Open Access Article
Open Access ArticleSe-catalyzed enantioselective lactamization enabled by a N-fluorosulfonyl group: total synthesis of diaporisoindole A
Daiki Yamamotoabc,
Shun Yamasakic and
Takuya Hashimoto *abd
*abd
aMolecular Synthesis and Function Laboratory, Pioneering Research Institute, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. E-mail: takuya.hashimoto@riken.jp
bGraduate School of Pharmaceutical Sciences, Chiba University, 1-8-1 Inohana, Chuo, Chiba 260-8675, Japan
cDepartment of Chemistry, Graduate School of Science, Chiba University, 1-33 Yayoi, Inage, Chiba 263-8522, Japan
dInstitute for Advanced Academic Research (IAAR), Chiba University, 1-33 Yayoi, Inage, Chiba 263-8522, Japan
First published on 15th December 2025
Abstract
Electrophilic cyclization of unsaturated carboxylic acids is a well-established strategy for the catalytic enantioselective synthesis of lactones. In contrast, analogous catalytic transformations of unsaturated carboxamides to access enantioenriched lactams remain underdeveloped due to the ambident nucleophilicity of carboxamides, favoring nucleophilic attack from their oxygen atom. Herein, we unveil N-(fluorosulfonyl)carboxamides as versatile and reactive substrates that overcome this limitation, as demonstrated by a selenium-catalyzed enantioselective lactamization, facile product derivatization, and application to the total synthesis of diaporisoindole A. To further showcase their synthetic potential, we briefly demonstrate their applicability in racemic iodo- and hydro-lactamizations.
Introduction
Forging C–O and C–N bonds within the same synthetic platform not only simplifies synthesis planning but also enables facile access to both O- and N-analogs of target molecules. Such synthetic strategies have been widely and often implicitly practiced, from classical SN2 alkylation and acylation to modern metal-catalyzed cross-coupling reactions.1 The intramolecular addition of a carboxylic acid to an electrophilically activated alkene is a common approach for the construction of a lactone ring via C–O bond formation,2,3 as represented by halolactonization. To date, dozens of catalytic enantioselective electrophilic lactonizations have been developed for these transformations, driven by the advent of new chiral catalysts.4–16While the direct application of electrophilic lactonization procedures to lactamization via C–N bond formation would be highly desirable, the intrinsically favored O-cyclization of carboxamides necessitates their modification prior to use (Fig. 1a).17–19 In stoichiometric electrophilic lactamization, carboxamides are classically modified as N,O-bis-trimethylsilyl, N-Boc or N-tosyl carboxamides to harness the ambident reactivity for forging a C–N bond (Fig. 1b).20–24 This prerequisite makes catalytic enantioselective electrophilic lactamization challenging, as it demands a fine balance between nucleophilic activation of the carboxamide and electrophilic activation of the internal alkene. To date, only two examples have been reported (Fig. 1c): Yeung and co-workers achieved an enantioselective bromolactamization using an amino carbamate catalyst,25 while the Denmark group reported a chiral Lewis base-catalyzed sulfenolactamization.26,27 In both cases, N-tosyl carboxamides were predominantly used, despite the removal of the N-tosyl group from the lactams being challenging, if not impossible. This dearth of catalytic electrophilic lactamization led to the development of several, mechanistically distinct, transition metal-catalyzed lactamizations of unsaturated carboxamides.28–31
Results and discussion
In our pursuit of chiral selenium π-acid catalysis,32–36 we faced the difficulty of adapting our selenium-catalyzed enantioselective electrophilic lactonization,37 that proceeds through an oxidative catalytic cycle,38 to lactamization (Fig. 1d).39–41 Our initial attempts using N-acyl and N-Boc carboxamides failed to yield the desired lactams. Even with the N-tosyl group, which has been validated not only in asymmetric lactamizations (Fig. 1c) but also selenium-catalyzed C–N bond formations,42–45 no desired product was obtained. In one case using N-methoxy carboxamides, the corresponding iminolactones were obtained exclusively.46 The breakthrough came from our separate project where N-(fluorosulfonyl)carbamates were identified as highly effective nitrogen nucleophiles.47–50 Incorporating the N-fluorosulfonyl group into unsaturated carboxamides led to clean conversion, producing the desired lactams with high enantioselectivities (Fig. 1d, this work). Herein, we detail the outcomes of this study, including its application to the enantioselective total synthesis of diaporisoindole A,51 and two other representative electrophilic lactamizations.We initially turned to computational studies to elucidate the drastic reactivity difference between N-tosyl and N-fluorosulfonyl amides (Fig. 1e). The key distinction lies in their pKa values, differing by more than 8 units between two model acetamides in acetonitrile.52 The local nucleophilicities of the nitrogen and oxygen atoms of each anion were assessed using condensed Fukui functions based on Hirshfeld charges, revealing that the nitrogen atoms exhibit higher values and thus greater nucleophilicity in both cases (Fig. 1e, middle).53–58 In contrast, neutral acetamide shows greater nucleophilicity at the oxygen atom, supporting the conventional knowledge as in Fig. 1a and b. Based on these observations, we reasoned that the higher acidity of an N-(fluorosulfonyl)amide facilitates the generation of the amide anion, which renders the nitrogen atom more nucleophilic, without interfering with electrophilic activation of the alkene by a selenium π-acid (key intermediate in Fig. 1d).59,60 Yet, the nitrogen atom of an N-(fluorosulfonyl)amide is less accessible than the oxygen atom as visually evident from the structure and quantitatively assessed by its smaller solvent accessible surface area (SASA) (Fig. 1e, right), posing a steric obstacle that could undermine the electronically harnessed pathway to lactamization.61,62
With the computational rationale in hand, we evaluated the substrate scope with minimal modification to the reaction conditions optimized for the lactonization,37 using 5 mol% diselenide 1 (Fig. 2a). The enantioselective lactamization of aliphatic N-(fluorosulfonyl)amides 3 proceeded in good yields and high enantioselectivities, tolerating primary and secondary alkyl (5a–5i), as well as benzyl (5j–5n) groups at the γ-position, although a small amount of O-cyclized iminolactones was observed in the crude mixtures. One limitation was the use of γ-t-alkyl amides which underwent O-cyclization exclusively (data not shown), highlighting the importance of steric factors in achieving N/O selectivity.
In the further substrate evaluation, we encountered lower enantioselectivities with γ-aryl-substituted carboxamides 4. It was concluded that the poorer enantioselectivity was caused by two factors: first, the formation of a desilylated catalyst which is still catalytically active but favors the opposite enantiomer (see the SI), and second, the gradual racemization of the more acidic product. The longer reaction times required for the full conversion of aromatic substrates exacerbated these issues. In light of these challenges, we re-optimized the reaction conditions (see the SI), making them applicable to various aromatic substrates (Fig. 2b). Key modifications were the use of more desilylation-resistant, TIPS-substituted catalyst 2, TMSOAc as the non-basic fluoride scavenger and CH3CN/H2O as the solvent. Under these optimized reaction conditions, unsaturated lactams 6a–6n were obtained in high yields and enantioselectivities, regardless of their substituent patterns or electronic properties. In case of aromatic substrates, O-cyclized products were not detected in the crude NMR, contributing to higher product yields. Notably, a tri-substituted alkene also participated in this lactamization, forming a tetra-substituted stereocenter with 78% ee (6o). Finally, the reaction was scaled up to 1 mmol, yielding 6a in 91% yield and 91% ee under 3 mol% of diselenide 2.
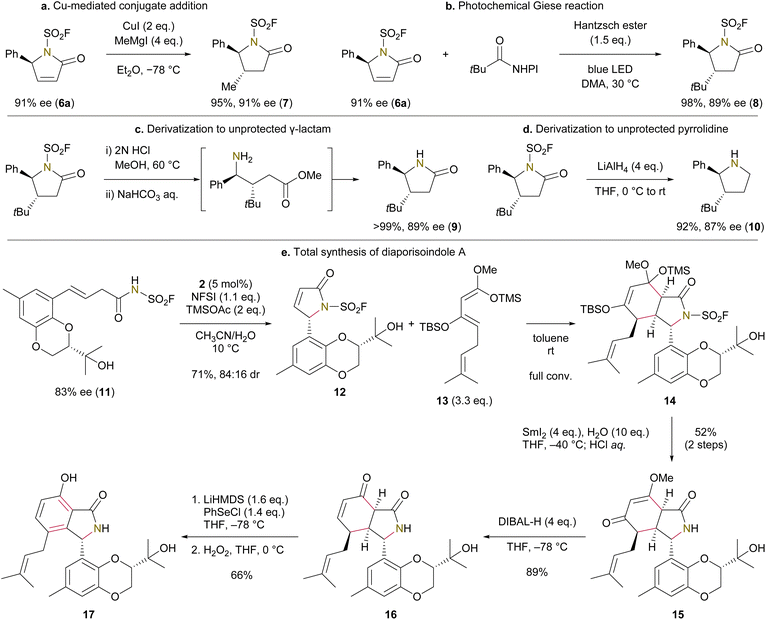
With the sufficient supply of enantioenriched unsaturated lactam 6a, we next explored its derivatization. A 1,4-addition of an organocuprate reagent proceeded smoothly, giving 4,5-trans-disubstituted γ-lactam 7 in 95% yield (Fig. 3a). Although the C5 stereocenter of γ-lactam 6a is prone to base-mediated racemization, its enantioselectivity was retained throughout the transformation. A Giese radical alkylation was also applicable to lactam 6a,63 enabling the installation of a bulky t-butyl group on the β-position (Fig. 3b). The N-fluorosulfonyl group of 8 was readily removed by sequential treatment with acid and base (Fig. 3c). Moreover, LiAlH4-mediated reduction of 8 furnished the corresponding N–H free pyrrolidine without opening the ring structure (Fig. 3d). These deprotection protocols further highlight the advantage of using the N-fluorosulfonyl group over the N-tosyl group.27
We next sought to demonstrate the feasibility of our selenium-catalyzed lactamization, and product derivatization and deprotection protocols in a more complex context—the enantioselective total synthesis of diaporisoindole A (Fig. 3e).51 This natural product was chosen due to our particular interest in exploring a Diels–Alder reaction, capitalizing on the strongly electron-deficient nature of our unsaturated lactam products.64 Literature reports indicate that successful Diels–Alder reactions of α,β-unsaturated γ-lactams typically require a low-lying LUMO on the lactam, presenting a significant challenge for this transformation.65–69 The lactamization precursor 11 was synthesized from cresol in enantioenriched form (see the SI), and was then subjected to our selenium catalysis, affording the key unsaturated lactam 12 in 71% yield with 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 dr. We next investigated the Diels–Alder reaction, identifying electron-rich 1,3-bis-silyloxybutadiene 13 which reacted efficiently with 12 to furnish bicyclic compound 14 with the concurrent installation of the prenyl group. The relative stereochemical relationship of this Diels–Alder reaction was tentatively assigned as shown in the scheme, based on SCXRD of a model compound (see the SI). Our attempt to deprotect the N-fluorosulfonyl group as described in Fig. 3c did not result in the formation of the N–H free lactam in satisfactory yield. To address this issue, we employed a single-electron reduction of the fluorosulfonyl group by SmI2 which cleanly provided the N–H free lactam 15 without compromising the molecule's structural integrity.70 Redox manipulation of this bicyclic intermediate led to diaporisoindole A 17, which matched the reported NMR data and optical rotation. Notably, the synthesized quantity of diaporisoindole A allowed us to characterize a broad 13C peak at C8 that was unassigned in the original isolation research (see the SI).
16 dr. We next investigated the Diels–Alder reaction, identifying electron-rich 1,3-bis-silyloxybutadiene 13 which reacted efficiently with 12 to furnish bicyclic compound 14 with the concurrent installation of the prenyl group. The relative stereochemical relationship of this Diels–Alder reaction was tentatively assigned as shown in the scheme, based on SCXRD of a model compound (see the SI). Our attempt to deprotect the N-fluorosulfonyl group as described in Fig. 3c did not result in the formation of the N–H free lactam in satisfactory yield. To address this issue, we employed a single-electron reduction of the fluorosulfonyl group by SmI2 which cleanly provided the N–H free lactam 15 without compromising the molecule's structural integrity.70 Redox manipulation of this bicyclic intermediate led to diaporisoindole A 17, which matched the reported NMR data and optical rotation. Notably, the synthesized quantity of diaporisoindole A allowed us to characterize a broad 13C peak at C8 that was unassigned in the original isolation research (see the SI).
Finally, to gain preliminary insight into the utility of N-fluorosulfonyl carboxamides in other electrophilic lactamizations, we examined racemic iodo- and hydro-lactamization using selected N-fluorosulfonyl carboxamides (Fig. 4).71 Exo- and endo-iodolactamizations using molecular iodine provided the corresponding lactams in moderate to good yields, along with minor amounts of iminolactones (Fig. 4a and b). Hydrolactamization catalyzed by H2SO4 proceeded cleanly to give 21 in nearly quantitative yield (Fig. 4c). These results would provide a basis for developing catalytic enantioselective variants.72,73 It should be noted that we observed the conversion of the initially formed iminolactone into the lactam in the hydrolactamization, but not in the seleno- or iodo-lactamizations, indicating the reversibility of this acid-catalyzed process and its thermodynamic preference for lactam formation.
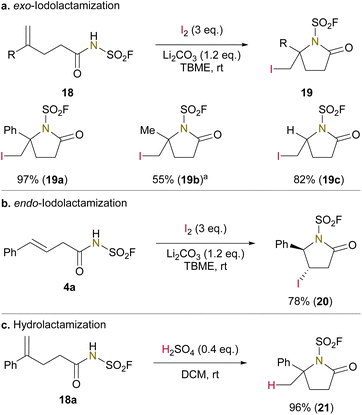 | ||
| Fig. 4 Iodo- and hydro-lactamizations of unsaturated N-(fluorosulfonyl)carboxamides. (a) exo-Iodolactamization. (b) endo-Iodolactamization. (c) Hydrolactamization. a1,4-Dioxane as solvent. | ||
Conclusions
The synthetic community has recognized the difficulty of achieving electrophilic lactamization since the 1950s.74 While pre-functionalization of amides has addressed this issue in stoichiometric contexts, the challenge continues to persist in asymmetric catalysis, widening the gap in progress between enantioselective electrophilic lactonization and lactamization. In this work, we demonstrated that N-(fluorosulfonyl)amides serve as effective substrates for chiral selenium-catalyzed electrophilic lactamization, a reactivity unattainable with other amides. The practicality of this method is underscored by the straightforward derivatization of the lactam products, including the enantioselective total synthesis of diaporisoindole A. This strategy also proved applicable to racemic iodo- and hydro-lactamizations, paving the way for the future development of enantioselective variants.Author contributions
D. Y. and S. Y. designed and conducted the experiments and prepared the SI. T. H. supervised the project and wrote the manuscript with input from D. Y. and S. Y.Conflicts of interest
There are no conflicts to declare.Data availability
CCDC 2482628–2482632 and 2484902 contain the supplementary crystallographic data for this paper.75a–fThe data that supports the findings of this study are available in the supplementary information (SI) of this article. Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc08502k.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers 19H02710 and 22H02072 (T. H.), and RIKEN Student Researcher (RSR) and Junior Research Associate (JRA) programs (D. Y.). The computations were performed using RIKEN's HOKUSAI BigWaterfall2.Notes and references
- B. Schlummer and U. Scholz, Adv. Synth. Catal., 2004, 346, 1599–1626 Search PubMed.
- M. D. Dowle and D. I. Davies, Chem. Soc. Rev., 1979, 8, 171–197 Search PubMed.
- S. Ranganathan, K. M. Muraleedharan, N. K. Vaish and N. Jayaraman, Tetrahedron, 2004, 60, 5273–5308 Search PubMed.
- C. K. Tan, L. Zhou and Y.-Y. Yeung, Synlett, 2011, 2011, 1335–1339 Search PubMed.
- S. E. Denmark, W. E. Kuester and M. T. Burk, Angew. Chem., Int. Ed., 2012, 51, 10938–10953 Search PubMed.
- U. Hennecke, Chem.–Asian J., 2012, 7, 456–465 Search PubMed.
- W. Zhang, N. Liu, C. M. Schienebeck, K. Decloux, S. Q. Zheng, J. B. Werness and W. P. Tang, Chem.–Eur. J., 2012, 18, 7296–7305 Search PubMed.
- J. M. J. Nolsoe and T. V. Hansen, Eur. J. Org Chem., 2014, 2014, 3051–3065 Search PubMed.
- A. Sakakura and K. Ishihara, Chem. Rec., 2015, 15, 728–742 Search PubMed.
- M. H. Gieuw, Z. Ke and Y.-Y. Yeung, Chem. Rec., 2017, 17, 287–311 Search PubMed.
- R. Kristianslund, J. E. Tungen and T. V. Hansen, Org. Biomol. Chem., 2019, 17, 3079–3092 Search PubMed.
- H. China, R. Kumar, K. Kikushima and T. Dohi, Molecules, 2020, 25, 6007 Search PubMed.
- S. De, A. Kumar Dan, R. Sahu and D. Das, Eur. J. Org Chem., 2022, 2022, e202200817 Search PubMed.
- S. Liu, B.-Q. Zhang, W.-Y. Xiao, Y.-L. Li and J. Deng, Adv. Synth. Catal., 2022, 364, 3974–4005 Search PubMed.
- L. Liao and X. Zhao, Acc. Chem. Res., 2022, 55, 2439–2453 Search PubMed.
- J. H. Yan, Z. Q. Zhou, Q. Q. He, G. Z. Chen, H. B. Wei and W. Q. Xie, Org. Chem. Front., 2022, 9, 499–516 Search PubMed.
- S. Robin and G. r. Rousseau, Tetrahedron, 1998, 54, 13681–13736 Search PubMed.
- M. Breugst, T. Tokuyasu and H. Mayr, J. Org. Chem., 2010, 75, 5250–5258 Search PubMed.
- H. Mayr, M. Breugst and A. R. Ofial, Angew. Chem., Int. Ed., 2011, 50, 6470–6505 Search PubMed.
- S. Knapp, K. E. Rodriques, A. T. Levorse and R. M. Ornaf, Tetrahedron Lett., 1985, 26, 1803–1806 Search PubMed.
- S. Knapp and A. T. Levorse, J. Org. Chem., 1988, 53, 4006–4014 Search PubMed.
- Y.-Y. Yeung and E. J. Corey, Tetrahedron Lett., 2007, 48, 7567–7570 Search PubMed.
- Y.-Y. Yeung, S. Hong and E. J. Corey, J. Am. Chem. Soc., 2006, 128, 6310–6311 Search PubMed.
- A. J. Biloski, R. D. Wood and B. Ganem, J. Am. Chem. Soc., 1982, 104, 3233–3235 Search PubMed.
- Y. A. Cheng, W. Z. Yu and Y. Y. Yeung, Angew. Chem., Int. Ed., 2015, 54, 12102–12106 Search PubMed.
- S. E. Denmark and H. M. Chi, J. Am. Chem. Soc., 2014, 136, 8915–8918 Search PubMed.
- J. L. Panger and S. E. Denmark, Org. Lett., 2020, 22, 2501–2505 Search PubMed.
- M. Hatano and T. Nishimura, Angew. Chem., Int. Ed., 2015, 54, 10949–10952 Search PubMed.
- M. Nagamoto, T. Yanagi, T. Nishimura and H. Yorimitsu, Org. Lett., 2016, 18, 4474–4477 Search PubMed.
- M. Freis, M. Balkenhohl, D. M. Fischer, T. Georgiev, R. C. Sarott and E. M. Carreira, Org. Lett., 2023, 25, 6380–6384 Search PubMed.
- X. Yang, P. Chen and G. Liu, Angew. Chem., Int. Ed., 2024, e202408305 Search PubMed.
- F. V. Singh and T. Wirth, Catal. Sci. Technol., 2019, 9, 1073–1091 Search PubMed.
- S. Ortgies and A. Breder, ACS Catal., 2017, 7, 5828–5840 Search PubMed.
- L. Liao and X. Zhao, Synlett, 2021, 32, 1262–1268 Search PubMed.
- L. Shao, Y. Li, J. Lu and X. Jiang, Org. Chem. Front., 2019, 6, 2999–3041 Search PubMed.
- J. T. Stadel and T. G. Back, Chem.–Eur. J., 2024, 30, e202304074 Search PubMed.
- Y. Kawamata, T. Hashimoto and K. Maruoka, J. Am. Chem. Soc., 2016, 138, 5206–5209 Search PubMed.
- D. M. Browne, O. Niyomura and T. Wirth, Org. Lett., 2007, 9, 3169–3171 Search PubMed.
- A. Toshimitsu, K. Terao and S. Uemura, J. Org. Chem., 1987, 52, 2018–2026 Search PubMed.
- M. Tiecco, L. Testaferri, M. Tingoli and F. Marini, J. Chem. Soc., Chem. Commun., 1994, 221, 10.1039/c39940000221.
- M. Tiecco, L. Testaferri, F. Marini, S. Sternativo, L. Bagnoli, C. Santi and A. Temperini, Tetrahedron: Asymmetry, 2001, 12, 1493–1502 Search PubMed.
- F. Krätzschmar, S. Ortgies, R. Y. N. Willing and A. Breder, Catalysts, 2019, 9, 153 Search PubMed.
- T. Lei, S. Graf, C. Schöll, F. Krätzschmar, B. Gregori, T. Appleson and A. Breder, ACS Catal., 2023, 13, 16240–16248 Search PubMed.
- Z. Tao, B. B. Gilbert and S. E. Denmark, J. Am. Chem. Soc., 2019, 141, 19161–19170 Search PubMed.
- E. M. Mumford, B. N. Hemric and S. E. Denmark, J. Am. Chem. Soc., 2021, 143, 13408–13417 Search PubMed.
- Y. Otsuka, Y. Shimazaki, H. Nagaoka, K. Maruoka and T. Hashimoto, Synlett, 2019, 30, 1679–1682 Search PubMed.
- C. Wata and T. Hashimoto, J. Am. Chem. Soc., 2021, 143, 1745–1751 Search PubMed.
- C. Wata and T. Hashimoto, Synthesis, 2021, 53, 2594–2601 Search PubMed.
- Y. Oe, R. Yoshida, A. Tanaka, A. Adachi, Y. Ishibashi, T. Okazoe, K. Aikawa and T. Hashimoto, J. Am. Chem. Soc., 2022, 144, 2107–2113 Search PubMed.
- H. Kimura, K. Aikawa and T. Hashimoto, Asian J. Org. Chem., 2023, 13, e202300522 Search PubMed.
- H. Cui, Y. Lin, M. Luo, Y. Lu, X. Huang and Z. She, Org. Lett., 2017, 19, 5621–5624 Search PubMed.
- A. Kütt, S. Tshepelevitsh, J. Saame, M. Lõkov, I. Kaljurand, S. Selberg and I. Leito, Eur. J. Org Chem., 2021, 2021, 1407–1419 Search PubMed.
- R. G. Parr and W. Yang, J. Am. Chem. Soc., 1984, 106, 4049–4050 Search PubMed.
- W. Yang and W. J. Mortier, J. Am. Chem. Soc., 1986, 108, 5708–5711 Search PubMed.
- S. Liu, C. Rong and T. Lu, J. Phys. Chem. A, 2014, 118, 3698–3704 Search PubMed.
- B. Wang, C. Rong, P. K. Chattaraj and S. Liu, Theor. Chem. Acc., 2019, 138, 124 Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 Search PubMed.
- T. Lu, J. Chem. Phys., 2024, 161, 082503 Search PubMed.
- Y. A. Cheng, W. Z. Yu and Y. Y. Yeung, J. Org. Chem., 2016, 81, 545–552 Search PubMed.
- T. Arai, O. Watanabe, S. Yabe and M. Yamanaka, Angew. Chem., Int. Ed., 2015, 54, 12767–12771 Search PubMed.
- T. Gensch, G. dos Passos Gomes, P. Friederich, E. Peters, T. Gaudin, R. Pollice, K. Jorner, A. Nigam, M. Lindner-D’Addario, M. S. Sigman and A. Aspuru-Guzik, J. Am. Chem. Soc., 2022, 144, 1205–1217 Search PubMed.
- C. H. Ng, E. R. Wearing, D. E. Blackmun, G. G. Terrones, J. Toigo, K.-M. Tong, K. C. Harper, J. C. Donovan, M. A. Gonley, E. A. Voight, B. D. Bergstrom, M. O. Wolf, H. J. Kulik and C. S. Schindler, J. Am. Chem. Soc., 2025, 147, 29722–29731 Search PubMed.
- C. Zheng, G.-Z. Wang and R. Shang, Adv. Synth. Catal., 2019, 361, 4500–4505 Search PubMed.
- N. Slavov, J. Cvengroš, J.-M. Neudörfl and H.-G. Schmalz, Angew. Chem., Int. Ed., 2010, 49, 7588–7591 Search PubMed.
- E. Merifield and E. J. Thomas, J. Chem. Soc., Chem. Commun., 1990, 464, 10.1039/c39900000464.
- M. J. Bamford, M. Beard, T. Cherry David and M. G. Moloney, Tetrahedron: Asymmetry, 1995, 6, 337 Search PubMed.
- J. H. Bailey, D. T. Cherry, K. M. Crapnell, M. G. Moloney, S. B. Shim, M. J. Bamford and R. B. Lamont, Tetrahedron, 1997, 53, 11731–11744 Search PubMed.
- K. Yoshida, T. Morikawa, N. Yokozuka, S. Harada and A. Nishida, Tetrahedron Lett., 2014, 55, 6907–6910 Search PubMed.
- K. R. Owens, S. V. McCowen, K. A. Blackford, S. Ueno, Y. Hirooka, M. Weber and R. Sarpong, J. Am. Chem. Soc., 2019, 141, 13713–13717 Search PubMed.
- M. Szostak, M. Spain and D. J. Procter, Chem. Soc. Rev., 2013, 42, 9155–9183 Search PubMed.
- T.-H. Chou, B.-H. Yu and R.-J. Chein, Chem. Commun., 2019, 55, 13522–13525 Search PubMed.
- R. Maji, S. Ghosh, O. Grossmann, P. Zhang, M. Leutzsch, N. Tsuji and B. List, J. Am. Chem. Soc., 2023, 145, 8788–8793 Search PubMed.
- N. Luo, M. Turberg, M. Leutzsch, B. Mitschke, S. Brunen, V. N. Wakchaure, N. Nöthling, M. Schelwies, R. Pelzer and B. List, Nature, 2024, 632, 795–801 Search PubMed.
- P. N. Craig, J. Am. Chem. Soc., 1952, 74, 129–131 Search PubMed.
- (a) CCDC 2482628: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbcs6; (b) CCDC 2482629: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbct7; (c) CCDC 2482630: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbcv8; (d) CCDC 2482631: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbcw9; (e) CCDC 2482632: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pbcxb; (f) CCDC 2484902: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pdr40.
| This journal is © The Royal Society of Chemistry 2026 |