charge separation (ηsep) and injection (ηinj) efficiency testing were performed in 0.5 M PBS/0.2 vol% H2O2 electrolyte. The ηsep and ηinj of the PbCrO4-FVCr and PbCrO4-RVCr film photoanodes were calculated according to the following equations:
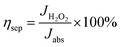 | (2) |
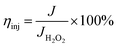 | (3) |
The applied bias photon-to-current efficiency (ABPE) of the PbCrO4-FVCr and PbCrO4-RVCr film photoanodes was calculated using the following equation:
 | (4) |
The Mott–Schottky plots of PbCrO4-FVCr and PbCrO4-RVCr film photoanodes were recorded in the potential range of 0.3 to 0.5 V vs. RHE under AM 1.5G illumination. The carrier concentration of the PbCrO4-FVCr and PbCrO4-RVCr films was calculated using the following equation:
| 1/C2 = (2/eεε0A2Nd)(Va − Vfb − kT/e) | (5) |
The electrochemical impedance spectroscopy (EIS) measurements were performed at 1.23 V vs. RHE. The testing frequency ranged from 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 0.01 Hz with an amplitude of 10 mV. The measured EIS data were fitted by Zview software under the proposed equivalent circuit model. Based on the EIS Bode plots, the hole lifetimes (τ) on the two types of PbCrO4 film photoanode were obtained using the following equation:
000 to 0.01 Hz with an amplitude of 10 mV. The measured EIS data were fitted by Zview software under the proposed equivalent circuit model. Based on the EIS Bode plots, the hole lifetimes (τ) on the two types of PbCrO4 film photoanode were obtained using the following equation:
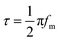 | (6) |
The water oxidation rate constant (kO2) and charge transfer rate constant (ktran) of the two types of PbCrO4 film photoanode were calculated on the basis of the fitted components' values for the equivalent circuit (the values shown in Table 2).18–21 kO2 was calculated through the following equation:
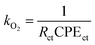 | (7) |
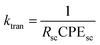 | (8) |
2.4 Density functional theory calculations
The charge density and the density of states of PbCrO4 (120) with and without Cr defects were calculated using the Vienna Ab initio Simulation Package (VASP).22,23 The exchange–correlation potential was described by the Perdew–Burke–Ernzerhof (PBE) generalized gradient approach (GGA).24 The electron–ion interactions were accounted for by the projector augmented wave (PAW) model.25 All density functional theory (DFT) calculations were performed with a cut-off energy of 400 eV, and the Brillouin zone was sampled using a 2 × 1 × 1 Gamma-centered k-point Monkhorst–Pack grid. The energy and force convergence criteria of the self-consistent iteration were set to 10−4 eV and 0.05 eV Å−1, respectively. The DFT-D3 method was used to describe van der Waals (vdW) interactions.26The Gibbs free energy changes (ΔG) of water oxidation on PbCrO4 (120) with and without Cr defects were calculated using the following formula:
| ΔG = ΔE + ΔZPE − TΔS + ΔGU + ΔGpH | (9) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 × pH, where kB is the Boltzmann constant.
10 × pH, where kB is the Boltzmann constant.
3 Results and discussion
3.1 Characterization results
Fig. 2a–f show the typical SEM images of PbCrO4-RVCr and PbCrO4-FVCr films. From the top and sectional SEM images shown in Fig. 2a–c, it can be seen that PbCrO4-RVCr films are not dense and flat enough, and obvious gaps are formed and evenly distributed throughout the whole film. By comparison, PbCrO4-FVCr films were denser and flatter, with few gaps (Fig. 2d–f). In terms of microscopic morphology, PbCrO4-RVCr and PbCrO4-FVCr films consist of connected nanoparticles, but the nanoparticle size of PbCrO4-RVCr films (average size ∼70 nm) was smaller than that of PbCrO4-FVCr films (average size ∼100 nm) (Fig. 2b, e and S1 in SI). In general, PbCrO4-RVCr films (∼230 nm, Fig. 2c) were slightly thicker than PbCrO4-FVCr films (∼200 nm, Fig. 2f), due to the difference in morphology and evenness, although their preparation used the same amount of Pb2+/Cr3+ precursor solution. In the HRTEM image of the PbCrO4-FVCr film, the crystalline feature was observed and the lattice spacing was ∼0.32 nm, which corresponds to the typical crystal feature of PbCrO4(120) (Fig. 2g). For the preparation of the two types of PbCrO4 films, the only condition difference was the presence or absence of a culture dish covering the Pb2+/Cr3+ precursor solution during annealing treatment. During the evaporation and pyrolysis of the Pb2+/Cr3+ precursor solution, the use of a culture dish cover enabled the precursor solution to gradually convert into a uniform colloid and film, in contrast to the process without a cover (Fig. S2). The morphology and thickness differences between the PbCrO4-RVCr and PbCrO4-FVCr films suggested that the formation of the PbCrO4 film was significantly impacted by the culture dish covering above the Pb2+/Cr3+ precursor solution during thermal treatment. In further XRD analyses, the typical diffraction peaks of monoclinic PbCrO4 (PDF#: 08-0209) were clearly observed for both types of PbCrO4 film, in addition to the signals for the FTO substrate (SnO2, PDF#:46-1088) (Fig. 2h). Significantly, the (120) diffraction peak intensity of the PbCrO4-FVCr film was 1.26 times that of the PbCrO4-RVCr film. According to the XRD data, the crystallinity of the PbCrO4-FVCr film was found to be 85.58%, which is higher than that of the PbCrO4-RVCr film (80.41%) (Table 1). It is generally believed that the crystallinity of metal oxides is closely influenced by the defect content.15 The crystallinity difference implied that the defect content in the PbCrO4-RVCr and PbCrO4-FVCr films is different.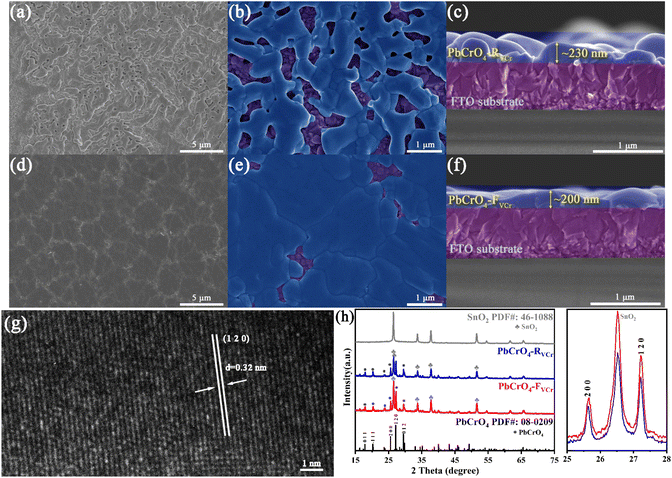 | ||
| Fig. 2 SEM images of (a–c) PbCrO4-RVCr and (d–f) PbCrO4-FVCr films. (g) HRTEM image of PbCrO4-FVCr film. (h) XRD patterns for PbCrO4-RVCr and PbCrO4-FVCr films. | ||
| Sample | hkl | 2Θ | FWHM | Crystallinity (%) |
|---|---|---|---|---|
| PbCrO4-FVCr film | (200) | 25.636 | 0.156 | 85.58 |
| (120) | 27.223 | 0.133 | ||
| PbCrO4-RVCr film | (200) | 25.624 | 0.118 | 80.41 |
| (120) | 27.218 | 0.107 |
To determine the chemical states of the constituent elements, the PbCrO4-RVCr and PbCrO4-FVCr films were further investigated using XPS. In the survey spectra, the XPS signals of O 1s, Pb 4f and Cr 2p were clearly detected on the PbCrO4-RVCr and PbCrO4-FVCr films (Fig. S3). In the high-resolution Pb 4f spectrum of both types of PbCrO4 film, the binding energy peaks at 138.27 and 143.12 eV were in good agreement with the Pb2+ in monoclinic PbCrO4 (Fig. 3a).27,28 For the high-resolution Cr 2p spectrum, the binding energy peaks of the PbCrO4-RVCr and PbCrO4-FVCr films generally matched the Cr 2p signals in monoclinic PbCrO4 (Fig. 3b).29,30 However, the Cr 2p binding energy peaks of PbCrO4-RVCr film (578.67 eV, 587.92 eV) negatively shifted relative to those of the PbCrO4-FVCr film (578.87 eV, 588.12 eV), hinting that their Cr have slightly different chemical states. Meanwhile, more obvious oxygen vacancy signals were found in the high-resolution O 1s spectrum of the PbCrO4-RVCr film in comparison with the PbCrO4-FVCr film, in addition to their similar lattice oxygen and adsorbed oxygen signals (Fig. 3c). Taking into account the Cr 2p and O 1s XPS differences between the PbCrO4-RVCr and PbCrO4-FVCr films, it can be inferred that the PbCrO4-RVCr film contains more defects than the PbCrO4-FVCr film. To detect the specific defects in PbCrO4-RVCr and PbCrO4-FVCr films, their EPR spectra were recorded and compared. As shown in Fig. 3d–f, two signal centers at g = 2.003 and g = 3.883 appeared for both types of PbCrO4 film, which are the typical EPR signals of oxygen vacancies and Cr defects, respectively.31–34 In comparison, the EPR signals of Cr defects were much stronger than those of oxygen vacancies, indicating that Cr defects are the predominant defects in PbCrO4-RVCr and PbCrO4-FVCr films and oxygen vacancies are companion species. Obviously, the PbCrO4-RVCr film had stronger Cr defects and oxygen vacancy signals than the PbCrO4-FVCr film, indicating a higher content of Cr defects in the PbCrO4-RVCr film. For the preparation of both types of PbCrO4 film, the only condition difference was the presence and absence of a culture dish covering above the Pb2+/Cr3+ precursor solution during the annealing treatment. The above characterizations show that PbCrO4 films with few Cr defects can be prepared using a culture dish covering above the Pb2+/Cr3+ precursor solution to reduce the loss of Cr during thermal treatment.
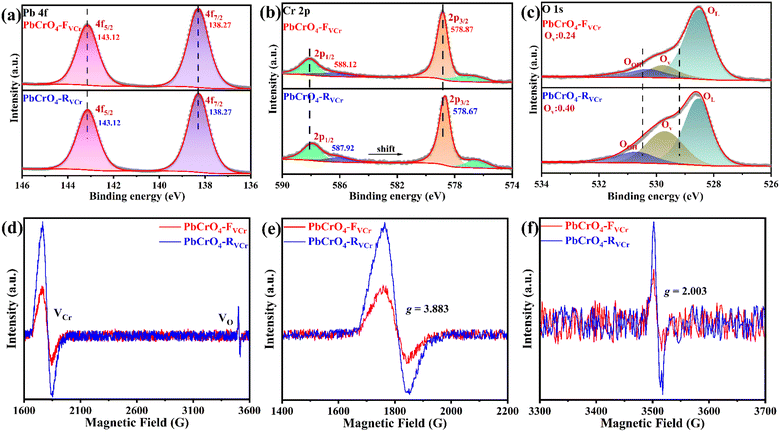 | ||
| Fig. 3 High-resolution XPS spectra for (a) Pb 4f, (b) Cr 2p and (c) O 1s of PbCrO4-RVCr and PbCrO4-FVCr films. (d–f) EPR spectra for PbCrO4-RVCr and PbCrO4-FVCr films. | ||
3.2 PbCrO4-RVCr and PbCrO4-FVCr films as photoanodes for solar water oxidation
In previous reports, PbCrO4 films have been demonstrated to work as photoanodes for solar water oxidation in PBS solution, and their activity can be directly reflected in the magnitude of the photocurrent.4,6 To check the influence of Cr defects on the solar water oxidation activity of PbCrO4 photoanodes, the photocurrent of the two types of PbCrO4 film was measured in 0.5 M PBS under AM 1.5G irradiation. As shown in Fig. 4a and S4, a higher photocurrent is produced on the PbCrO4-FVCr film than on the PbCrO4-RVCr film. At 1.23 V vs. RHE, the PbCrO4-FVCr film had a higher photocurrent of 1.13 mA cm−2 compared to the PbCrO4-RVCr film (0.55 mA cm−2), indicating its higher solar water oxidation activity. In ABPE measurement, the higher activity was further observed on the PbCrO4-FVCr film photoanode; their maximum ABPE (0.073%) was nearly double that of the PbCrO4-RVCr film photoanode (0.038%) (Fig. 4b). It is important to note that the PbCrO4-FVCr film in the present work has impressive activity compared with recently reported PbCrO4 films (Table S1). In ηsep detection, PbCrO4-FVCr and PbCrO4-RVCr film photoanodes were found to have ηsep of 65% and 45% at 1.23 V vs. RHE, respectively (Fig. 4c). Meanwhile, the ηinj of PbCrO4-FVCr and PbCrO4-RVCr film photoanodes were 29% and 21% at 1.23 V vs. RHE, respectively (Fig. 4d). Taking the ηsep and ηinj differences between the two types of PbCrO4 film photoanodes as comparisons, it can be found that the PbCrO4-FVCr and PbCrO4-RVCr film photoanodes exhibit a more significant difference in charge separation than in charge injection. The ηsep and ηinj results suggested that PbCrO4 films with fewer Cr defects can achieve charge separation more effectively.35 From the morphology observations shown in Fig. 2a–f, the PbCrO4-RVCr film appears to have a larger specific surface area than the PbCrO4-FVCr film, due to the presence of more gaps. However, electrochemically active surface area (ECSA) testing showed that the PbCrO4-FVCr films have a larger ECSA (10.31) than the PbCrO4-RVCr film (8.60) (Fig. 4e and S6). In addition, higher electrochemical water oxidation activity was observed on the PbCrO4-FVCr film than on the PbCrO4-RVCr film under dark conditions (Fig. S7). In terms of the electron configuration of transition metal electrocatalysts,36 Cr would have higher catalytic activity for water splitting than Pb, since Cr has a half-filled 3d orbital (1s22s22p63s23p63d54s1) to provide active sites for reaction, while Pb has a full-filled 3d and 5d orbitals (1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p2).37 Therefore, it can be understood that PbCrO4 with more Cr defects has lower electrochemical activity for water oxidation. Based on the photocurrent and ECSA data, it was found that the PbCrO4-FVCr film has a higher ECSA-normalized photocurrent than the PbCrO4-RVCr film (Fig. 4f). The above findings jointly demonstrate that PbCrO4 films with fewer Cr defects can work as higher activity photoanodes for solar water splitting.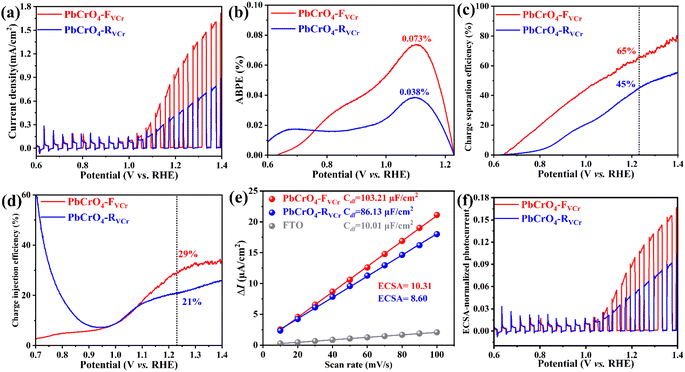 | ||
| Fig. 4 (a) Chopped LSV curves, (b) ABPE, (c) ηsep, (d) ηinj, (e) ECSA, and (f) ECSA-normalized photocurrent for PbCrO4-RVCr and PbCrO4-FVCr film photoanodes in 0.5 M PBS. | ||
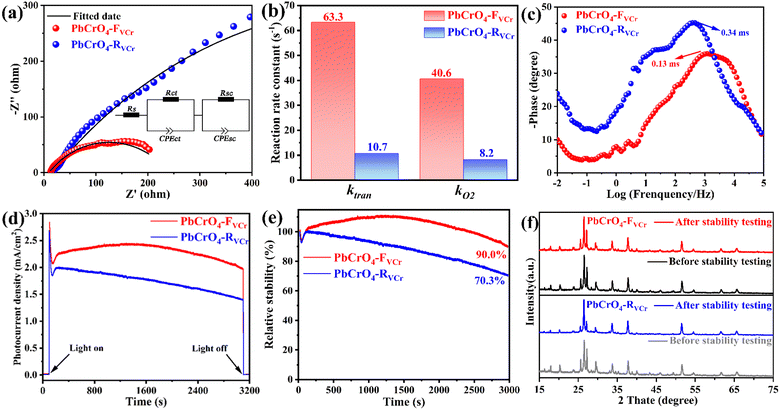
To get insight into the water oxidation kinetics on the two types of PbCrO4 film photoanode, their Nyquist and Bode plots were recorded at 1.23 V vs. RHE in 0.5 M PBS under AM 1.5G irradiation. As displayed in Fig. 5a, the Nyquist plot of the PbCrO4-FVCr film photoanode had a smaller arc than that of the PbCrO4-RVCr film photoanode, suggesting faster kinetics in solar water oxidation. Data fitting indicated that the charge transfer (Rsc) and water oxidation (Rct) resistance on the PbCrO4-FVCr film photoanode were 290 Ω and 186 Ω, respectively (Table 2).38,39 By comparison, the PbCrO4-RVCr film photoanode had higher Rsc (1253 Ω) and Rct (962 Ω). Based on the fitted components' equivalent circuit values, the PbCrO4-FVCr film photoanode was found to have higher kO2 and ktran (kO2: 40.6 s−1; ktran: 63.3 s−1) than the PbCrO4-RVCr film photoanode (kO2: 8.2 s−1; ktran: 40.6 s−1) (Fig. 5b), indicating faster water oxidation and charge transfer rates on the PbCrO4-FVCr film photoanode. According to the Bode plots, the hole lifetimes (τ) on the PbCrO4-FVCr and PbCrO4-RVCr film photoanodes were calculated to be 0.13 ms and 0.34 ms, respectively (Fig. 5c). These outcomes confirmed that the holes on the PbCrO4-FVCr film photoanode can react with H2O molecules quickly.40,41 As previously analyzed, Cr has a half-filled 3d orbital that could provide active sites for water oxidation. Therefore, it is not surprising that the PbCrO4 film photoanode with fewer Cr defects is kinetically favorable for solar water oxidation.
| Sample | Rs/Ω (error) | Rct/Ω (error) | CPEct/F (error) | Rsc/Ω (error) | CPEsc/F (error) |
|---|---|---|---|---|---|
| PbCrO4-RVCr | 17.67 (1.98%) | 962 (6.26%) | 9.72 × 10−5 (5.15%) | 1253 (4.39%) | 1.50 × 10−4 (7.20%) |
| PbCrO4-FVCr | 10.67 (1.42%) | 186 (2.62%) | 8.47 × 10−5 (5.23%) | 290 (7.93%) | 2.80 × 10−4 (7.62%) |
In terms of the stability for solar water oxidation, it was unfortunate that unsatisfactory activity decay was observed on the PbCrO4-FVCr and PbCrO4-RVCr film photoanodes in 0.5 M PBS during continuous testing at 1.23 V vs. RHE (Fig. S8). Further investigations found that dissolution and photocorrosion issues are the main causes of activity decay on both types of PbCrO4 film photoanode in 0.5 M PBS (please see the result discussions for Fig. S9 to S11 and Table S2 in the SI). According to these findings, the PEC stability testing for the PbCrO4-FVCr and PbCrO4-RVCr films was conducted in 0.1 M KHP/0.05 M Na2SO3 aqueous solution at 1.23 V vs. RHE. As shown in Fig. 5d and e, the PbCrO4-FVCr film has a higher photocurrent than the PbCrO4-RVCr film, showing its higher activity for SO32− oxidation. After 3000 s of continuous reaction, 90.0% activity was retained on the PbCrO4-FVCr film, while the PbCrO4-RVCr film was 70.3% (Fig. 5e). Contrastive XRD, Raman and SEM investigations indicated that the crystalline phases, molecular structure and morphology of the two types of PbCrO4 film photoanode show slight changes after stability testing (Fig. 5f, S10 and S11). However, the PbCrO4-RVCr film after stability testing had a more pronounced decrease in the XRD peaks' intensity, relative to the PbCrO4-FVCr film (Fig. 5f). From the stability difference between the PbCrO4-FVCr and PbCrO4-RVCr film photoanodes, it can be seen that PbCrO4 with fewer Cr defects is more stable to drive PEC reactions.
3.3 The mechanism for the higher activity of PbCrO4 films with fewer Cr defects
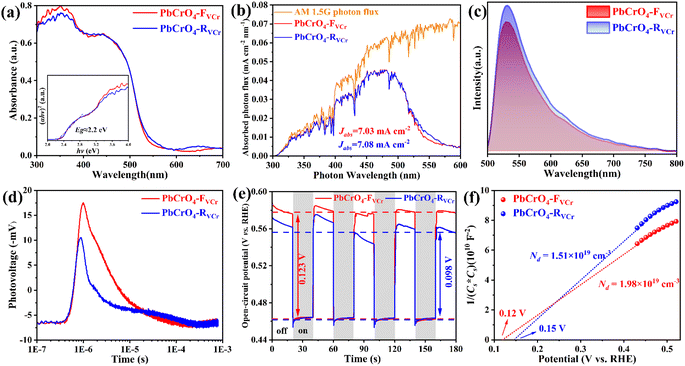
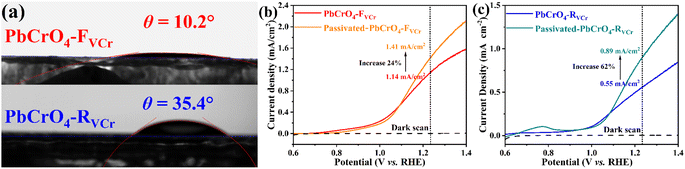
The above investigations demonstrated that PbCrO4 films with fewer Cr defects have higher solar water oxidation activity. To reveal the mechanism for PbCrO4 films with fewer Cr defects having higher activity, the physicochemical properties of the PbCrO4-FVCr and PbCrO4-RVCr films involving solar water oxidation were contrastively checked. First of all, the UV-vis light absorption property of the two types of PbCrO4 film was investigated. As shown in Fig. 6a, the PbCrO4-FVCr and PbCrO4-RVCr films have a close absorption edge around 550 nm, which corresponds to the typical band gap of monoclinic PbCrO4 (∼2.2 eV).1,42 Meanwhile, the light absorption intensity of the two types of PbCrO4 film was very close too, and the absorbed photon fluxes of the PbCrO4-FVCr and PbCrO4-RVCr films were 7.03 mA cm−2 and 7.08 mA cm−2, respectively (Fig. 6b). These observations indicated that the obvious activity difference between PbCrO4-FVCr and PbCrO4-RVCr films is not be caused by a difference in their light absorption properties. In PL spectrum investigations, weaker PL peak intensity was detected on the PbCrO4-FVCr film compared with the PbCrO4-RVCr film, reflecting lower carrier recombination in/on the PbCrO4-FVCr film (Fig. 6c). In addition, stronger surface photovoltage signals were detected on the PbCrO4-FVCr films than on the PbCrO4-RVCr film, further confirming better carrier separation and transfer in/on the PbCrO4-FVCr film (Fig. 6d and S12). As mentioned in the Introduction, the presence of a high concentration of Cr defects in monoclinic PbCrO4 could disrupt its CrO4 tetrahedron network and form defect-level traps, which are disadvantageous to the delocalized separation and transfer of electrons/holes in theory. To confirm the effect of Cr defects on carrier separation and transfer, the open-circuit potential (OCP) variations were detected on PbCrO4-FVCr and PbCrO4-RVCr films under and without AM 1.5G irradiation. As displayed in Fig. 6e, an OCPlight of ∼0.461 V vs. RHE was observed on both types of PbCrO4 film, while the OCPdark of the PbCrO4-FVCr and PbCrO4-RVCr films are 0.582 and 0.552 V vs. RHE, respectively. Accordingly, a more obvious OCP variation was produced on the PbCrO4-FVCr film (0.123 V) than the PbCrO4-RVCr film (0.098 V), suggesting that PbCrO4 films with fewer Cr defects have better charge separation and transfer properties.43,44 In the Mott–Schottky plots (Fig. 6f), a more negative Efb of 0.12 V vs. RHE was observed on the PbCrO4-FVCr film, relative to the PbCrO4-RVCr film (0.15 V). Considering the relevance of Efb in n-type semiconductors to their Fermi level position (Ef), the PbCrO4-FVCr film, having a more negative Efb, indicates that PbCrO4 with fewer Cr defects has a higher Ef and carrier concentration (PbCrO4-FVCr film: 1.98 × 1019 cm−3; PbCrO4-RVCr film: 1.51 × 1019 cm−3).In terms of chargeability, one Cr6+ defect carries three effective negative charges 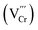 in monoclinic PbCrO4. From a unit cell structural perspective, the presence of Cr defects could cause the formation of unsaturated O dangling bonds on the PbCrO4 surface. To check the presence and content difference of O dangling bonds on the two types of PbCrO4 film, their hydrophilicity was detected and compared (Fig. 7a). Interestingly, weaker hydrophilicity was observed on the PbCrO4-RVCr film (θ = 35.4°) relative to the PbCrO4-FVCr film (θ = 10.2°), although the PbCrO4-RVCr film had a rougher surface (SEM images shown in Fig. 2a–f). In essence, the hydrophilicity of the film depends on the interaction intensity between H2O molecules and the surface compounds.45 It has been reported that high concentrations of Al defects in α-Al2O3 can result in the reconstitution of unsaturated O dangling bonds and the decrease of surface energy, thereby α-Al2O3 with rich Al defects has weaker hydrophilicity than pristine α-Al2O3.46 The hydrophilicity difference suggested that PbCrO4-FVCr and PbCrO4-RVCr films have different contents of O dangling bonds on the surface, owing to their different Cr defect contents. The presence of O dangling bonds usually has a negative effect on carrier separation on the surface of semiconductors.47,48 Through the –SH groups of 1-octadecanethiol passivating the O dangling bonds on PbCrO4 films,49–51 the photocurrent of the PbCrO4-RVCr film was found to increase by 62%, but there was only a 24% increase on the PbCrO4-FVCr film (Fig. 7b and c). The photocurrent enhancement on 1-octadecanethiol-passivated PbCrO4 films confirmed the presence of O dangling bonds and the negative effect of O dangling bonds on carrier separation on PbCrO4. Meanwhile, the more obvious photocurrent improvement on the 1-octadecanethiol-passivated PbCrO4-RVCr film reflected their higher content of O dangling bonds, due to rich Cr defects.
in monoclinic PbCrO4. From a unit cell structural perspective, the presence of Cr defects could cause the formation of unsaturated O dangling bonds on the PbCrO4 surface. To check the presence and content difference of O dangling bonds on the two types of PbCrO4 film, their hydrophilicity was detected and compared (Fig. 7a). Interestingly, weaker hydrophilicity was observed on the PbCrO4-RVCr film (θ = 35.4°) relative to the PbCrO4-FVCr film (θ = 10.2°), although the PbCrO4-RVCr film had a rougher surface (SEM images shown in Fig. 2a–f). In essence, the hydrophilicity of the film depends on the interaction intensity between H2O molecules and the surface compounds.45 It has been reported that high concentrations of Al defects in α-Al2O3 can result in the reconstitution of unsaturated O dangling bonds and the decrease of surface energy, thereby α-Al2O3 with rich Al defects has weaker hydrophilicity than pristine α-Al2O3.46 The hydrophilicity difference suggested that PbCrO4-FVCr and PbCrO4-RVCr films have different contents of O dangling bonds on the surface, owing to their different Cr defect contents. The presence of O dangling bonds usually has a negative effect on carrier separation on the surface of semiconductors.47,48 Through the –SH groups of 1-octadecanethiol passivating the O dangling bonds on PbCrO4 films,49–51 the photocurrent of the PbCrO4-RVCr film was found to increase by 62%, but there was only a 24% increase on the PbCrO4-FVCr film (Fig. 7b and c). The photocurrent enhancement on 1-octadecanethiol-passivated PbCrO4 films confirmed the presence of O dangling bonds and the negative effect of O dangling bonds on carrier separation on PbCrO4. Meanwhile, the more obvious photocurrent improvement on the 1-octadecanethiol-passivated PbCrO4-RVCr film reflected their higher content of O dangling bonds, due to rich Cr defects.
To understand the influence of Cr defects on the band structure and carrier localization of PbCrO4, the density of states and electron localization function of monoclinic PbCrO4 with and without Cr defects were simulated by DFT calculations. As shown in Fig. 8a, PbCrO4 and PbCrO4-VCr had similar total density of state (TDOS), indicating that their theoretical carrier density is relatively close. From the partial density of state (PDOS) (Fig. 8b and c), the valence and conduction bands of PbCrO4 and PbCrO4-VCr can be observed to be mainly composed of O 2p and Cr 3d orbitals, respectively. However, two small peaks corresponding to O 2p and Cr 3d orbitals were found at the Fermi level of PbCrO4-VCr (Fig. 8c), indicating that a deep energy level is formed in the band structure of PbCrO4-VCr due to the presence of Cr defects. The presence of a deep energy level in PbCrO4 with Cr defects was actually reflected in the surface photovoltage spectrum of PbCrO4-RVCr films (shown in Fig. 6d), in which small photovoltage trailing in the time scale of 10−5–10−4 s was displayed. It has been reported that the formation of a deep energy level in semiconductors usually results in high non-radiative carrier recombination.52,53 In terms of structural theory, the presence of Cr defects in PbCrO4 could trigger strong non-radiative carrier recombination, as the d–d transition of Cr6+ is limited by spin selectivity and its radiation rate is low. Electron localization comparisons suggested that the presence of Cr defects increases the degree of carrier localization in PbCrO4 (Fig. 8d), hinting at the negative impact of Cr defects on carrier transfer in PbCrO4.54,55 Combined with the experimental findings of higher carrier recombination in/on PbCrO4-RVCr film than PbCrO4-FVCr film (Fig. 6c and d), it is certain that the presence of Cr defects in PbCrO4 can result in the formation of a deep energy level, which is not conducive to carrier transfer in the bulk of PbCrO4.
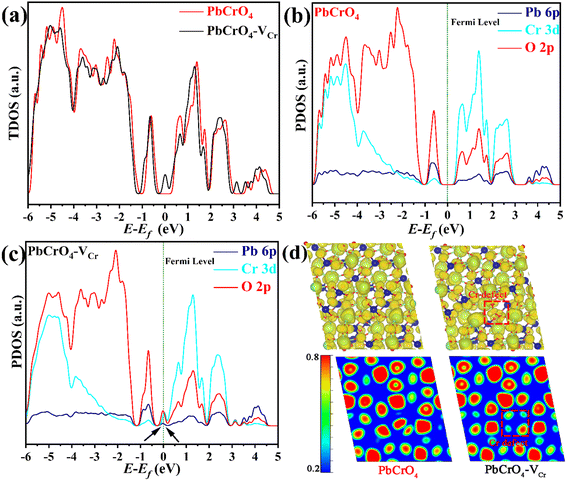 | ||
| Fig. 8 (a) Total density of states, (b and c) partial density of states, and (d) electron localization function of PbCrO4 with and without Cr defects. | ||
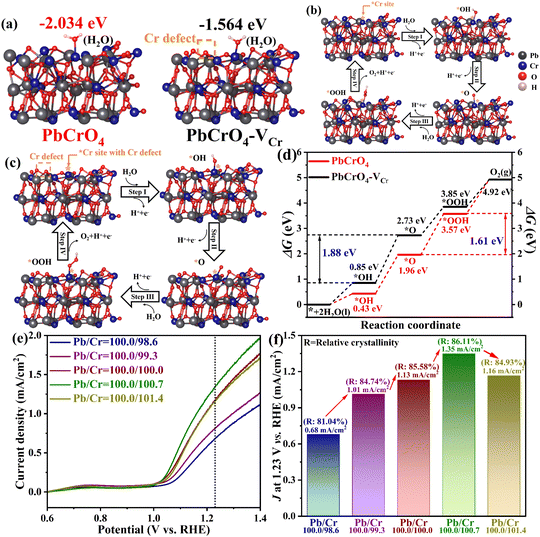
The Gibbs free energy (ΔG) of water oxidation on PbCrO4 with and without Cr defects was further simulated by DFT calculations based on a typical four-electron pathway (reactions (1)–(4)).56,57
| H2O(l) + * → *OH + H2O2 + H+ + e− | (1) |
| *OH + H2O(l) + H+ + e− → *O + H2O(l) + 2H+ + 2e− | (2) |
| *O + 2H2O(l) + 2H+ + 2e− → *OOH + 3H+ + 3e− | (3) |
| *OOH + 3H+ + 3e− → O2 + 4H+ + 4e− | (4) |
For the adsorption of H2O on PbCrO4, it was observed that H2O molecules preferentially adsorbed on the Cr sites of PbCrO4 (Fig. 9a), as Cr has a half-filled 3d orbital to provide active sites.36 The adsorption energy of H2O molecules on PbCrO4 was −2.034 eV, which is more negative than that on PbCrO4-VCr (−1.564 eV). PbCrO4 having a more negative H2O adsorption energy means that PbCrO4 has better properties for H2O adsorption than PbCrO4-VCr, which is consistent with the hydrophilicity results shown in Fig. 7a. For water oxidation, strong H2O adsorption on photoanodes is energetically favorable for the subsequent dissociation.58–61 In subsequent calculations for the dehydrogenation of H2O into O2 on PbCrO4 and PbCrO4-VCr, different ΔG values were found in each elementary step (Fig. 9b–d). Significantly, the rate-limiting step of water oxidation on PbCrO4-VCr was found to be the dehydrogenation of *OH into *O, and its energy barrier was 1.88 eV. For PbCrO4, its rate-limiting step of water oxidation was the reaction of *OH with H2O to form *OOH, and its energy barrier was 1.61 eV. This comparison indicates that the presence of Cr defects makes the rate-limiting step of water oxidation on PbCrO4 the dehydrogenation of *OH into *O, which has a higher energy barrier of 1.88 eV. Based on the EIS experimental results (Fig. 5a–c and Table 2), it can be further stated that the presence of Cr defects weakens the catalytic activity of PbCrO4 for water oxidation, and turns the rate-limiting step to the dehydrogenation of *OH into *O, which has a high energy barrier of 1.88 eV. Considering the negative effect of Cr defects and the ease of formation of Cr defects in PbCrO4 films using the drop-coating/thermal treatment approach, we discovered that PbCrO4 films with higher water oxidation activity can be prepared using a slightly excess of Cr precursor to compensate for the loss of Cr in thermal treatment, in addition to using a culture dish to cover the precursor solution. As shown in Fig. 9e and f, the highest water oxidation photocurrent was achieved on the PbCrO4 films that were prepared using the precursor solution with a Pb/Cr atomic ratio of 100.0/100.7. Meanwhile, the PbCrO4 film prepared with a Pb/Cr atomic ratio of 100.0/100.7 had the highest crystallinity (86.11%) (Fig. S13 and Table S3). The above findings provide a solution for the preparation of high-activity PbCrO4 films.
Summarizing the findings from experimental observations and DFT calculations, the mechanism for the higher activity of PbCrO4 films with fewer Cr defects can be explained as follows: (i) the presence of Cr defects results in the formation of a deep energy level in PbCrO4, which is unfavorable to carrier transfer in the bulk of PbCrO4 films; (ii) the presence of Cr defects leads to the formation of unsaturated O dangling bonds on PbCrO4, which are harmful traps for carrier separation on the surface of PbCrO4 films; (iii) Cr has a half-filled 3d5 orbital to provide active sites for reaction, so the presence of Cr defects weakens the catalytic activity of PbCrO4 for water oxidation, and makes the rate-limiting step the dehydrogenation of *OH into *O, which requires a high energy barrier of 1.88 eV.
4 Conclusions
In summary, monoclinic PbCrO4 films with few Cr defects (PbCrO4-FVCr) were prepared on FTO substrate through drop-coating Pb2+/Cr3+ precursor solution to reduce the thermal loss of Cr during annealing treatment. Compared with the monoclinic PbCrO4 film with rich Cr defects (PbCrO4-RVCr), the PbCrO4-FVCr film as a photoanode had higher solar water oxidation activity. A higher water oxidation photocurrent of 1.13 mA cm−2 at 1.23 V vs. RHE was produced on the PbCrO4-FVCr film photoanode, while the PbCrO4-RVCr film photoanode was 0.55 mA cm−2. Meanwhile, faster water oxidation kinetics were observed on the PbCrO4-FVCr film photoanode, relative to the PbCrO4-RVCr film photoanode. The PbCrO4-FVCr film photoanode had a water oxidation rate constant (kO2) of 40.6 s−1, and the kO2 of PbCrO4-RVCr film photoanode was 8.2 s−1. The higher solar water oxidation activity of PbCrO4 films with fewer Cr defects can be attributed to following reasons: (i) the presence of Cr defects can result in the formation of a deep energy level in PbCrO4, which is unfavorable for carrier transfer in the bulk of PbCrO4 films; (ii) the presence of Cr defects can cause the formation of unsaturated O dangling bonds on PbCrO4, which are harmful traps for carrier separation on the surface of PbCrO4 films; (iii) Cr has a half-filled 3d5 orbital to provide active sites for reaction, so the presence of Cr defects weakens the catalytic activity of PbCrO4 for water oxidation, and makes the dehydrogenation of *OH into *O the rate-limiting step, which requires a high energy barrier of 1.88 eV. The present work provides insight into monoclinic PbCrO4 film photoanodes in terms of preparation conditions, Cr defects, water oxidation activity and reaction mechanism.Author contributions
Jiahe Li designed and performed the experiments, and analysed most of the photoelectrochemical data (65%). Gaili Ke analysed partial photoelectrochemical data (35%). Minji Yang assisted with the analysis of DFT calculations data. Guoliang Lv and Lanyi Cao assisted with the XRD and SEM characterizations of samples. Wenjun Li and Wenrong Wang assisted with the XPS and EPR characterizations of samples. Tao Han and Yong Zhou assisted with the preparation and revision of manuscript. Huichao He conceived and directed this work. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data supporting this manuscript are available from the corresponding author upon reasonable request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5sc08541a.
Acknowledgements
This work was supported by National Natural Science Foundation of China (41702037), Natural Science Foundation of Chongqing (no. CSTB2023NSCQ-MSX0765), and Research Foundation of Chongqing University of Science and Technology (ckrc2022003).References
- A. E. Lindberg, W. Wang, S. Zhang, G. Galli and K. S. Choi, ACS Appl. Energy Mater., 2020, 3, 8658–8666 Search PubMed.
- H. C. Lee, S. K. Cho, H. S. Park, K. M. Nam and A. J. Bard, J. Phys. Chem. C, 2017, 121, 17561–17568 Search PubMed.
- S. K. Cho, R. Akbar, J. Kang, W. Lee and H. S. Park, J. Mater. Chem. A, 2018, 6, 13312–13320 Search PubMed.
- H. Zhou, D. Zhang, X. Gong, Z. Feng, M. Shi, Y. Liu, C. Zhang, P. Luan, P. Zhang, F. Fan, R. Li and C. Li, Adv. Mater., 2022, 34, 2110610 Search PubMed.
- Y. R. Gwon, J. Kang, S. Choe and S. K. Cho, J. Electrochem. Soc., 2023, 170, 106504 Search PubMed.
- H. Zhou, D. Zhang, H. Xie, Y. Liu, C. Meng, P. Zhang, F. Fan, R. Li and C. Li, Adv. Mater., 2023, 35, 2300914 CrossRef CAS PubMed.
- J. Kang, Y. R. Gwon and S. K. Cho, J. Electroanal. Chem., 2020, 878, 114601 CrossRef CAS.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS.
- Y. Zhang and J. Yan, Chem. Eng. J., 2023, 472, 144831 CrossRef CAS.
- J. Xiong, J. Di, J. Xia, W. Zhu and H. Li, Adv. Funct. Mater., 2018, 28, 1801983 CrossRef.
- H. Effenberger and F. Pertlik, Z. Kristallogr., 1986, 176, 75 CrossRef CAS.
- D. Errandonea, A. Muñoz, P. Rodríguez-Hernández, J. E. Proctor, F. Sapiña and M. Bettinelli, Inorg. Chem., 2015, 54, 7524–7535 CrossRef CAS PubMed.
- M. Lamers, S. Fiechter, D. Friedrich, F. F. Abdi and R. van de Krol, J. Mater. Chem. A, 2018, 6, 18694–18700 RSC.
- T. K. van Leeuwen, R. Dowdy, A. Guerrero and P. Gannon, J. Power Sources, 2023, 572, 233065 Search PubMed.
- K. Chen, X. Yuan, Z. Tian, M. Zou, Y. Yuan, Z. Chen, Q. Zhang, Y. Zhang, X. Jin, T. Wu, R. Shahbazian-Yassar and G. Liu, Nat. Mater., 2025, 24, 835–842 CrossRef CAS PubMed.
- H. Kato, K. Asakura and A. Kudo, J. Am. Chem. Soc., 2003, 125, 3082–3089 CrossRef CAS.
- K. Sivula, R. Zboril, F. Le Formal, R. Robert, A. Weidenkaff, J. Tucek, J. Frydrych and M. Grätzel, J. Am. Chem. Soc., 2010, 132, 7436–7444 CrossRef CAS.
- J. Xiao, L. Fan, F. Zhao, Z. Huang, S. F. Zhou and G. Zhan, J. Catal., 2020, 381, 139–149 Search PubMed.
- G. M. Carroll and D. R. Gamelin, J. Mater. Chem. A, 2016, 4, 2986–2994 RSC.
- J. Zhang, R. García-Rodríguez, P. Cameron and S. Eslava, Energy Environ. Sci., 2018, 11, 2972–2984 RSC.
- C. Debraj, K. Ouchi, Y. Tsubonouchi, Z. N. Zahran and M. Yagi, Appl. Catal., B, 2026, 380, 125733 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 47, 558 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- H. Abdel-Samad and P. R. Watson, Appl. Surf. Sci., 1998, 136, 46–54 CrossRef CAS.
- H. Bi, G. Han, M. Guo, C. Ding, H. Zou, Q. Shen, S. Hayase and W. Hou, ACS Appl. Mater. Interfaces, 2022, 14, 35513–35521 CrossRef CAS PubMed.
- M. C. Biesinger, C. Brown, J. R. Mycroft, R. D. Davidson and N. S. McIntyre, Surf. Interface Anal., 2004, 36, 1550–1563 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. St. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- L. Wang, S. Zhang, X. Lin, Q. Chen and H. Chen, Chem. Eng. J., 2024, 481, 147955 Search PubMed.
- D. Zu, Y. Ying, Q. Wei, P. Xiong, M. S. Ahmed, Z. Lin, M. M. Li, M. Li, Z. Xu, G. Chen, L. Bai, S. She, Y. Tsang and H. Huang, Angew. Chem., Int. Ed., 2024, 63, e202405756 CrossRef CAS.
- B. Qu and L. Chinese, J. Lumin., 2023, 43, 1815–1822 Search PubMed.
- F. Gan, H. Deng and H. Liu, J. Non-Cryst. Solids, 1982, 52, 135–141 CrossRef CAS.
- K. Tsai, C. Lai, Y. Chen, L. Ing-Chi, J. Chang, C. Kuo, S. Tseng, Y. Li and Y. Pu, Appl. Catal., B, 2024, 341, 123288 Search PubMed.
- L. Han, S. Dong and E. Wang, Adv. Mater., 2016, 28, 9266–9291 CrossRef CAS.
- J. Chen, H. Li, S. Chen, J. Fei, C. Liu, Z. Yu, K. Shin, Z. Liu, L. Song, H. Graeme, L. Wei and Y. Chen, Adv. Energy Mater., 2021, 11, 2003412 Search PubMed.
- S. Ye, W. Shi, Y. Liu, D. Li, H. Yin, H. Chi, Y. Luo, N. Ta, F. Fan, X. Wang and C. Li, J. Am. Chem. Soc., 2021, 143, 12499–12508 Search PubMed.
- W. Nabgan, B. Nabgan, A. A. Jalil, M. Ikram, I. Hussain, M. B. Bahari, T. V. Tran, M. Alhassan, A. H. K. Owgi, L. Parashuram, A. H. Nordin and F. Medina, Int. J. Hydrogen Energy, 2024, 52, 358–380 CrossRef CAS.
- S. Corby, R. R. Rao, L. Steier and J. R. Durrant, Nat. Rev. Mater., 2021, 6, 1136–1155 CrossRef CAS.
- J. Zhang, Y. Deng, D. Gu, S. Wang, L. She, R. Che, Z. S. Wang, B. Tu, S. Xie and D. Zhao, Adv. Energy Mater., 2011, 1, 241–248 CrossRef CAS.
- M. Ma, K. Zhang, P. Li, M. S. Jung and M. J. Jeong, Angew. Chem., Int. Ed., 2016, 55, 11819–11823 CrossRef CAS.
- K. H. Ye, H. Li, D. Huang, S. Xiao, W. Qiu, M. Li, Y. Hu, W. Mai, H. Ji and S. Yang, Nat. Commun., 2019, 10, 3687 CrossRef PubMed.
- F. A. L. Laskowski, M. R. Nellist, J. Qiu and S. W. Boettcher, J. Am. Chem. Soc., 2019, 141, 1394–1405 CrossRef CAS PubMed.
- R. J. Good, J. Adhes. Sci. Technol., 1992, 6, 1269 CrossRef CAS.
- K. P. D. Lagerlöf and R. W. Grimes, Acta Mater., 1998, 46, 5689–5700 CrossRef.
- H. Wang, S. Jiang, H. Yu, K. Deng, Z. Wang, X. Li, Y. Xu and L. Wang, J. Mater. Chem. A, 2023, 11, 13633–13639 RSC.
- X. Zhong, H. He, M. Yang, G. Ke, Z. Zhao, F. Dong, B. Wang, Y. Chen, X. Shi and Y. Zhou, J. Mater. Chem. A, 2018, 6, 10456–10465 Search PubMed.
- M. Yang, H. He, H. Zhang, G. Ke, X. Zhong, F. Dong, Y. Chen, J. Du and Y. Zhou, Electrochim. Acta, 2018, 283, 871–881 CrossRef CAS.
- G. Yang, Y. Li, Y. Liu, F. Xie, G. Yan, X. Yang, C. Wei, B. Bian, X. Zhang and N. Lu, IEEE Trans. Electron Devices, 2022, 69, 195–200 CAS.
- X. Min, Q. Xie, Z. Wang, X. Wang and M. Chen, Mater. Chem. Phys., 2022, 276, 125404 CrossRef CAS.
- J. Ma, H. Chi, A. Wang, P. Wang, H. Jing and T. Yao, J. Am. Chem. Soc., 2022, 144, 17540–17548 CrossRef CAS.
- Z. Zhu, K. Mao, K. Zhang, W. Peng, J. Zhang, H. Meng, S. Cheng, T. Li, H. Lin, Q. Chen, X. Wu and J. Xu, Joule, 2022, 6, 2849–2868 CrossRef CAS.
- S. Wang, L. Pan, J. Song, W. Mi, J. Zou, L. Wang and X. Zhang, J. Am. Chem. Soc., 2015, 137, 2975–2983 CrossRef CAS PubMed.
- P. Fuentealba, E. Chamorro, J. Santos and A. Toro-Labbé, Comput. Theor. Chem., 2007, 19, 57–85 CAS.
- S. Wang, T. He, P. Chen, A. Du, K. Ostrikov, W. Huang and L. Wang, Adv. Mater., 2020, 32, 2001385 CrossRef CAS.
- M. Gerosa, F. Gygi, M. Govoni and G. Galli, Nat. Mater., 2018, 17, 1122–1127 CrossRef CAS PubMed.
- P. Li, X. Chen, H. He, X. Zhou, Y. Zhou and Z. Zou, Adv. Mater., 2018, 30, 1703119 CrossRef.
- S. Wan, C. Dong, J. Jin, J. Li, Q. Zhong, K. Zhang and J. H. Park, ACS Energy Lett., 2022, 7, 3024–3031 CrossRef CAS.
- P. Yue, H. She, L. Zhang, B. Niu, R. Lian, J. Huang, L. Wang and Q. Wang, Appl. Catal., B, 2021, 286, 119875 CrossRef CAS.
- X. Wei, J. Zhang, L. Wang, Y. Bai, J. Huang, H. She and Q. Wang, Chem. Eng. J., 2024, 482, 149114 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |