Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of chiral lactams by asymmetric nitrogen insertion
Jasmin
Hammes†
,
Clara
Mañas†
 ,
Abhilash
Pedada
,
Abhilash
Pedada
 ,
Marlene
Arnold
and
Johannes M.
Wahl
,
Marlene
Arnold
and
Johannes M.
Wahl
 *
*
Department Chemie, Johannes Gutenberg-Universität, Duesbergweg 10-14, 55128 Mainz, Germany. E-mail: wahl@uni-mainz.de
First published on 10th November 2025
Abstract
Asymmetric nitrogen insertion into prochiral and meso cycloalkanones is achieved using diphenylphosphinyl hydroxylamine (DPPH) as the nitrogen source. A combination of Brønsted acid catalyst and Lewis acid promoter enables high yields and selectivity in the syntheses of a range of 5- to 7-membered lactams (19 examples, up to 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er). Mechanistically, the sequence follows a Beckmann pathway involving an asymmetric condensation followed by a stereospecific rearrangement. The utility of the method is showcased by the syntheses of the drugs phenotropil, rolipram, pregabalin, and baclofen.
3 er). Mechanistically, the sequence follows a Beckmann pathway involving an asymmetric condensation followed by a stereospecific rearrangement. The utility of the method is showcased by the syntheses of the drugs phenotropil, rolipram, pregabalin, and baclofen.
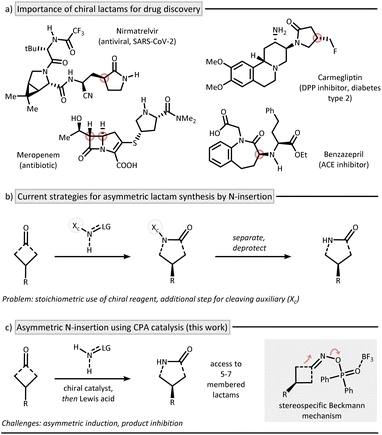
Introduction
The direct insertion of a nitrogen atom into a cyclic ketone represents a powerful synthetic strategy to forge lactams as an important class of nitrogen heterocycles.1,2 A prominent example is the large-scale production of ε-caprolactam from cyclohexanone, which serves as a key precursor in Nylon manufacturing.3 Traditionally, nitrogen atom insertion is achieved via the venerable Beckmann4 and Schmidt5 reactions. The relatively harsh reaction conditions of these methods have hindered the development of enantioselective protocols, yet the prevalence of chiral lactams in modern drugs makes asymmetric variants particularly compelling (Fig. 1a). Most existing asymmetric protocols thus rely on chiral reagents for induction (Fig. 1b).6–9 It is striking that, while the related catalytic asymmetric oxygen insertion via Baeyer–Villiger rearrangement has been successfully implemented both enzymatically10,11 and by using small molecule catalysts,12 no broadly applicable approach exists for nitrogen insertion.13 This absence of catalytic asymmetric strategies may stem from the fundamental mechanistic differences between the Beckmann and Baeyer–Villiger rearrangements, along with the difficulties in establishing catalysis for nitrogen insertion.14 One major obstacle is product inhibition, an inherent issue in any ketone-to-amide conversion.15,16 In addition, no enzymatic pathways for nitrogen insertion are known, eliminating Nature as a potential blueprint for catalyst design.Inspiration for our work can be found in the pioneering reports on asymmetric oxime formation by Antilla and co-workers,17 as well as on the elegant multistep chirality transfer approaches from oxime ethers to lactams recently described by the groups of Tan18 and Shi.19 However, a general, two-step Beckmann process has to the best of our knowledge not been disclosed and represents a long-time goal in nitrogen insertion chemistry providing access to chiral 3D-nitrogen heterocycles. In this work, we address this challenge by presenting a mild and enantioselective nitrogen insertion method for synthesizing 5- to 7-membered lactams, which operates within the Beckmann mechanistic framework (Fig. 1c).
Results and discussion
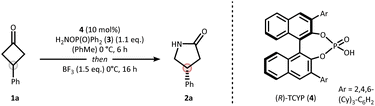
Our strategy for synthesizing chiral lactams relies on the use of hydroxylamine-derived reagents.20,21 We recently discovered that O-phosphinyl hydroxylamines undergo efficient nitrogen insertion, offering a practical approach due to their commercial availability.22 Nitrogen insertion at cyclobutanones with hydroxylamine derivatives has been shown to proceed via an aza-Baeyer–Villiger pathway.22–24 Similar findings were also reported for cyclopropanones by Lindsay and coworkers.25 Given this mechanistic peculiarity, along with the synthetic importance of γ-lactams in drug discovery,26 we deemed prochiral cyclobutanones as an ideal starting point for developing a catalytic asymmetric nitrogen insertion protocol.Based on the privileged role of chiral phosphoric acids (CPAs) in organocatalyzed condensation27 and insertion reactions,28–31 we initiated a systematic evaluation of the reaction conditions using prochiral phenylcyclobutanone 1a as the model substrate (see SI for full details). The optimization identified diphenylphosphinyl hydroxylamine (3) as the optimal nitrogen source, R-TCYP (4) as the most effective CPA catalyst, and boron trifluoride as essential promoter to forge γ-lactam 2a in 82% yield and an enantiomeric ratio (er) of 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 (Table 1, entry 1). Several key insights emerged from the optimization process. Importantly, the combination of Brønsted acid and Lewis acid was crucial for a successful outcome. Low yields were obtained in the absence of boron trifluoride (entry 2), while alternative promoters such as para-toluenesulfonic acid (pTsOH) or heat afforded the lactam with low selectivity (entries 3 & 4). High enantioselectivity was also dependent on the sequential addition of Brønsted and Lewis acids as can be extracted from the simultaneous addition experiment (entry 5).
13 (Table 1, entry 1). Several key insights emerged from the optimization process. Importantly, the combination of Brønsted acid and Lewis acid was crucial for a successful outcome. Low yields were obtained in the absence of boron trifluoride (entry 2), while alternative promoters such as para-toluenesulfonic acid (pTsOH) or heat afforded the lactam with low selectivity (entries 3 & 4). High enantioselectivity was also dependent on the sequential addition of Brønsted and Lewis acids as can be extracted from the simultaneous addition experiment (entry 5).
| Entry | Changes from standard conditiona | Yieldb | erc |
|---|---|---|---|
| a Reactions were run on a 0.1 mmol scale using 2.0 mL of solvent (0.05 M). b Determined by 1H NMR using CH2Br2 as an internal standard. c Determined by HPLC analysis using a chiral column. | |||
| 1 | — | 82% | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 2 | No BF3 was added after 6 h | 4% | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 3 | pTsOH instead of BF3 | 41% | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
| 4 | Heated to 80 °C instead of BF3 addition | 14% | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
| 5 | BF3 added from the beginning | 94% | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
| 6 | 0.5 equivalents (eq.) BF3 used | <5% | — |
| 7 | Wet PhMe was used | 80% | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 8 | 4 Å MS added from the beginning | 56% | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
Moreover, BF3 must be used in stoichiometric amounts to effectively promote the insertion; catalytic quantities proved insufficient (entry 6). Interestingly, the reaction showed notable tolerance to residual water (entry 7). In contrast, strictly anhydrous conditions using 4 Å molecular sieves (MS) led to a reduced yield (entry 8), providing an initial hint about the underlying mechanism (vide infra).
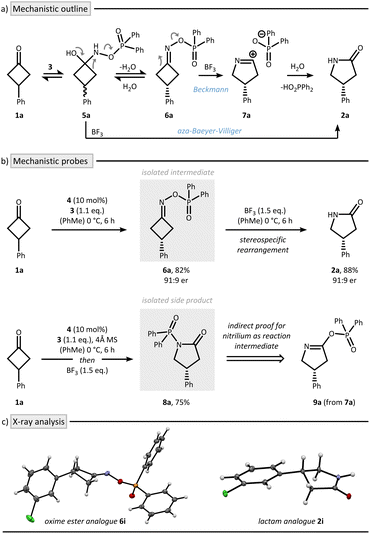
Two mechanistic scenarios are plausible for the nitrogen insertion sequence: (i) asymmetric condensation followed by stereospecific rearrangement (Beckmann pathway), or (ii) enantioselective insertion prior to condensation from prochiral hemiaminal 5a (aza-Baeyer–Villiger pathway) (Fig. 2a). To probe these possibilities, we conducted a series of carefully designed control experiments (Fig. 2b). Isolation of oxime ester intermediate 6a was possible in 82% yield and 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er when conducting the reaction in the absence of BF3. The role of the CPA within this asymmetric condensation process was recently elucidated by density functional theory (DFT) calculations, suggesting the formation of the depicted absolute configuration of 6a.30 Upon treatment with BF3 a stereospecific rearrangement to lactam 2a was initiated. X-ray crystallographic analysis of the respective chloro-derivatives 6i and 2i enabled assignment of the absolute configuration and confirmed their structural identity (Fig. 2c).
9 er when conducting the reaction in the absence of BF3. The role of the CPA within this asymmetric condensation process was recently elucidated by density functional theory (DFT) calculations, suggesting the formation of the depicted absolute configuration of 6a.30 Upon treatment with BF3 a stereospecific rearrangement to lactam 2a was initiated. X-ray crystallographic analysis of the respective chloro-derivatives 6i and 2i enabled assignment of the absolute configuration and confirmed their structural identity (Fig. 2c).
These observations support a Beckmann pathway, in which the CPA catalyzes the initial asymmetric condensation,31,32 while BF3 promotes the subsequent stereospecific rearrangement, explaining the importance of sequential addition which circumvents the problem of product inhibition of the CPA. Under rigorously anhydrous conditions, we were successful in isolating side product 8a. This species likely arises from nitrilium ion capture by the phosphinic acid (7a → 9a), followed by Chapman-type rearrangement33,34 a process only consistent with a Beckmann mechanism.35,36
During the mechanistic analysis, we realized that performing the reaction in a two-step fashion led to a small increase in enantioselectivity for lactam 2a, improving from 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 er to 91
13 er to 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er (cf.Table 1 and Fig. 2b). We attribute this enhancement to the avoidance of undesired racemization of the chiral oxime ester intermediate, likely caused by the formation of a highly acidic environment from residual Brønsted acid and BF3.37 We further found that lowering the temperature to −40 °C during the initial enantio-determining condensation further enhances the enantioselectivity to 95
9 er (cf.Table 1 and Fig. 2b). We attribute this enhancement to the avoidance of undesired racemization of the chiral oxime ester intermediate, likely caused by the formation of a highly acidic environment from residual Brønsted acid and BF3.37 We further found that lowering the temperature to −40 °C during the initial enantio-determining condensation further enhances the enantioselectivity to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
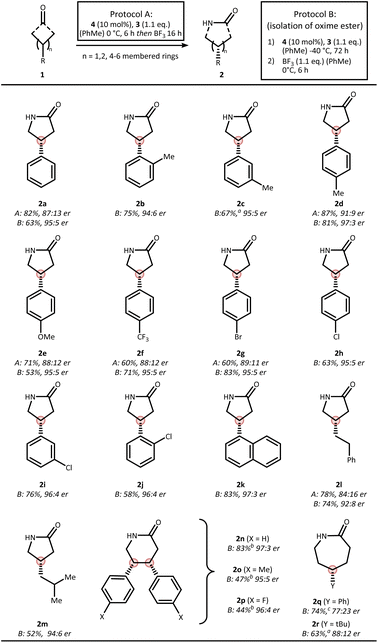
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er. Based on these observations, we decided to explore the scope using both a one-pot protocol A (as described in the optimization) as well as a two-step protocol B, which involves low temperature condensation and removal of the residual CPA after the initial step by a short silica gel purification (Fig. 3).
5 er. Based on these observations, we decided to explore the scope using both a one-pot protocol A (as described in the optimization) as well as a two-step protocol B, which involves low temperature condensation and removal of the residual CPA after the initial step by a short silica gel purification (Fig. 3).
During our scope evaluation, we found that steric and electronic perturbations on the aromatic ring were well tolerated using both Beckmann protocols, affording the corresponding γ-lactams 2a–f in >87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 er using protocol A and consistently >94
13 er using protocol A and consistently >94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er when using protocol B. Halogenation at various positions of the aryl ring – offering synthetic handles for further derivatization – proceeded smoothly to furnish lactams 2g–j with high selectivity observed in all cases (protocol A > 89
6 er when using protocol B. Halogenation at various positions of the aryl ring – offering synthetic handles for further derivatization – proceeded smoothly to furnish lactams 2g–j with high selectivity observed in all cases (protocol A > 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er, protocol B > 95
11 er, protocol B > 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). A naphthyl-substituted substrate was also compatible, delivering γ-lactam 2k in 83% yield and 97
5 er). A naphthyl-substituted substrate was also compatible, delivering γ-lactam 2k in 83% yield and 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er using the two-step protocol. Alkyl-substituted cyclobutanones were tolerated as well, affording 2l and 2m in good yields, albeit with slightly lower enantioselectivity. Notably, the nitrogen insertion was not restricted to cyclobutanones
3 er using the two-step protocol. Alkyl-substituted cyclobutanones were tolerated as well, affording 2l and 2m in good yields, albeit with slightly lower enantioselectivity. Notably, the nitrogen insertion was not restricted to cyclobutanones![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) meso-cyclopentanones also participated in the transformation when using protocol B, providing δ-lactam 2n, 2o, and 2p in good yield and excellent enantioselectivity (>95
meso-cyclopentanones also participated in the transformation when using protocol B, providing δ-lactam 2n, 2o, and 2p in good yield and excellent enantioselectivity (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). Extension to six-membered prochiral cyclohexanones was also feasible using either Et2AlCl or BF3 as Lewis acids. Under these conditions, ε-lactam 2q and 2r were obtained in 74% and 63% yield, albeit with a reduced selectivity of 77
5 er). Extension to six-membered prochiral cyclohexanones was also feasible using either Et2AlCl or BF3 as Lewis acids. Under these conditions, ε-lactam 2q and 2r were obtained in 74% and 63% yield, albeit with a reduced selectivity of 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 er and 88
23 er and 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 er. A key advantage of this protocol lies in its straightforward applicability to less strained cycloalkanones as evidenced by the successful synthesis of lactams 2n–r.
12 er. A key advantage of this protocol lies in its straightforward applicability to less strained cycloalkanones as evidenced by the successful synthesis of lactams 2n–r.
The reaction was also found to be scalable as shown by the synthesis of lactam 2a on 1 mmol scale using protocol B (Fig. 4a). We were able to recover 91% of CPA 4 during this process.38 Interestingly, lactam 2a can be transferred to R-phenotropil (10) in one additional step.39 To further highlight the synthetic utility of an asymmetric Beckmann reaction, we applied protocol B to the synthesis of the phosphordiesterase-4 (PDE-4) inhibitor rolipram (12) starting from isovanillin (11) (Fig. 4b).40–42 The nitrogen insertion was achieved at the final stage of the synthesis in 48% yield and 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er highlighting the applicability of the method for late-stage functionalization (1s → 12). Furthermore, baclofen (13, trade name: Lioresal®), a clinically used drug for muscle spasticity, can be accessed in its enantiopure form from lactam 2h (Fig. 4c).43 This is particularly noteworthy given that baclofen is typically administered as a racemate, despite the well-established differences in biological activity between its enantiomers.44 Similarly, pregabalin (14, trade name: Lyrica®), another γ-aminobutyric acid (GABA) derivative used primarily as an anticonvulsant, was obtained via hydrolysis of lactam 2m in 79% yield.45–47
3 er highlighting the applicability of the method for late-stage functionalization (1s → 12). Furthermore, baclofen (13, trade name: Lioresal®), a clinically used drug for muscle spasticity, can be accessed in its enantiopure form from lactam 2h (Fig. 4c).43 This is particularly noteworthy given that baclofen is typically administered as a racemate, despite the well-established differences in biological activity between its enantiomers.44 Similarly, pregabalin (14, trade name: Lyrica®), another γ-aminobutyric acid (GABA) derivative used primarily as an anticonvulsant, was obtained via hydrolysis of lactam 2m in 79% yield.45–47
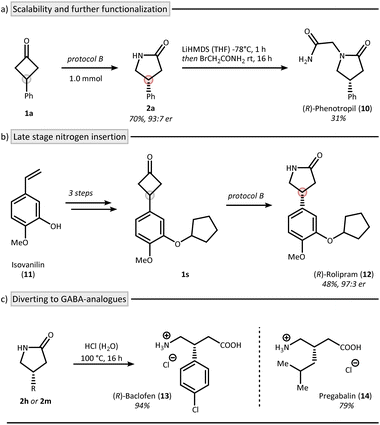 | ||
| Fig. 4 Application of the method through the syntheses of bioactive γ-lactams. Synthesis of phenotropil (a) and rolipram (b), as well as the GABA-derivatives baclofen and pregabalin (c). Protocol B: see Fig. 3. | ||
Conclusions
In summary, this study introduces an asymmetric Beckmann strategy for nitrogen insertion into cyclic ketones via a sequence of enantioselective condensation and stereospecific rearrangement. Key to its success is the combination of DPPH as the nitrogen source, along with a CPA organocatalyst and BF3 as a stereospecific Lewis acid promoter. The sequence can be run in a slightly less selective one-pot protocol as well as a in a two-step process based on the good stability of the oxime ester intermediates. Thus, the developed protocol provides robust access to enantioenriched γ-, δ-, and ε-lactams. The utility of the method is highlighted through the enantioselective synthesis of four pharmaceutically relevant compounds. In light of the fact that 81% of all small-molecule drugs feature nitrogen-containing heterocycles, this work is expected to stimulate further advances in the development of mild and selective nitrogen insertion methods.Author contributions
M. A. and J. M. W. conceived the project. J. H., C. M., A. P., and M. A. carried out the method development and experimental investigations. J. M. W. supervised the project and secured funding. All authors contributed to data analysis, participated in writing the manuscript, and approved the final version.Conflicts of interest
There are no conflicts to declare.Data availability
Experimental data including detailed procedures, characterization of new compounds as well as NMR and HPLC spectra is accessible in the SI.CCDC 2465049 (6i) and 2465050 (2i) contain the supplementary crystallographic data for this paper.48a,b
Supplementary information: experimental data including detailed procedures, characterisation of new compounds as well as NMR and HPLC spectra is accessible in the SI. See DOI: https://doi.org/10.1039/d5sc08417b.
Acknowledgements
Generous financial support from the Johannes Gutenberg-Universität Mainz is acknowledged. MA is grateful to the Sibylle Kalkhof-Rose Stiftung for a PhD fellowship. This work is further supported by an exploration grant of the Boehringer Ingelheim foundation, by the Deutsche Forschungsgemeinschaft (CRC 1552, Project 465145163), and by the European Research Council (ERC, project 101165618 – NINSERT). Luisa Ruhland is thanked for the synthesis of relevant starting materials.Notes and references
- C. M. Marshall, J. G. Federice, C. N. Bell, P. B. Cox and J. T. Njardarson, J. Med. Chem., 2024, 67, 11622 CrossRef CAS.
- J. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong and M. D. Levin, Nat. Synth., 2022, 1, 352 CrossRef CAS PubMed.
- J. Tinge, M. Groothaert, H. op het Veld, J. Ritz, H. Fuchs, H. Kieczka and W. C. Moran, Ullmann's Encycl. Ind. Chem., 2018, 1–31 Search PubMed.
- E. Beckmann and B. Dtsch, Chemurg. Dig., 1886, 19, 988 Search PubMed.
- K. F. Schmidt, Ber. Dtsch. Chem. Ges., 1924, 57, 704 CrossRef.
- A. Lattes, E. Oliveros, M. Riviere, C. Belzeck, D. Mostowicz, W. Abramskj, C. Piccinni-Leopardi, G. Germain and M. van Meerssche, J. Am. Chem. Soc., 1982, 104, 3929 CrossRef CAS.
- J. Aubé, Y. Wang, S. Ghosh and K. L. Langhans, Synth. Commun., 1991, 21, 693 CrossRef.
- K. Sahasrabudhe, V. Gracias, K. Furness, B. T. Smith, C. E. Katz, D. S. Reddy and J. Aubé, J. Am. Chem. Soc., 2003, 125, 7914 CrossRef CAS PubMed.
- J. Sietmann, M. Ong, C. Mück-Lichtenfeld, C. G. Daniliuc and J. M. Wahl, Angew. Chem., Int. Ed., 2021, 60, 9719 CrossRef CAS PubMed.
- M. D. Mihovilovic, B. Müller and P. Stanetty, Eur. J. Org Chem., 2002, 3711 CrossRef CAS.
- M. J. L. J. Fürst, A. Gran-Scheuch, F. S. Aalbers and M. W. Fraaije, ACS Catal., 2019, 9, 11207 CrossRef.
- C. Liu, K.-G. Wen, X.-P. Zeng and Y.-Y. Peng, Adv. Synth. Catal., 2020, 362, 1015 CrossRef CAS.
- F. Capitta, A. Frongia, J. Ollivier, P. P. Piras and F. Secci, Synlett, 2011, 89 CAS.
- R. E. Gawley, in Organic Reactions, 2004, pp. 1–420 Search PubMed.
- H. F. Motiwala, C. Fehl, S.-W. Li, E. Hirt, P. Porubsky and J. Aubé, J. Am. Chem. Soc., 2013, 135, 9000 CrossRef CAS PubMed.
- X. Mo, T. D. R. Morgan, H. T. Ang and D. G. Hall, J. Am. Chem. Soc., 2018, 140, 5264 CrossRef CAS PubMed.
- S. K. Nimmagadda, S. C. Mallojjala, L. Woztas, S. E. Wheeler and J. C. Antilla, Angew. Chem., Int. Ed., 2017, 56, 2454 CrossRef CAS PubMed.
- S.-J. He, S. Zhu, S.-Q. Qiu, W.-Y. Ding, J. K. Cheng, S.-H. Xiang and B. Tan, Angew. Chem., Int. Ed., 2023, 62, e202213914 CrossRef CAS.
- B. Shao, B. Ren, W. Jiang and L. Shi, Org. Lett., 2024, 26, 2646 CrossRef CAS PubMed.
- Y. Tamura, J. Minamikawa and M. Ikeda, Synthesis, 1977, 1 CrossRef CAS.
- V. C. M. Gasser, S. Makai and B. Morandi, Chem. Commun., 2022, 58, 9991 RSC.
- M. Ong, M. Arnold, A. W. Walz and J. M. Wahl, Org. Lett., 2022, 24, 6171 CrossRef CAS PubMed.
- M. Lachia, F. Richard, R. Bigler, A. Kolleth-Krieger, M. Dieckmann, A. Lumbroso, U. Karadeniz, S. Catak and A. de Mesmaeker, Tetrahedron Lett., 2018, 59, 1896 CrossRef CAS.
- P. Jin, P.-J. Liu, Y. Chong, S. Pruksawan, L. Li, Y. Wen, H. Wei and F. Wang, Comput. Theor. Chem., 2024, 1237, 114635 CrossRef CAS.
- C. M. Poteat, Y. Jang, M. Jung, J. D. Johnson, R. G. Williams and V. N. G. Lindsay, Angew. Chem., Int. Ed., 2020, 59, 18655 CrossRef CAS PubMed.
- S. Shorvon, Lancet, 2001, 358, 1885 CrossRef CAS.
- J. Jia, Q. Xu, H. Fan, Y. Lv and D. Li, Org. Chem. Front., 2025, 12, 960 RSC.
- S. Xu, Z. Wang, X. Zhang, X. Zhang and K. Ding, Angew. Chem., Int. Ed., 2008, 47, 2840 CrossRef CAS PubMed.
- M. Yang, Y.-M. Zhao, S.-Y. Zhang, Y.-Q. Tu and F.-M. Zhang, Chem.–Asian J., 2011, 6, 1344 CrossRef CAS PubMed.
- M. Arnold, J. Hammes, M. Ong, C. Mück-Lichtenfeld and J. M. Wahl, Angew. Chem., Int. Ed., 2025, 64, e202503056 CrossRef CAS PubMed.
- G. Cheng, J. Zheng, Y. Zhu, Y. Yang, G. Qiu, W.-Y. Ding and S. Li, Org. Lett., 2025, 27, 2509 CrossRef CAS PubMed.
- X. Zhang, J.-Q. Zhang, Z.-H. Sun, H.-M. Shan, J.-C. Su, X.-P. Ma, G.-F. Su, L.-P. Xu and D.-L. Mo, Angew. Chem., Int. Ed., 2025, 64, e202420390 CrossRef CAS PubMed.
- A. W. Chapman, J. Chem. Soc., 1925, 127, 1992 RSC.
- B. C. Challis and A. D. Frenkel, J. Chem. Soc., Chem. Commun., 1972, 303 RSC.
- K. Maruoka, T. Miyazaki, M. Ando, Y. Matsumura, S. Sakane, K. Hattori and H. Yamamoto, J. Am. Chem. Soc., 1983, 105, 2831 CrossRef CAS.
- Y. Yamamoto, H. Hasegawa and H. Yamataka, J. Org. Chem., 2011, 76, 4652 CrossRef CAS PubMed.
- J. Mas-Roselló, T. Smejkal and N. Cramer, Science, 2020, 368, 1098 CrossRef PubMed.
- M. Klussmann, L. Ratjen, S. Hoffmann, V. Wakchaure, R. Goddard and B. List, Synlett, 2010, 2010, 2189 CrossRef.
- A. N. Reznikov, E. V. Golovin and Y. N. Klimochkin, Russ. J. Org. Chem., 2013, 49, 663 CrossRef CAS.
- W. Krause, G. Kühne and N. Sauerbrey, Eur. J. Clin. Pharmacol, 1990, 38, 71 CrossRef CAS PubMed.
- K. Daishiro, T. Hideo, I. Fumihiro, K. Kazuo, S. Shigeki and H. Toshio, Heterocycles, 1996, 42, 109 CrossRef.
- J. Zhu, E. Mix and B. Winblad, CNS Drug Rev., 2001, 7, 387 CrossRef CAS PubMed.
- N. G. Bowery, D. R. Hill, A. L. Hudson, A. Doble, D. N. Middlemiss, J. Shaw and M. Turnbull, Nature, 1980, 283, 92 CrossRef CAS PubMed.
- H.-R. Olpe, H. Demiéville, V. Baltzer, W. L. Bencze, W. P. Koella, P. Wolf and H. L. Haas, Eur. J. Pharmacol., 1978, 52, 133 CrossRef CAS.
- E. Ben-Menachem, Epilepsia, 2004, 45, 13 CrossRef CAS PubMed.
- C. P. Taylor, T. Angelotti and E. Fauman, Epilepsy Res., 2007, 73, 137 CrossRef CAS PubMed.
- A. Leyva-Pérez, P. García-García and A. Corma, Angew. Chem., Int. Ed., 2014, 53, 8687 CrossRef.
- (a) CCDC 2465049: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc12kl18; (b) CCDC 2465050: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nr2r8.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |