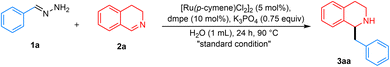Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A general aqueous synthetic strategy towards 1-benzylTHIQs enabled by umpolung hydrazone
Manpreet
Kaur
 ,
Evan F. W.
Chen
,
Evan F. W.
Chen
 ,
Jan Michael
Salgado
,
Jan Michael
Salgado
 ,
Ruofei
Cheng
and
Chao-Jun
Li
,
Ruofei
Cheng
and
Chao-Jun
Li
 *
*
Department of Chemistry, and FRQNT Centre for Green Chemistry and Catalysis, McGill University, 801 Sherbrooke Street West, Montreal, QC H3A 0B8, Canada. E-mail: cj.li@mcgill.ca
First published on 9th January 2026
Abstract
Primarily found in plants, 1-benzyltetrahydroisoquinoline (1-benzylTHIQ) alkaloids are a diverse class of N-heterocyclic natural products with biological activity against various infectious diseases and neurodegenerative pathologies. Traditionally, 1-benzylTHIQs are synthesized using commercially inaccessible pre-functionalized materials or hazardous organometallic reagents, making their synthesis challenging. Herein, we developed an environmentally benign synthetic strategy to synthesize 1-benzylTHIQs, which aligns with green chemistry principles. This method utilizes abundant, renewable aldehydes as sustainable alkyl carbanion equivalents, thereby eliminating the use of highly reactive or hazardous organometallic reagents. This reaction is catalyzed by ruthenium, using water as a greener solvent, eliminating the need for organic solvents, thereby reducing its environmental impact. Moreover, only N2 and H2O are produced as by-products, which minimizes waste generation. A diverse array of substituted 1-benzylTHIQs was synthesized, showing good functional group tolerance (without the need to protect the functional groups) and resulting in moderate to excellent yields. The sustainability of our method was further demonstrated through the synthesis of natural 1-benzylTHIQ based alkaloids and late-stage functionalization of pharmacologically relevant molecules.
Introduction
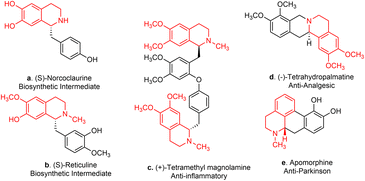
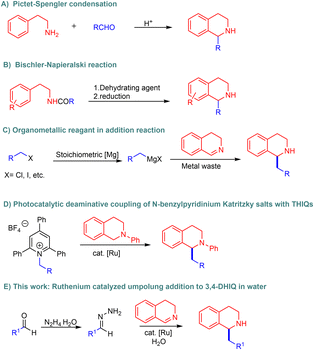
Tetrahydroisoquinolines (THIQ) are defined as the saturated derivatives of isoquinoline, which are structurally distinguished by a fused piperidine ring. Their derivatives have been the subject of extensive research for decades due to their wide therapeutic potential and pharmacological applications.1–4 Notably, C1-functionalization of THIQs serves as a highly effective and versatile technique for the synthesis of polycyclic and multi-substituted derivatives, such as 1-benzylTHIQs.5 These important motifs are commonly found in medicinally relevant bioactive compounds and naturally derived alkaloids.3,6,7 Well-known examples include tetrahydropalmatine (Fig. 1d) and morphine, which serve as analgesics, and berberine, valued for its antiseptic activity.8,9 Other 1-benzylTHIQs, such as norcoclaurine and reticuline, serve as significant precursors in the biosynthesis of morphine and codeine (Fig. 1a and b).7Owing to the pharmaceutical relevance of C1-functionalized compounds and their utility as precursors in alkaloid biosynthesis, over the past decades, synthetic chemists have shown considerable interest in developing complex THIQs by functionalizing simple isoquinolines. Traditional approaches, such as the Pictet–Spengler condensation10 (Fig. 2A) and the Bischler–Napieralski reaction (Fig. 2B), are commonly used to synthesize 1-benzylTHIQs.11,12 However, these methods have notable drawbacks, including low yields, the need for higher temperatures and extended reaction times, and the use of hazardous reagents such as strong acids.
Alternatively, other pathways to access 1-benzylTHIQs include the Grignard addition of benzyl organometallic reagents to 3,4-dihydroisoquinolines (3,4-DHIQs) (Fig. 2C).13 However, most organometallic reagents are synthesized using stoichiometric amounts of the corresponding metals and organic halides. Their use also generates copious amounts of metal halide waste while exhibiting high moisture sensitivity and requires special handling under inert conditions. Moreover, as reported in previous literature, catalytic hydrogenation of isoquinolines has also been explored for the synthesis of THIQs.14–16 In recent years, photocatalytic strategies have emerged as promising alternatives. For example, visible-light driven functionalization of N-aryl tetrahydroisoquinolines has been reported, providing access to structurally diverse derivatives.17–19 Additionally, a Ru-photocatalyzed coupling of N-aryl THIQs with Katritzky salts has recently been developed to produce 1-benzylTHIQs (Fig. 2D).20 Despite this advancement, the method suffers from several limitations, including poor reaction efficiency, a narrow substrate scope, and limited functional group tolerance. Furthermore, commercially inaccessible pyridinium salts hinder the accessibility of this methodology. Given the significant limitations in current methods, a simple and sustainable methodology for the synthesis of 1-benzylTHIQs is highly desired.
Recently, our research group pioneered methodologies for using hydrazones as organometallic equivalents (HOME) in nucleophilic addition and cross-coupling reactions.21,22 In this approach, carbonyl-derived hydrazones act as alkyl carbanion equivalents, coupling with diverse electrophilic partners in the presence of a catalytic amount of transition metals. This strategy involves preparing hydrazones from naturally abundant alcohols and carbonyl compounds derived from renewable feedstocks, generating water and nitrogen gas as innocuous by-products under mild reaction conditions. Previously, our group reported the Ru and Fe-catalyzed umpolung addition of hydrazones to imines to synthesize secondary amines.23,24 In addition, ruthenium has been shown to tolerate aqueous environments.25 Recent efforts have focused on developing green solvents to replace petroleum-derived alternatives, with emphasis on low toxicity, abundance, safety, cost-effectiveness, and ease of separation. Water, an inexpensive and abundant solvent, aligns with global demands for sustainable and green chemistry.26–29 However, the application of this system in THIQ synthesis remains unexplored. Herein, we report a Ru-catalyzed umpolung addition of aryl aldehyde hydrazones onto 3,4-DHIQs in water (Fig. 2E).
Results and discussion
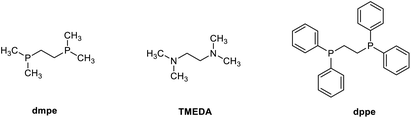
At the beginning of our investigation, benzaldehyde hydrazone 1a (2 equiv.) was chosen as the model nucleophile reagent, reacting with 3,4-DHIQ 2a (0.2 mmol) as the model electrophile under an atmosphere of N2 in THF as solvent. Based on previous literature,23,24,30 we initially screened Ru, Mn, and Fe catalysts. We observed that [Ru(p-cymene)Cl2]2 in combination with bis(dimethylphosphino)ethane (dmpe) afforded 3aa in 20% yield (Table 1, entry 1), while no product was observed for the other metals. Then, we explored a variety of monodentate and bidentate phosphine ligands. Monodentate phosphines such as PMe3 gave 3aa in 23% yield (entry 2), whereas no product 3aa was observed when 1,2-bis(diphenylphosphino)ethane (dppe) was employed (entry 3). Moreover, amine-based bidentate ligands such as N,N,N′,N′-tetramethyl ethylenediamine (TMEDA) also yielded 3aa in 16% yield (entry 4). These results indicate that the reaction was favored by electron-rich and non-bulky phosphine and nitrogen ligands. When the reaction was carried out in other organic solvents such as dioxane and toluene, no improvement in the yield of 3aa was observed (entries 5 and 6). To our surprise, switching the solvent from organic to H2O improved the yield of 3aa to 70% (entry 7). This improvement in yield could be due to the hydrophobic effect in water. This effect increases the reaction rate, even when the reactants have poor or no solubility in the solvent.31–34 To further improve the yield, catalytic amounts of different phase transfer catalysts were added. Tetrabutylammonium iodide (TBAI) and TPGS-750M were added in catalytic amounts to give 3aa in 86% and 71% yield, respectively (entries 8 and 9). Different bases were also screened for this reaction, with K3PO4 being the most efficient (see SI and Table 1). Control experiments showed that both catalyst and ligand are required for the reaction (entries 10 and 11). Without surfactant, the reaction gave a 70% yield of product 3aa (entry 7). When carried out under air, the reaction yielded 73% of 3aa (entry 12).| Entry | Deviation from standard conditionsa | 3aa (yield%) |
|---|---|---|
| a Standard conditions: benzaldehyde hydrazone 1a (0.4 mmol, 2 equiv.), 3,4-DHIQ 2 (0.2 mmol), [Ru(p-cymene)Cl2]2 (5 mol%), ligand (10 mol% for bidentate, 20 mol% for monodentate), surfactant (20 mol%), and K3PO4 (0.15 mmol, 0.75 equiv.) in 1 mL H2O for 24 h at 90 °C under Ar. Yields were determined by 1H NMR with 1,3,5-trimethoxybenzene as an internal standard. | ||
| 1 | THF as solvent | 20 |
| 2 | PMe3 as ligand and THF as solvent | 23 |
| 3 | dppe as ligand and THF as solvent | 0 |
| 4 | TMEDA as ligand and THF as solvent | 16 |
| 5 | Dioxane as solvent | 20 |
| 6 | Toluene as solvent | 18 |
| 7 | None | 70 |
| 8 | TBAI as surfactant | 86 |
| 9 | TPGS-750M as surfactant | 71 |
| 10 | Without [Ru(p-cymene)Cl2]2 | 3 |
| 11 | Without dmpe | 0 |
| 12 | Under air | 73 |
|
||
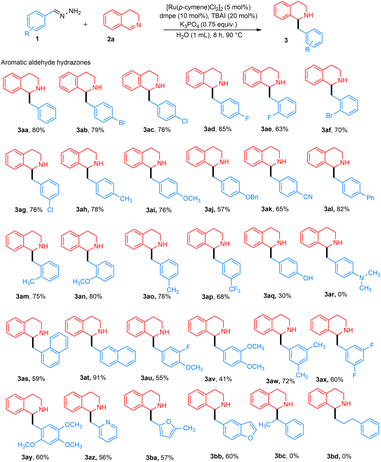
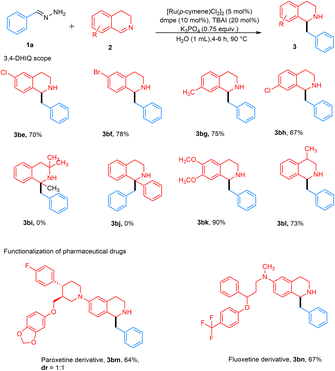
With our optimized conditions from Table 1, entry 8, we next investigated the substrate scope. Various aryl-substituted hydrazones were explored (Fig. 3). Although complete conversion was achieved with benzaldehyde hydrazone 1 and 3,4-DHIQ 2a within 4 h, other substituted hydrazones led to incomplete conversion of 2a. Therefore, the reaction time was extended to 8 h to achieve complete conversion. In general, aryl hydrazones with various substitution patterns afforded moderate to excellent yields.
Hydrazones containing different para-substituted halogens, such as chloro, bromo, and fluoro-groups, gave the desired products 3ab–3ad with 65–79% yields. Similarly, meta- and ortho-halogenated substrates yielded products 3ae–3ag with 63–76% yields. para-Substituted hydrazones with electron-donating groups, such as methyl, methoxy, and benzyloxy, produced desired products 3ah–3aj in moderate to high yields of 57–78%. Hydrazones containing para-substituted electron-withdrawing groups, such as cyano and phenyl, yielded the desired products 3ak and 3al in high yields ranging from 65–82%. These results indicate that electron-withdrawing groups contribute to improved reaction efficiency, likely due to carbanion stabilization, while both electron-donating and withdrawing groups enable successful product formation. ortho-Substituted electron-donating groups such as methoxy and methyl performed well, resulting in the desired products 3am and 3an with yields of 75–80%. meta-Substituted benzaldehyde hydrazones were also investigated and gave desired products 3ao–3ap in moderately high yields of 68–76%. In the case of a strongly electron-donating group such as a para-substituted hydroxy group, product 3aq was obtained in a 30% NMR yield. In contrast, no product 3ar was detected for the NMe2 group, due to the unfavorable formation of the carbanion. 1- and 2-Naphthaldehyde hydrazones worked well, giving the desired products 3as and 3at in yields of 59% and 91%, respectively. The lower yield for 1-naphthaldehyde hydrazone might be due to steric hindrance. Benzaldehyde hydrazones with multiple substituents also proved to be effective substrates, yielding products 3au–3ay in moderate yields of 41–72%.
Heteroaryl aldehyde hydrazones also worked well, affording products 3az–3bb in moderate yields of 56–60%. Acetophenone hydrazones were also investigated, but no product 3bc was observed. The reason could be due to increased bulkiness around the benzylic position of the generated carbanion, as well as the steric hindrance from 3,4-DHIQ, which resulted in no reaction. Consequently, aliphatic hydrazones like 3-phenylpropanal hydrazone did not yield product 3bd due to the absence of an aromatic ring to stabilize the carbanion intermediate.
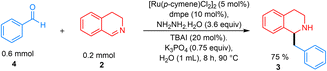
Subsequently, various substituted 3,4-DHIQs were investigated (Fig. 4). 6-Substituted chloro and bromo 3,4-DHIQs gave desired products 3be–3bf in high yields of 70–78%. Likewise, 7-substituted chloro and methyl 3,4-DHIQs afforded the desired products 3bg and 3bh in high yields of 67–75%. We also tested our conditions with 1-substituted 3,4-DHIQs, which did not give product 3bi and 3bj. This substitution pattern introduced significant steric hindrance around the 1-position, possibly preventing nucleophilic addition. 6,7-Disubstituted dimethoxy 3,4-DHIQ gave 3bk with an excellent yield of 90%. The reason for this high yield could be that the chelation of methoxy groups with either the ruthenium catalyst or the K cation increases the electrophile's reactivity, making it more susceptible to a carbanion attack. 4-Substituted methyl 3,4-DHIQ also gave a good yield of around 73%. Late-stage functionalization of drugs was then investigated. The reaction with paroxetine and fluoxetine derivatives proceeded well, affording 3bm and 3bn in 64% and 67% yields, respectively. Finally, this method was assessed on a larger scale, and gratifyingly, a 79% yield of 3 was obtained from 1.00 g (7.62 mmol) of 3,4-DHIQ (Fig. 5).
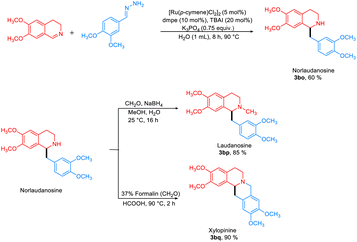
The hydrazine-mediated addition of benzaldehyde to 3,4-DHIQ gave the desired product in 75% yield (Fig. 6). To demonstrate the synthetic applicability of this approach, norlaudanosine (3bo) was synthesized in 60% yield, which was subsequently converted to laudanosine 3bp (85%) and xylopinine 3bq (90%), respectively (Fig. 7).
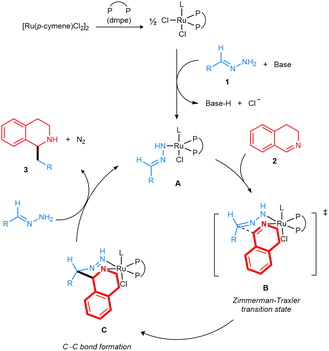
Based on the previous literature23–25 we have proposed the following mechanism (Fig. 8). The Ru complex was generated from [Ru(p-cymene)Cl2]2 with the dmpe ligand. Then, the coordination of deprotonated hydrazone 1a to the dmpe-Ru catalyst formed complex A. The resulting intermediate would combine with 3,4-DHIQ to form a Zimmerman–Traxler chair-like transition state B. Next, intramolecular nucleophilic attack of the carbanion generated from the hydrazone forms the C–C bond, leading to complex C. Finally, by the release of N2 under the assistance of the base and coordination of another deprotonated hydrazone, the addition product 3aa was generated, and complex A was reformed.
Conclusions
In summary, we have developed a method for the umpolung addition of hydrazones to 3,4-DHIQs mediated by a Ru(dmpe) catalytic system. This method facilitates the synthesis of 1-benzylTHIQs through the functionalization of simple 3,4-DHIQs using water as the solvent. The synthetic potential of this method is demonstrated by its broad substrate scope, excellent functional group tolerance, and its applicability to the synthesis of natural alkaloids and the late-stage functionalization of drugs. Earlier methods use DMA, acetonitrile, or THF as solvents, whereas this approach employs a greener solvent. Our method shows a significant improvement over previously reported protocols by adhering to several green chemistry principles, such as waste prevention, the use of renewable feedstocks, safer solvents, and catalysis. Consequently, the method provides a convenient and sustainable route for the functionalization of 3,4-DHIQ.Author contributions
MK optimized the reaction, synthesized and purified the substrate scope, and prepared the manuscript. EFWC contributed to the purification of the compounds, general guidance, and manuscript editing. RC provided general guidance and manuscript editing. JMS assisted in the substrate scope and helped in manuscript editing. CJL conceptualized the idea, provided general guidance and edited the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
Supplementary information (SI): all experimental procedures, optimization data and characterisation data of all synthesized compounds. See DOI: https://doi.org/10.1039/d5sc08310a.Acknowledgements
We sincerely acknowledge the Canada Research Chair (Tier I) Foundation, the E. B. Eddy Endowment Fund, Centre en Chimie Verte et Catalyse, the Canada Foundation for Innovation, and the Natural Sciences and Engineering Research Council of Canada for supporting our research. EFWC acknowledges the funding provided by NSERC (Canada Graduate Research Scholarship – Doctoral), McGill Faculty of Science (Dr Lawrence Light Fellowship in Sustainability) and the Walter C. Sumner Foundation (Walter C. Sumner Memorial Fellowship). The authors also thank A. Wahba for assistance with HRMS analysis and the McGill Magnetic Resonance Facility for their support.Notes and references
- D. Thangeswaran, S. Shamsuddin and V. Balakrishnan, A comprehensive review on the progress and challenges of tetrahydroisoquinoline derivatives as a promising therapeutic agent to treat Alzheimer’s disease, Heliyon, 2024, 10(10), e30788 CrossRef CAS PubMed
.
- Faheem, B. Karan Kumar, K. Venkata Gowri Chandra Sekhar, S. Chander, S. Kunjiappan and S. Murugesan, 1,2,3,4-Tetrahydroisoquinoline (THIQ) as privileged scaffold for anticancer de novo drug design, Expert Opin. Drug Discovery, 2021, 16(10), 1119–1147 CrossRef CAS
.
- I. P. Singh and P. Shah, Tetrahydroisoquinolines in therapeutics: a patent review (2010-2015), Expert Opin. Ther. Pat., 2017, 27(1), 17–36 CrossRef CAS PubMed
.
- A. N. Kim, A. Ngamnithiporn, E. Du and B. M. Stoltz, Recent advances in the total synthesis of the tetrahydroisoquinoline alkaloids (2002–2020), Chem. Rev., 2023, 123(15), 9447–9496 Search PubMed
.
- Z. Tashrifi, M. Mohammadi Khanaposhtani, B. Larijani and M. Mahdavi,
C1-functionalization of 1,2,3,4-tetrahydroisoquinolines (THIQs), Asian J. Org. Chem., 2021, 10(10), 2421–2439 CrossRef CAS
.
- B. K. Kumar, K. V. G. C. Sekhar, S. Chander, S. Kunjiappan and S. Murugesan, Medicinal chemistry perspectives of 1,2,3,4-tetrahydroisoquinoline analogs–biological activities and SAR studies, RSC Adv., 2021, 11(20), 12254–12287 RSC
.
- A. K. Tanwar, N. Sengar, N. Mase and I. P. Singh, Tetrahydroisoquinolines–an updated patent review for cancer treatment (2016–present), Expert Opin. Ther. Pat., 2024, 34(10), 873–906 Search PubMed
.
- J. M. Hagel and P. J. Facchini, Benzylisoquinoline alkaloid metabolism: a century of discovery and a brave new world, Plant Cell Physiol., 2013, 54(5), 647–672 Search PubMed
.
- P. J. Facchini, C. Penzes, A. G. Johnson and D. Bull, Molecular characterization of berberine bridge enzyme genes from opium poppy, Plant Physiol., 1996, 112(4), 1669–1677 Search PubMed
.
- C. Kuhakarn, N. Panyachariwat and S. Ruchirawat, A variation of the Pictet–Spengler reaction via a sequential reduction–cyclization reaction of N-acylcarbamates: synthesis of 1-substituted tetrahydroisoquinoline derivatives, Tetrahedron Lett., 2007, 48(46), 8182–8184 Search PubMed
.
- M. Mihoubi, N. Micale, A. Scala, R. M. Jarraya, A. Bouaziz, T. Schirmeister, F. Risitano, A. Piperno and G. Grassi, Synthesis of C3/C1-substituted tetrahydroisoquinolines, Molecules, 2015, 20(8), 14902–14914 Search PubMed
.
- M. Nicoletti, D. O'Hagan and A. M. Slawin, The asymmetric Bischler–Napieralski reaction: preparation of 1,3,4-trisubstituted 1,2,3,4-tetrahydroisoquinolines, J. Chem. Soc., Perkin Trans. 1, 2002, 116–121 CAS
.
- Y. Al-Hiari, B. Sweileh, A. Shakya, G. A. Sheikha and S. Almuhtaseb, Synthesis of 1-benzyl-1,2,3,4-tetrahydroisoquinoline, Part I: Grignard synthesis of 1-(substitutedbenzyl)-1,2,3,4-tetrahydroisoquinoline models with potential antibacterial activity, Jordan J. Pharm. Sci., 2009, 2(1) Search PubMed
.
- L. Shi, Z. S. Ye, L. L. Cao, R. N. Guo, Y. Hu and Y. G. Zhou, Enantioselective Iridium-catalyzed hydrogenation of 3,4-disubstituted isoquinolines, Angew. Chem., Int. Ed., 2012, 51(33), 8286 CrossRef CAS PubMed
.
- Y. Wang, L. Zhu, Z. Shao, G. Li, Y. Lan and Q. Liu, Unmasking the ligand effect in manganese-catalyzed hydrogenation: mechanistic insight and catalytic application, J. Am. Chem. Soc., 2019, 141(43), 17337–17349 Search PubMed
.
- A. N. Kim and B. M. Stoltz, Recent advances in homogeneous catalysts for the asymmetric hydrogenation of heteroarenes, ACS Catal., 2020, 10(23), 13834–13851 CrossRef CAS PubMed
.
- Z. Li, P. Ma, Y. Tan, Y. Liu, M. Gao, Y. Zhang, B. Yang, X. Huang, Y. Gao and J. Zhang, Photocatalyst-and transition-metal-free α-allylation of N-aryl tetrahydroisoquinolines mediated by visible light, Green Chem., 2020, 22(3), 646–650 RSC
.
- Y. Zhao, X. Hou, M. He, Y. Wang, S. Yang, W. Wang, M. Bao and X. Yu, Visible-light-driven α-substituted amines enabled by in situ formation of amine substrate aggregates, Org. Lett., 2023, 25(40), 7344–7348 CrossRef CAS PubMed
.
- W. J. Zhou, G. M. Cao, G. Shen, X. Y. Zhu, Y. Y. Gui, J. H. Ye, L. Sun, L. L. Liao, J. Li and D. G. Yu, Visible-light-driven Palladium-catalyzed radical alkylation of C–H bonds with unactivated alkyl bromides, Angew. Chem., 2017, 129(49), 15889–15893 Search PubMed
.
- D. Schönbauer, C. Sambiagio, T. Noël and M. Schnürch, Photocatalytic deaminative benzylation and alkylation of tetrahydroisoquinolines with N-alkylpyrydinium salts, Beilstein J. Org. Chem., 2020, 16(1), 809–817 CrossRef PubMed
.
- X.-J. Dai, C.-C. Li and C.-J. Li, Carbonyl umpolung as an organometallic reagent surrogate, Chem. Soc. Rev., 2021, 50(19), 10733–10742 RSC
.
- C.-J. Li, HOME-Chemistry: hydrazone as organo-metallic equivalent, Pure Appl. Chem., 2023, 95(5), 465–474 CrossRef CAS
.
- N. Chen, X. J. Dai, H. Wang and C. J. Li, Umpolung addition of aldehydes to aryl imines, Angew. Chem., 2017, 129(22), 6356–6359 CrossRef
.
- C.-C. Li, X.-J. Dai, H. Wang, D. Zhu, J. Gao and C.-J. Li, Iron-catalyzed nucleophilic addition reaction of organic carbanion equivalents via hydrazones, Org. Lett., 2018, 20(13), 3801–3805 CrossRef CAS PubMed
.
- Y.-Z. Wang, S.-D. Liu, L. Cheng, L. Liu and C.-J. Li, Asymmetric addition of hydrazones as alkyl carbanion equivalents with aryl imines in water, Org. Chem. Front., 2023, 10(12), 3021–3026 RSC
.
- R. N. Butler and A. G. Coyne, Water: nature’s reaction enforcer—comparative effects for organic synthesis “In-water” and “On-water”, Chem. Rev., 2010, 110(10), 6302–6337 CrossRef CAS
.
-
C.-J. Li and T.-H. Chan, Comprehensive Organic Reactions in Aqueous Media, John Wiley & Sons, New York, 2007 Search PubMed
.
-
P. Dixneuf and V. Cadierno, Metal-Catalyzed Reactions in Water, John Wiley & Sons, New York, 2013 Search PubMed
.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 selection guide of classical-and less classical-solvents, Green Chem., 2016, 18(1), 288–296 RSC
.
- J. M. Salgado, D. J. Castillo-Pazos, J. D. Lasso, K. L. Stock and C.-J. Li, Manganese-catalyzed nucleophilic addition of aldehydes to carbonyl compounds via hydrazone umpolung on water, Green Chem., 2024, 26(12), 7357–7362 RSC
.
- F. Zhou, Z. Hearne and C.-J. Li, Water—the greenest solvent overall, Curr. Opin. Green Sustainable Chem., 2019, 18, 118–123 CrossRef
.
- D. C. Rideout and R. Breslow, Hydrophobic acceleration of Diels-Alder reactions, J. Am. Chem. Soc., 1980, 102(26), 7816–7817 CrossRef CAS
.
- R. N. Butler, W. J. Cunningham, A. G. Coyne and L. A. Burke, The influence of water on the rates of 1,3-dipolar cycloaddition reactions: trigger points for exponential rate increases in water–organic solvent mixtures. water-super versus water-normal dipolarophiles, J. Am. Chem. Soc., 2004, 126(38), 11923–11929 CrossRef CAS
.
- C.-J. Li and L. Chen, Organic chemistry in water, Chem. Soc. Rev., 2006, 35(1), 68–82 RSC
.
| This journal is © The Royal Society of Chemistry 2026 |